Abstract
DNA polymerase substrate specificity is fundamental to genome integrity and to polymerase applications in biotechnology. In the current paradigm, active site geometry is the main site of specificity control. Here, we describe the discovery of a distinct specificity checkpoint located over 25 Å from the active site in the polymerase thumb subdomain. In Tgo, the replicative DNA polymerase from Thermococcus gorgonarius, we identify a single mutation (E664K) within this region that enables translesion synthesis across a template abasic site or a cyclobutane thymidine dimer. In conjunction with a classic “steric-gate” mutation (Y409G) in the active site, E664K transforms Tgo DNA polymerase into an RNA polymerase capable of synthesizing RNAs up to 1.7 kb long as well as fully pseudouridine-, 5-methyl-C–, 2′-fluoro–, or 2′-azido–modified RNAs primed from a wide range of primer chemistries comprising DNA, RNA, locked nucleic acid (LNA), or 2′O-methyl–DNA. We find that E664K enables RNA synthesis by selectively increasing polymerase affinity for the noncognate RNA/DNA duplex as well as lowering the Km for ribonucleotide triphosphate incorporation. This gatekeeper mutation therefore identifies a key missing step in the adaptive path from DNA to RNA polymerases and defines a previously unknown postsynthetic determinant of polymerase substrate specificity with implications for the synthesis and replication of noncognate nucleic acid polymers.
Keywords: processivity, protein engineering, second gate
Replicative polymerases require extraordinary specificity in substrate selection, incorporation, and replication both to ensure fidelity and to exclude noncognate and/or damaged nucleotides from the genome. A particular threat to DNA genome integrity are ribonucleotide triphosphates (NTPs), which are present in the cell at concentrations up to 100-fold in excess of the cognate deoxyribonucleotide triphosphates (dNTPs) (1–3) yet differ from them only by the presence of a 2′-hydroxyl(-OH) group. Indeed, although DNA polymerases have evolved to exclude NTPs from their active sites, incorporation does occur to a detectable degree, with significant implications for genome stability and repair (2, 4). This issue may be even more acute for thermophilic organisms, because high temperatures further increase genome instability by accelerating the spontaneous degradation of RNA (5). Control of NTP incorporation by DNA polymerases is therefore a paradigmatic case of the link between polymerase substrate specificity and genome stability.
DNA polymerases from all three domains of life are known to use a common strategy to prevent NTP incorporation into the nascent strand, by exerting stringent geometric control of the chemical nature of the 2′ position of the incoming nucleotide through a single active site residue, the “steric gate” (6). This strategy is so efficient that mutation of the steric gate alone (e.g., to an amino acid with a smaller side chain) can reduce discrimination against NTP incorporation by several orders of magnitude (6–13). However, although steric-gate mutations generally render DNA polymerases permissive for NTP incorporation, they do not by themselves enable synthesis of RNAs beyond short termination products. Indeed, engineering efforts using rational design (9), in vitro and in vivo screening (14, 15), or directed evolution by phage display (16) and compartmentalized self-replication (17) have so far only yielded polymerases that can synthesize RNAs up to 58 nucleotide incorporations (nt) long (14) and more commonly stall at +6–7 nt (14–16, 18) even after prolonged incubation. At the same time, there is compelling structural and phylogenetic evidence for an adaptive path linking DNA to RNA polymerase activity in the evolution of the single-subunit RNA polymerases (ssRNAPs) of mitochondria and T-odd bacteriophages (e.g., T7 RNA polymerase), which are thought to derive from an ancestral polA-family DNA polymerase (19–22). Thus, we (17) and others (6, 10, 16) have argued that there must be a determinant of polymerase substrate specificity that has remained unidentified and that precludes synthesis of longer RNAs in the steric-gate mutants.
Here, we describe the discovery and characterization of a plausible candidate for such a specificity checkpoint. Using Tgo DNA polymerase, the replicative DNA polymerase from Thermococcus gorgonarius, as our model system, we identify a region in the thumb subdomain, and a single key residue (E664) within it. Mutation of E664 to lysine (K) relieves the synthetic block for RNA polymerization and, in the context of a steric-gate mutation (Y409G) and four previously described auxiliary mutations, enables the primer-dependent synthesis of long RNAs. Characterization of the phenotype suggests a molecular mechanism based on an enhanced primer/template duplex interaction interface.
Results
Polymerase Region Enabling RNA Synthesis.
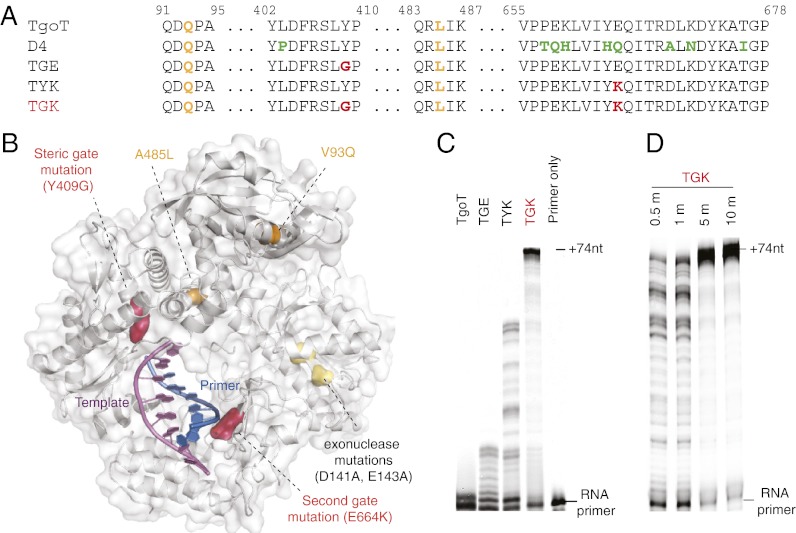
Recent work in our group has focused on the engineering of polymerases for the synthesis and replication of unnatural nucleