Abstract
The dynamic character of G protein-coupled receptors is essential to their function. However, the details of how ligands stabilize a particular conformation to selectively activate a signaling pathway and how signaling proteins affect this conformational repertoire remain unclear. Using a prototypical peptide-activated class A G protein-coupled receptor (GPCR), the ghrelin receptor, reconstituted as a monomer into lipid discs and labeled with a fluorescent conformational reporter, we demonstrate that ligand efficacy and functional selectivity are directly related to different receptor conformations. Of importance, our data bring direct evidence that distinct effector proteins affect the conformational landscape of the ghrelin receptor in different ways. Whereas G proteins affect the balance between active and inactive receptor substates in favor of the active state, agonist-induced arrestin recruitment is accompanied by a marked change in the structural features of the receptor that adopt a conformation different from that observed in the absence of arrestin. In contrast to G proteins and arrestins, μ-AP2 has no significant effect on the organization of the transmembrane core of the receptor. Such a modulation of a GPCR conformational landscape by pharmacologically distinct ligands and effectors provides insights into the structural bases that decisively affect ligand efficacy and subsequent biological responses. This is also likely to have major implications for the design of drugs activating specific GPCR-associated signaling pathways.
Keywords: membrane protein, structural repertoire, biased ligands, constitutive activity
Major progress has been made in past years in understanding the molecular bases of G protein-coupled receptor (GPCR)-mediated signaling. Spectacular developments in GPCR stabilization and crystallization have resulted in many different crystal structures of active and inactive receptors (1) and ultimately led to the structure of the agonist-activated β2-adrenergic receptor in complex with its cognate Gs protein (2). However, these structures still represent unique states, whereas the dynamic character of receptors is essential for their physiological functions. Indeed, many reports point at a model where GPCRs are highly dynamic proteins capable of adopting a large number of conformational states that signal with different efficacies to various pathways (3). The most detailed description of the conformational repertoire that a GPCR can adopt has been obtained in the case of the β2-adrenergic receptor with an experimental demonstration of a complex conformational landscape where ligands with distinct pharmacological properties shift the conformation of the receptor from one state to another (4–11). In the same way, we showed that ligands with different efficacies stabilize different conformations of the serotonin receptor (12). Despite this wealth of data, understanding of GPCR activation mechanisms, and in particular of the necessary influence of signaling proteins on the receptor conformational repertoire, is still incomplete.
We have used the purified ghrelin receptor GHS-R1a (Growth Hormone Secretagogue Receptor 1a) to analyze how ligands and signaling proteins impact the conformation of this receptor. Ghrelin is a neuroendocrine peptide hormone that acts through its cognate GPCR to control diverse processes such as growth hormone secretion, food intake, or reward-seeking behaviors (13). In addition to its importance for drug design, GHS-R1a is a prototype for peptide-activated class A GPCRs that allows the molecular processes of ghrelin-mediated signaling to be extended to this major class of receptors. We reconstituted into lipid-disk particles a monomeric ghrelin receptor labeled in either its extracellular or intracellular regions with a fluorescent conformational reporter. Combining conventional fluorescence spectroscopy, which represents an average intensity over a population of fluorescently labeled proteins, and fluorescence lifetime spectroscopy, which can distinguish substates within a population of fluorescent molecules, we bring direct evidence that ligands with distinct pharmacological properties stabilize specific receptor conformations. Importantly, we demonstrate that effector proteins such as G proteins and arrestins affect this conformational repertoire in different ways, either by modifying the relative substate populations or by stabilizing a different GHS-R1a conformation.
Results
Bimane Fluorescence Emission Reveals Distinct Ligand-Dependent GHS-R1a Conformations in the Absence of Effector Proteins.
To analyze ligand- and protein-induced conformational changes of the ghrelin receptor, we devised a fluorescence-based approach that relies on the use of receptor preparations with a unique bimane fluorescent probe. Bimane is a particularly well-adapted probe because of its small size and its sensitivity to the polarity of its immediate environment (7). GHS-R1a was assembled as a monomer into lipid discs (14) and labeled through unique reactive cysteines either in its intracellular region (C146 in the proximal part of the second intracellular loop ICL2) or in its extracellular domain (C304 in the extracellular tip of TM7) (SI Appendix, Fig. S1). Labeling the receptor at either of these positions did not affect the pharmacological properties of the purified receptor (SI Appendix, Figs. S2–S5) and provides valuable sensors to report on conformational changes on the basis of molecular modeling experiments. Indeed, a significant reorientation of the bimane moiety, associated with a modification of the environment of the probe, was observed along the activation pathway of GHS-R1a for both C146 and C304 positions (SI Appendix, Figs. S6 and S7).
We first explored the influence of ligands on the conformational repertoire of GHS-R1a in the absence of effector proteins. Ligands with distinct pharmacological properties were selected: full agonists (ghrelin, MK0677, JMV 1843) (15) that trigger G protein activation (Gi/Gq) and arrestin recruitment (SI Appendix, Fig. S2); biased agonists (JMV 3002, JMV 3018) (16) that trigger Gq activation with a lower efficacy than ghrelin and do not trigger Gi activation or arrestin recruitment (SI Appendix, Fig. S2); Substance P Analog (SPA), which is an inverse agonist that reverts basal Gq activation and does not induce any arrestin recruitment (SI Appendix, Fig. S2) (14, 17). All these ligands also display different behaviors toward recruitment of the purified μ-subunit of the adaptor protein AP2: the ligand-free receptor interacts with μ-AP2, full agonists and partial agonists do not affect this interaction, and SPA binding is accompanied by a dissociation of the GHS-R1a:μ-AP2 complex (14).
As shown in Fig. 1, binding of the full agonists in the absence of any effector was accompanied by a significant change in bimane emission properties. Emission intensity decreased whether the probe was attached to C146 or to C304. This indicates that binding of these compounds was associated with a change in receptor conformation that impacted on the environment of bimane in both the extracellular and the intracellular regions of GHS-R1a. The same change was observed for three molecules that differ in their chemical structure, i.e., the natural ghrelin peptide and the two unrelated synthetic compounds MK0677 and JMV 1843 (Fig. 1). This suggests that these three pharmacologically related but structurally different ligands trigger similar receptor conformational changes.
Fig. 1.
Functionally different ligands trigger different GHS-R1a conformations. (A) Fluorescence emission spectra of bimane attached to C146 in the absence of ligand or in the presence of ghrelin, of JMV 3018, or of SPA. (B) Fluorescence emission spectra of bimane attached to C304 in the absence of ligand or in the presence of ghrelin or of JMV 3018. (C) Fluorescence emission spectra of bimane attached to C304 in the absence of ligand or in the presence of SPA. (D and E) Percentage changes in maximum emission intensity induced by functionally different ligands in the absence of protein effector. The data are representative of two experiments performed in duplicate, and the error bar represents the SEM.
JMV 3002 and JMV 3018 also induced a decrease in bimane emission intensity whether the probe was located in TM7 or in ICL2 (Fig. 1), indicating that binding of these ligands was again accompanied by a change in GHS-R1a conformation. Both compounds triggered the same changes, suggesting that they stabilize similar conformations. Interestingly, the decrease in emission intensity in the presence of JMV 3002 or JMV 3018 was of lower amplitude than that observed with ghrelin, JMV 1843, or MK0677, in particular when the bimane probe was attached to C304 (9 vs. 18% decrease). This suggests that ligands with different efficacies at activating Gq and recruiting arrestin trigger different conformational changes.
Finally, binding of the inverse agonist SPA induced an increase in the emission intensity of bimane located in either TM7 or ICL2 (Fig. 1). Even if qualitatively similar, the increase in bimane emission intensity was quantitatively very different depending whether the probe was attached to C146 or C304 (∼9 and 120% increase, respectively). Of importance, bimane emission increased in the presence of SPA whereas it decreased with either full or partial agonists, suggesting that the inverse agonist stabilizes a conformation that is different from that reached in the presence of a compound with agonistic properties.
Even if indicative of different conformations, fluorescence intensity measurements reflect only the weighted average of one or more discrete states so the emission spectra could account for discrete conformations or for mixtures of different conformations. To discriminate between these two possibilities, we carried out fluorescence lifetime measurements. Fluorescence lifetime not only reveals averaged microenvironmental properties around the probe, as does the maximum emission of the fluorescent dye, but also discloses molecular population distributions of the probed system. In principle, observation of a biexponential decay process for a protein containing a single fluorophore is indicative of the existence of two conformations of the protein.
The same trends were observed in the fluorescence decay experiments whether the probe was attached to C146 or C304; therefore, we will not make any distinction between the decay data obtained with the two labeling positions, unless otherwise indicated. Typical decay profiles are given in the SI Appendix, Fig. S8, and all of the decay parameters are presented in Table 1. As shown in Table 1, the fluorescence decay of the bimane-labeled receptor in the absence of any ligand was best described by a single exponential, indicating the occurrence of one major receptor population (R). In contrast, in the presence of any of the functionally active ligands, the fluorescence decay was best described by two significant exponential components, indicating the occurrence of at least two different substate populations. Whatever the ligand was, one substate population displayed the same lifetime value as the R substate. The second one was unique and different from each other depending on whether the ligand was a full agonist (ghrelin), a partial agonist (JMV 3018), or an inverse agonist (SPA). This is evidence that each of these ligands stabilize the receptor in a specific, well-defined, conformational substate (Rg, R*1, and R*2 for the inverse, partial, and full agonist stabilized conformations, respectively). In contrast, within one class of pharmacologically related ligands, compounds with different chemical structures stabilize a similar conformation (SI Appendix, Table S2).
Table 1.
Influence of ligands and effector proteins on GHS-R1a conformation
| C146 |
C304 |
|||||||
| α1 | τ1 (ns) | α2 | τ2 (ns) | α1 | τ1 (ns) | α2 | τ2 (ns) | |
| No ligand | 1.00 | 11.1 | 1.00 | 4.1 | ||||
| No ligand, Gαqβγ | 0.59 | 7.8 | 0.41 | 11.1 | 0.57 | 2.0 | 0.43 | 4.1 |
| No ligand, Gαqβγ, GTPγS | 1.00 | 11.1 | 1.00 | 4.1 | ||||
| No ligand, Gαi2βγ | 1.00 | 11.1 | 0.99 | 4.1 | ||||
| No ligand, Gαi2βγ, GTPγS | 1.00 | 11.1 | 1.00 | 4.1 | ||||
| No ligand, arrestin 2 | 1.00 | 11.1 | 1.00 | 4.1 | ||||
| No ligand, μ-AP2 | 1.00 | 11.1 | 1.00 | 4.1 | ||||
| Ghrelin | 0.48 | 7.8 | 0.52 | 11.1 | 0.51 | 1.9 | 0.49 | 4.1 |
| Ghrelin, Gαqβγ | 0.78 | 7.8 | 0.22 | 11.1 | 0.77 | 1.9 | 0.23 | 4.1 |
| Ghrelin, Gαqβγ, GTPγS | 0.51 | 7.8 | 0.49 | 11.1 | 0.48 | 1.9 | 0.52 | 4.1 |
| Ghrelin, Gαi2βγ | 0.60 | 7.8 | 0.40 | 11.1 | 0.58 | 2.0 | 0.42 | 4.1 |
| Ghrelin, Gαi2βγ, GTPγS | 0.50 | 7.8 | 0.50 | 11.1 | 0.49 | 1.9 | 0.51 | 4.1 |
| Ghrelin, arrestin 2 | 0.09 | 11.1 | 0.91 | 13.2 | 0.11 | 4.1 | 0.89 | 4.5 |
| Ghrelin, μ-AP2 | 0.46 | 7.8 | 0.54 | 11.1 | 0.49 | 1.9 | 0.51 | 4.1 |
| JMV 3018 | 0.38 | 10.6 | 0.62 | 11.1 | 0.38 | 2.7 | 0.62 | 4.1 |
| JMV 3018, Gαqβγ | 0.67 | 10.6 | 0.33 | 11.1 | 0.68 | 2.7 | 0.32 | 4.1 |
| JMV 3018, Gαqβγ, GTPγS | 0.40 | 10.6 | 0.60 | 11.1 | 0.36 | 2.7 | 0.64 | 4.1 |
| JMV 3018, Gαi2βγ | 0.35 | 10.6 | 0.65 | 11.1 | 0.39 | 2.7 | 0.61 | 4.1 |
| JMV 3018, Gαi2βγ, GTPγS | 0.34 | 10.6 | 0.66 | 11.1 | 0.34 | 2.7 | 0.66 | 4.1 |
| JMV 3018, arrestin 2 | 0.36 | 10.6 | 0.64 | 11.1 | 0.36 | 2.7 | 0.64 | 4.1 |
| JMV 3018, μ-AP2 | 0.39 | 10.6 | 0.61 | 11.1 | 0.35 | 2.7 | 0.65 | 4.1 |
| SPA | 0.11 | 11.1 | 0.89 | 12.1 | 0.11 | 4.1 | 0.89 | 5.0 |
| SPA, Gαqβγ | 0.57 | 7.8 | 0.43 | 12.0 | 0.58 | 2.0 | 0.42 | 5.0 |
| SPA, Gαqβγ, GTPγS | 0.09 | 11.1 | 0.91 | 12.1 | 0.10 | 4.1 | 0.90 | 5.0 |
| SPA, Gαi2βγ | 0.12 | 11.1 | 0.88 | 12.1 | 0.10 | 4.1 | 0.90 | 5.0 |
| SPA, Gαi2βγ, GTPγS | 0.10 | 11.1 | 0.90 | 12.1 | 0.08 | 4.1 | 0.92 | 5.0 |
| SPA, arrestin 2 | 0.11 | 11.1 | 0.89 | 12.1 | 0.09 | 4.1 | 0.91 | 5.0 |
| SPA, μ-AP2 | 0.12 | 11.1 | 0.88 | 12.1 | 0.13 | 4.1 | 0.87 | 5.0 |
Monoexponential and double-exponential fluorescence lifetime analysis of bimane-labeled GHS-R1a in the absence or in the presence of ligands and in the absence or in the presence of effector proteins. τ1 and τ2 are the lifetimes in nanoseconds, α1 and α2 are the fractional amplitudes of each lifetime τ1 and τ2, respectively (the sum of the pre-exponential factors α is normalized to 1). The 11.1-ns (C146 labeling) and 4.1-ns (C304 labeling) lifetimes are attributed to the R state, 12.1-ns (C146) and 5.0-ns (C304) lifetimes to Rg, 10.6-ns (C146) and 2.7-ns (C304) lifetimes to R*1, 7.8-ns (C146) and 1.9-ns (C304) lifetimes to R*2, and 13.2-ns (C146) and 4.5-ns (C304) lifetimes to R*3.
Signaling Proteins Affect GHS-R1a Conformational Landscape.
We then analyzed the effects of different associated proteins [Gαqβ1γ2 (Gq), Gαi2β1γ2 (Gi), arrestin-2, and μ-AP2] on the conformational features of the purified ghrelin receptor in the absence of ligand and in the presence of full, partial, or inverse agonists. In all of the experiments reported below, the receptor and the effector protein were first incubated together before the ligand was added and fluorescence measured, unless otherwise indicated.
Ligand-free state.
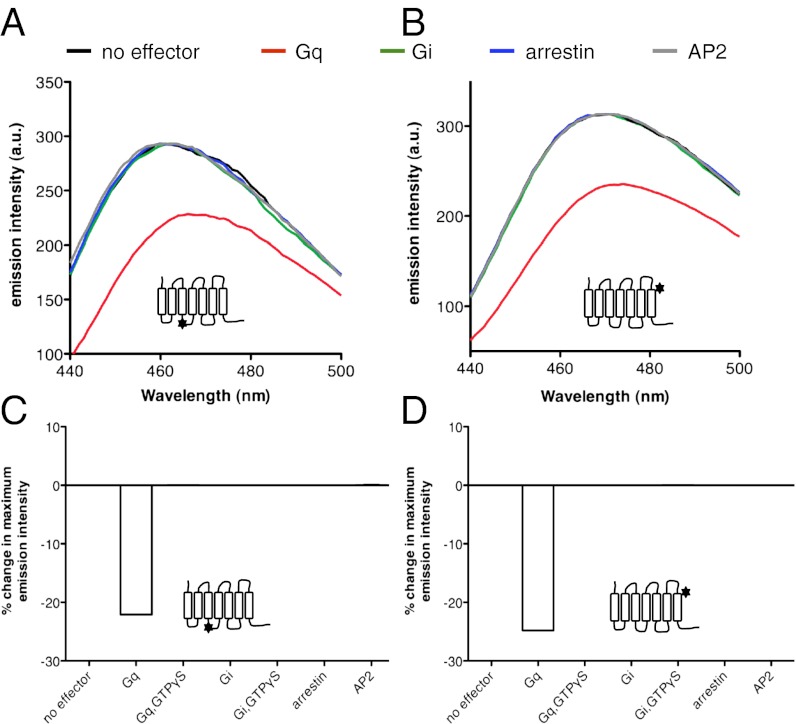
We observed a significant decrease in bimane emission intensity upon adding Gq to the ligand-free receptor whether bimane was located in TM7 or in ICL2 (Fig. 2). This indicates that coupling of the ligand-free receptor to Gq is accompanied by a significant change in GHS-R1a conformation. In agreement with such an assumption, adding GTPγS was accompanied by a reversal in the Gq-induced effects on bimane emission. Consistent with the intensity measurements, the conformational landscape inferred from the fluorescence decay was significantly different whether Gq was present or not (Table 1). Whereas the lifetime decay in the absence of Gq was best described by a single exponential, that in the presence of the G-protein trimer was best described by two exponential components. One lifetime value was identical to that of R and the other very close to that of R*2. This indicates that coupling of the ligand-free receptor to Gq stabilizes GHS-R1a in a presumably active conformation that is closely related, if not the same as that observed in the presence of full agonists. Consistent with the absence of constitutive arrestin recruitment and Gi activation (14), no change in the emission properties of bimane was observed upon adding arrestin-2 or Gi to the ligand-free receptor (Fig. 2). We observed also no change in bimane fluorescence in the presence of μ-AP2 (Fig. 2), although the ligand-free receptor interacts with this adaptor protein (14).
Fig. 2.
Effector proteins affect GHS-R1a conformation. (A and B) Fluorescence emission spectra of bimane attached to C146 (A) or C304 (B) in the absence of ligand and in the absence or in the presence of effector proteins. (C and D) Percentage changes in maximum emission intensity in the absence of ligand and in the absence or in the presence of effector proteins.
Full-agonists.
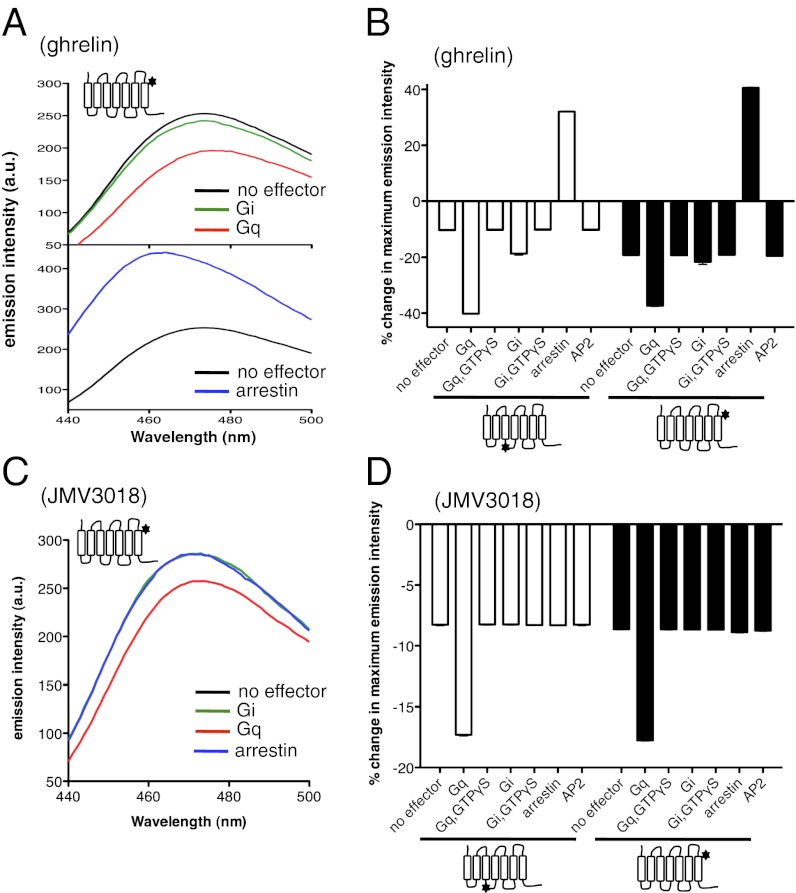
We then investigated the effects of signaling proteins on full agonist-induced conformational changes. Results are presented here for ghrelin, but similar data were obtained with MK0677 (SI Appendix, Fig. S9) and JMV 1843 (SI Appendix, Fig. S10). A significant change in the emission properties of the bimane probe coupled to C146 or C304 was observed when the receptor was incubated with Gq and ghrelin was subsequently added (Fig. 3 A and B). This emission intensity change was larger than that observed with ghrelin or Gq alone. The additional decrease in bimane emission intensity observed upon preincubation with Gq was not observed when GTPγS was added, indicating that it results from the coupling of the agonist-activated receptor to the G protein. A similar pattern of bimane fluorescence changes was observed when using Gi instead of Gq (Fig. 3). These variations are not the result of the mere addition of an exogenous G protein; indeed, adding the purified Gs protein that is not activated by the ghrelin receptor under any ligand condition (14) was not accompanied by a change in bimane fluorescence (SI Appendix, Fig. S11).
Fig. 3.
Effector proteins affect agonist-induced GHS-R1a conformations. (A and C) Fluorescence emission spectra of bimane attached to C304 in the presence of ghrelin (A) or JMV3018 (C) and in the absence or in the presence of effector proteins. Similar profiles were obtained with bimane attached to C146 (SI Appendix, Fig. S15). (B and D) Percentage changes in maximum emission intensity in the presence of ghrelin (B) or JMV3018 (D) and in the absence or in the presence of effector proteins.
Analysis of the fluorescence lifetime decay in the presence of ghrelin and Gq gave a lifetime distribution closely related to that obtained in the presence of ghrelin alone (Table 1). Only the relative populations of the short and long lifetimes changed, with an increase in the short lifetime population. This suggests that coupling to Gq in the presence of the full agonist ghrelin does not stabilize an additional receptor conformation but rather further increases the stability of the R*2 conformation. This is also the case, although to a lesser extent, upon coupling to Gi (Table 1).
Incubation of GHS-R1a with arrestin before adding ghrelin also affected bimane emission intensity (Fig. 3 A and B). Under such conditions, a well-defined arrestin:receptor complex is formed, as assessed through size-exclusion chromatography (SI Appendix, Fig. S12), in agreement with the bimane-based arrestin recruitment assay (SI Appendix, Fig. S2). In contrast to G-protein coupling, however, an increase in bimane emission intensity was observed in this case for both labeling positions. As is the case for G-protein–induced effects, this variation is not the consequence of the simple addition of arrestin. Indeed, no effect was observed when replacing arrestin-2 with its polar core mutant that does not interact with receptors (18) (SI Appendix, Fig. S11). Moreover, the changes in receptor-bound bimane fluorescence vary in a strict ligand- and arrestin-dose–dependent manner (SI Appendix, Fig. S13) that strictly parallels ghrelin-induced arrestin recruitment (14). This is a strong indication that the observed changes in bimane emission result from a specific full agonist-induced arrestin recruitment. Altogether, these data suggest that coupling of the ghrelin-activated receptor to arrestin is accompanied by the occurrence of a receptor conformation different from that observed in the presence of ghrelin alone. Consistently, the lifetime decay measurements in the presence of arrestin showed the occurrence of a specific major population (R*3) with a longer lifetime than that of the R and R*2 substates (Table 1). Finally, no difference in bimane emission intensity (Fig. 3B) and lifetime decay (Table 1) was observed whether the receptor was incubated or not with μ-AP2 before ghrelin addition.
Partial agonists.
We next investigated the effects of effector proteins on the conformational transitions triggered by partial agonists. Data are presented for JMV 3018, but very similar results were obtained with JMV 3002 (SI Appendix, Fig. S14). As in the case of full agonists, a larger decrease in bimane emission intensity was observed in the presence of JMV 3018 and Gq compared with what was measured in the presence of only JMV 3018 (Fig. 3 C and D). This additional decrease results from the coupling to the G protein because it was not observed when GTPγS was added. This indicates that coupling of GHS-R1a to Gq in the presence of JMV 3018 either gives rise to a different receptor conformation or affects the distribution of the different conformations observed in the absence of G protein. The former hypothesis is, however, ruled out by the lifetime decay measurements. Indeed, adding JMV 3018 to the ghrelin receptor in the presence of Gq gave rise to the same species as those observed in the absence of G protein but with a different distribution (Table 1). In contrast to what occurs with Gq, arrestin-2 and Gi did not affect JMV 3018-triggered bimane emission changes (Fig. 3 C and D; Table 1). This is consistent with the observation that this biased agonist neither triggers arrestin recruitment nor activates Gi (SI Appendix, Fig. S2). No additional change was observed in bimane emission intensity in the presence of μ-AP2 either (Fig. 3D; Table 1).
Inverse agonist.
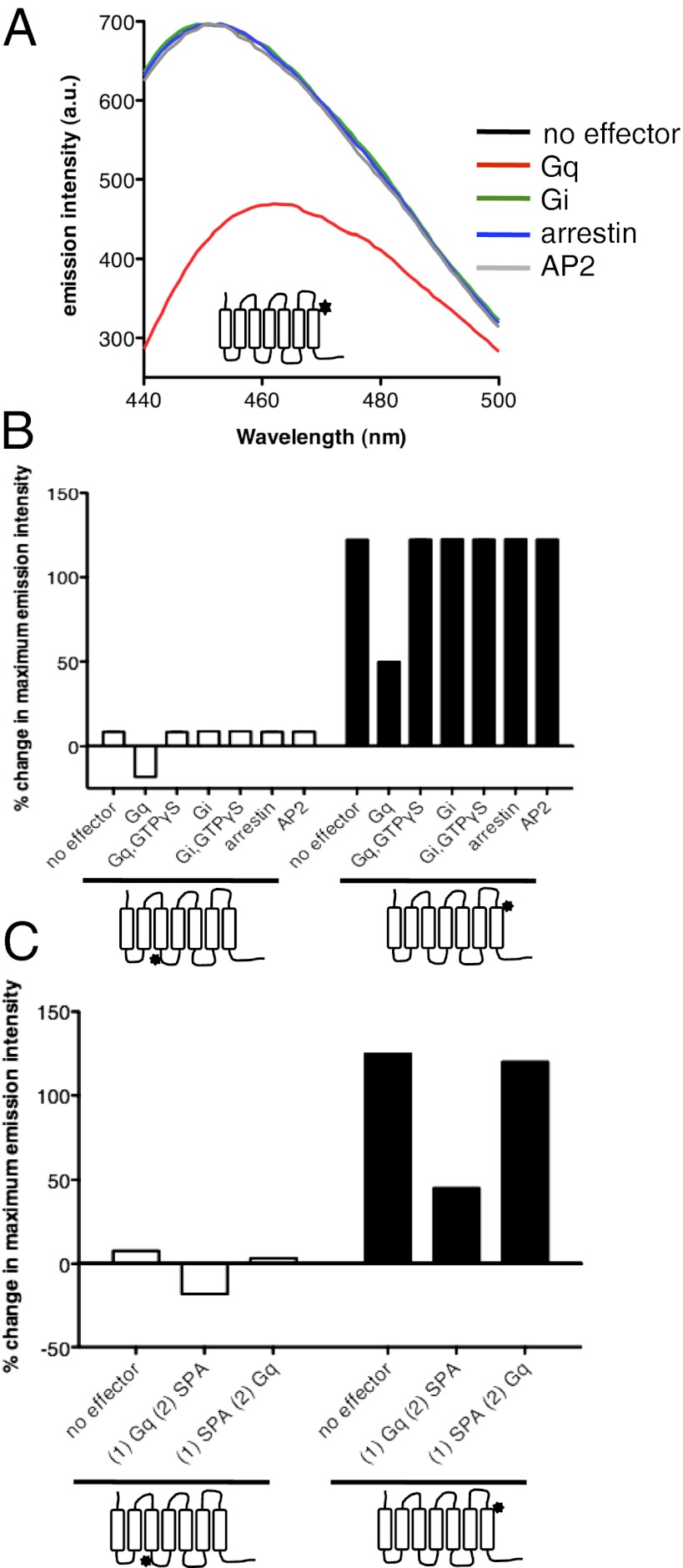
Incubating the purified receptor with Gq before SPA addition resulted in fluorescence changes significantly different from those in the absence of Gq (Fig. 4 A and B). It was only after adding GTPγS that the change in fluorescence equaled that observed in the absence of Gq. As shown in Table 1, lifetime decay in the presence of SPA and Gq was still best described by two exponential components. One lifetime value was similar to that of R*2 and the other was similar to that of Rg. However, in the presence of Gq, the Rg state was significantly less populated than in the absence of the G protein. Importantly, no significant population with a lifetime comparable to that of the R state was observed. A possible reason for this might be that the Rg population observed in the presence of SPA and Gq essentially originates from the residual uncoupled receptor fraction and the preformed complex between the nucleotide-free Gq and GHS-R1a could not undergo the conformational transition from the R*2 to the Rg states. In agreement with this assumption, the subsequent addition of GTPγS led to a receptor population distribution totally indistinguishable from that observed in the absence of Gq (Table 1).
Fig. 4.
Effector proteins affect inverse agonist-induced GHS-R1a conformations. (A) Fluorescence emission spectra of bimane attached to C304 in the presence of SPA and in the absence or in the presence of effector proteins. Similar profiles were obtained with bimane attached to C146 (SI Appendix, Fig. S15). (B) Percentage change in maximum emission intensity in the presence of SPA and in the absence or in the presence of effector proteins. (C) Percentage change in maximum emission intensity in the presence of SPA and in the absence of Gq, in the presence of SPA after preincubation of the receptor with Gq, or in the presence of SPA with subsequent addition of Gq.
The limited effect of SPA on the preformed receptor–G-protein complex is not consistent with its inhibitory effect in signaling assays. To monitor if SPA binding affected the association of the ghrelin receptor and Gq, we first incubated the purified GHS-R1a with SPA and then added the purified G protein. As shown in Fig. 4C, when SPA addition preceded incubation with the G protein, the changes in bimane emission intensity were closely related to those observed in the absence of Gq, indicating that the inverse agonist SPA is most efficient at preventing Gq-induced changes in GHS-R1a conformation rather than at reverting a preformed receptor:G-protein complex.
Finally, the same fluorescence profiles were observed upon addition of SPA to the bimane-labeled receptor whether the latter was or was not incubated previously with Gi, arrestin-2, or μ-AP2 (Fig. 4 and Table 1). This is in agreement with the fact that neither Gi activation nor arrestin and μ-AP2 recruitment occur in the presence of this inverse agonist (14).
Discussion
Using the purified monomeric ghrelin receptor, we explored the influence of ligands and associated signaling proteins on the conformational landscape that can explore a GPCR. GHS-R1a can be taken here as a model for peptide-activated class A receptors so that the conclusions inferred from our work are likely to apply to this entire class of receptors.
All ligands and effector proteins affected the emission properties of bimane whether the probe was attached to C146 or C304, indicating that ligand binding and coupling to effector proteins affect the conformation of the ghrelin receptor in both its extracellular and intracellular domains. In agreement with this conclusion, modeling experiments indicate that GHS-R1a activation is associated with a change in C146 and C304 microenvironments. At this stage of the analysis, we cannot exclude, however, that proximity with the bound signaling protein contributes, at least to some extent, to the changes in bimane fluorescence when the probe was attached to C146. Indeed, ICL2 has been shown to be involved in G-protein and arrestin binding to class A GPCRs (2, 19, 20).
Surprisingly, similar qualitative changes were observed whether the bimane probe was attached to C146 or to C304. Bimane emission essentially reports on the polarity of the immediate environment surrounding the probe (21). The observation of similar qualitative changes is thus simply indicative that the conformational transitions trigger changes in the polarity of the environment that are of similar nature for both probes. However, even if qualitatively similar, the effects of ligands and signaling proteins on each probe are very different in their quantitative aspects (e.g., upon binding partial agonists in the absence of signaling proteins, upon binding inverse agonists in the absence and in the presence of effectors, or upon coupling to Gi in the presence of full agonists). Moreover, dose–response experiments at varying agonist or arrestin concentration were strictly similar whether C146 or C304 labeling was considered. Altogether, this indicates that both positions report, in a different manner, on the same conformational event.
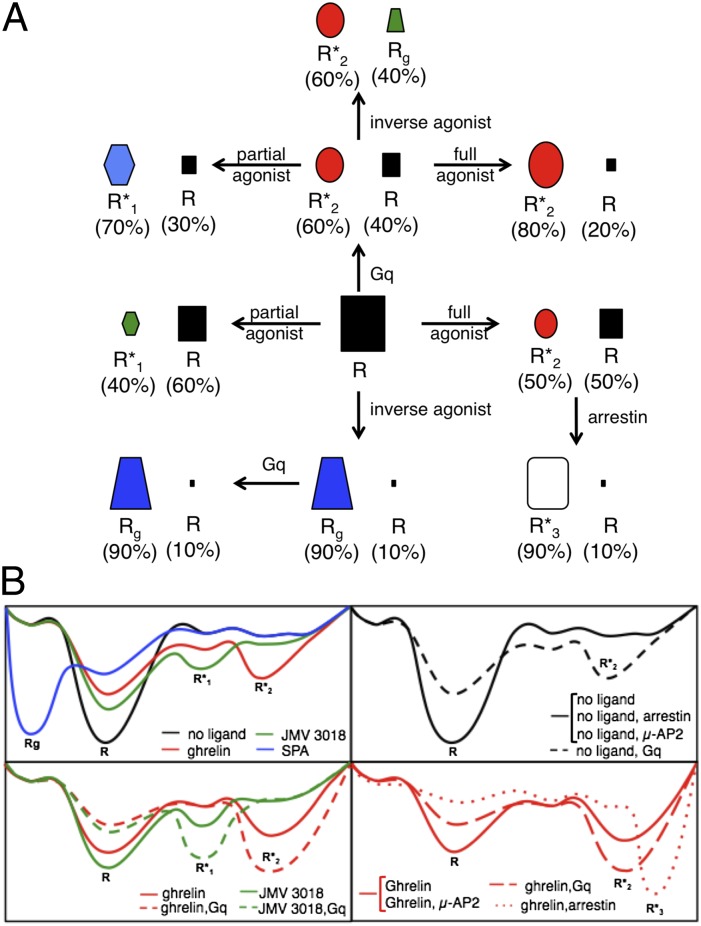
One of the main characteristics of the ghrelin receptor is its high constitutive activity in vitro (14), in heterologous expression systems (22), and in vivo (23). It is particularly striking that the receptor in the absence of an effector is characterized by a single, major conformation R. Indeed in the presence of Gq only does the receptor adopt a more complex conformational landscape with at least two different substates, R and R*2 (Fig. 5). Because constitutive Gq activation logically occurs only when the G protein is present, this more complex population distribution, and not the homogeneous one observed in the absence of Gq, is the one associated with GHS-R1a constitutive activity. A reason for this might be that R is an inherently flexible state and can spontaneously undergo a transition toward R*2, the latter being stabilized by the G protein, as predicted by the conformational selection model. However, if one considers such an inherent flexibility to be associated with the high constitutive activity of GHS-R1a, it is surprising that we observed a homogeneous conformational landscape in the absence of G proteins, with no spontaneous transitions between R and R*2. Perhaps this was because the fluorescence method that we used did not allow us to detect rare preexisting conformations. Scarcity of the R*2 population in the absence of Gq could be associated with high-energy barriers that would limit interconversion between these different substates in the absence of any effector. Binding of the G protein to the ligand-free state would decrease this interconversion energy barrier, leading to a significant increase in the R*2 population.
Fig. 5.
Allosteric effects of ligands and effector proteins on the conformational landscape of the ghrelin receptor. (A) Schematic representation of the receptor conformational substates observed in the absence or in the presence of pharmacologically distinct ligands and effector proteins. The size of the symbol representing each GHS-R1a substate is directly proportional to the relative proportion of this conformation in the total population. (B) Schematic representation of the possible energy landscape of GHS-R1a.
Our data directly establish that pharmacologically distinct ligands stabilize different, specific conformations of the ghrelin receptor (Fig. 5). We observed a complex conformational landscape with all of the ligands with agonistic properties, indicating that these ligands increase the dynamics of the receptor. In contrast, in the presence of the inverse agonist, a single major state was observed, indicating that it decreased the dynamic behavior of GHS-R1a. Of importance, the conformational diversity observed here is to be linked to the relative efficacy and selectivity of ligands in activating different signaling pathways. Indeed, ghrelin, which is a full agonist for Gq that activates Gi and triggers arrestin recruitment, and JMV 3018, which is a biased agonist that activates only Gq, induce different landscapes. Overall, this is experimental evidence for the fact that the conformational landscape of GHS-R1a is directly related to the way in which the receptor will interact with its different signaling/trafficking partners and thereby activate divergent signaling pathways. As far as the ligand-induced conformational changes are considered, we observed no difference for structurally different but pharmacologically related ligands. This means that the compounds that we used follow similar trends of receptor conformational signature that are dependent upon their pharmacological properties rather than upon their chemical structure. This is different from what has been reported with the β2-adrenergic receptor where distinct conformational patterns have been observed for pharmacologically similar ligands (6, 10).
Although ligand-induced conformational changes in GPCRs are well documented, there is little direct description of the changes in GPCR conformation induced by associated signaling proteins. Comparison of the crystal structure of the β2-adrenergic receptor in complex with an agonist (24) or associated with a Gs-mimicking nanobody (25) or to Gs itself (2) indicates that the binding energy from a G protein is required to stabilize the active conformation. Our data bring further experimental evidence to this model. Indeed, the G-protein trimer does not stabilize a GHS-R1a conformation significantly different from that stabilized by the agonist, whether the latter is full or partial, but rather affects the distribution of the different substates, significantly populating the agonist-activated conformation (Fig. 5). This is consistent with the energy landscape model proposed by Deupi and Kobilka (26).
Unexpectedly, different signaling proteins affected the conformational landscape of the ghrelin receptor in different ways. In contrast to G proteins that essentially populate the agonist-stabilized conformation, coupling of the ghrelin-activated receptor to arrestin-2 populates an R*3 conformation that is significantly different from that stabilized by the agonist. Notably, R*3 was not observed in the conformational landscape of the receptor in the presence of ghrelin alone. A possibility would be that this substate preexists in this landscape of the agonist-loaded GHS-R1a but remains undetected either because of its scarcity or because interpretation of the decay profiles is limited and provides only a minimal number of preexisting conformational substates. This preexisting R*3 state would not be present in the conformational landscape of the ligand-free GHS-R1a or in that of the partial agonist-loaded receptor. Indeed, no arrestin-2 recruitment was observed in the absence of ligand or in the presence of JMV 3018. Binding of arrestin to this rare R*3 population would stabilize it further, making it the predominant conformation in the ghrelin:GHS-R1a:arrestin ternary complex. Whatever the exact mechanism is, such a difference in the conformational features of the receptor bound to G proteins or arrestin is likely to be related to the subsequent effects on signaling.
Finally, a different behavior was observed in the presence the μ-subunit of the adaptor AP2 protein. Whereas the G protein populates the agonist-induced active conformations and arrestin-2 stabilizes an additional R*3 state, no effect on bimane emission was observed upon complex formation between the ghrelin receptor and μ-AP2. It has been proposed that the μ2 subunit binds directly to the C-terminal region of the ghrelin receptor (27). If this is the case, complex formation could essentially impact on this region, with no influence on the TM core. Binding of μ-AP2 to GHS-R1a nevertheless is, at least to some extent, dependent on the conformational transitions occurring in the TM domains because the R-to-Rg transition triggered by SPA leads to the dissociation of the complex. Another possibility would be to consider that this transition also has an impact on the conformational features of the C-terminal domains, as observed for the β2-adrenergic receptor upon binding different ligands (8).
In summary, we provide here evidence that ligand efficacy and functional selectivity can be directly linked to distinct receptor conformations. More important, we directly established that coupling to signaling proteins also affects receptor conformation, but in a different way that is dependent on the signaling protein considered. Such an effect is very likely to have a major impact on the way in which distinct signaling pathways are activated by pharmacologically different ligands and can ultimately have significant implications for the design of drugs specifically targeting a given signaling pathway.
Materials and Methods
Sample Preparation and Functional Assays.
Human ghrelin receptor was expressed and assembled as a monomer into lipid discs as described (14). Receptor labeling was carried out by incubating the purified receptor protein with monobromobimane at 4 °C for either 2 or 16 h. Unreacted dye was removed by dialysis. Ligand and GTPγS binding as well as arrestin-2 and μ-AP2 recruitment assays were carried out as described (14).
Spectroscopy.
The ghrelin receptor, at a concentration of 5 μM, was incubated with saturating concentrations of the different ligands (50 μM) for 30 min. In the experiments with the purified effector proteins, the latter were added to the ligand-free receptor (receptor-to-G-protein ratio was 1:5, receptor-to-arrestin ratio was 1:2, and receptor-to-AP2 ratio was 1:2) and incubated for 30 min. The different ligands were then added and incubation was pursued for 30 min more. Fluorescence emission spectra were recorded using a Cary Eclipse spectrofluorimeter (Varian) with an excitation wavelength at 380 nm. The percentage of change in maximum emission was calculated as follows: I − I(0)/I(0) × 100, where I is the maximum emission intensity under the conditions considered and I(0) is the maximum emission intensity in the absence of ligand and in the absence of any effector. An EasyLife TCSP module by Horiba spectrofluorimeter with a NanoLED pulsed laser-diode was used to measure bimane lifetime decay, with 375-nm excitation pulses (200 ps). Lifetime decay curves were collected between 200 ps and 3 μs. Data were analyzed using the commercial software.
Supplementary Material
Acknowledgments
This work was supported by Universities of Montpellier 1 and Montpellier 2, Centre National de la Recherche Scientifique, and Agence Nationale pour la Recherche Contract PCV08_323163.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119881109/-/DCSupplemental.
References
- 1.Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr Opin Struct Biol. 2011;21:541–551. doi: 10.1016/j.sbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luttrell LM, Kenakin TP. Refining efficacy: Allosterism and bias in G protein-coupled receptor signaling. Methods Mol Biol. 2011;756(1):3–35. doi: 10.1007/978-1-61779-160-4_1. [DOI] [PubMed] [Google Scholar]
- 4.Ghanouni P, et al. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor. J Biol Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 5.Peleg G, Ghanouni P, Kobilka BK, Zare RN. Single-molecule spectroscopy of the beta(2) adrenergic receptor: Observation of conformational substates in a membrane protein. Proc Natl Acad Sci USA. 2001;98:8469–8474. doi: 10.1073/pnas.151239698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swaminath G, et al. Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem. 2004;279:686–691. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- 7.Yao X, et al. Coupling ligand structure to specific conformational switches in the beta2-adrenoceptor. Nat Chem Biol. 2006;2:417–422. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- 8.Granier S, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: Insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 9.Yao XJ, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc Natl Acad Sci USA. 2009;106:9501–9506. doi: 10.1073/pnas.0811437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahsai AW, et al. Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat Chem Biol. 2011;7:692–700. doi: 10.1038/nchembio.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West GM, et al. Ligand-dependent perturbation of the conformational ensemble for the GPCR β2 adrenergic receptor revealed by HDX. Structure. 2011;19:1424–1432. doi: 10.1016/j.str.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banères JL, et al. Molecular characterization of a purified 5-HT4 receptor: A structural basis for drug efficacy. J Biol Chem. 2005;280:20253–20260. doi: 10.1074/jbc.M412009200. [DOI] [PubMed] [Google Scholar]
- 13.Kojima M, Kangawa K. Ghrelin: More than endogenous growth hormone secretagogue. Ann N Y Acad Sci. 2010;1200:140–148. doi: 10.1111/j.1749-6632.2010.05516.x. [DOI] [PubMed] [Google Scholar]
- 14.Damian M, et al. High constitutive activity is an intrinsic feature of ghrelin receptor protein: A study with a functional monomeric GHS-R1a receptor reconstituted in lipid discs. J Biol Chem. 2012;287:3630–3641. doi: 10.1074/jbc.M111.288324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerlavais V, et al. New active series of growth hormone secretagogues. J Med Chem. 2003;46:1191–1203. doi: 10.1021/jm020985q. [DOI] [PubMed] [Google Scholar]
- 16.Moulin A, et al. New trisubstituted 1,2,4-triazole derivatives as potent ghrelin receptor antagonists. 3. Synthesis and pharmacological in vitro and in vivo evaluations. J Med Chem. 2008;51:689–693. doi: 10.1021/jm701292s. [DOI] [PubMed] [Google Scholar]
- 17.Holst B, et al. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem. 2004;279:53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- 18.Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV. Visual and both non-visual arrestins in their “inactive” conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem. 2006;281:21491–21499. doi: 10.1074/jbc.M603659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro O, Lameh J, Högger P, Sadée W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biol Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 20.Marion S, Oakley RH, Kim KM, Caron MG, Barak LS. A beta-arrestin binding determinant common to the second intracellular loops of rhodopsin family G protein-coupled receptors. J Biol Chem. 2006;281:2932–2938. doi: 10.1074/jbc.M508074200. [DOI] [PubMed] [Google Scholar]
- 21.Kosower EM, Giniger R, Radkowsky A, Hebel D, Shusterman A. Bimanes 22. Flexible fluorescent molecules. Solvent effects on the photophysical properties of syn-bimanes (1,5-diazabicyclo[3.3.0]octa-3,6-diene-2,8-diones) J Phys Chem. 1986;90:5552–5557. [Google Scholar]
- 22.Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004;25:113–117. doi: 10.1016/j.tips.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Petersen PS, et al. In vivo characterization of high basal signaling from the ghrelin receptor. Endocrinology. 2009;150:4920–4930. doi: 10.1210/en.2008-1638. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum DM, et al. Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deupi X, Kobilka BK. Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology (Bethesda) 2010;25:293–303. doi: 10.1152/physiol.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holliday ND, Holst B, Rodionova EA, Schwartz TW, Cox HM. Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol Endocrinol. 2007;21:3100–3112. doi: 10.1210/me.2007-0254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.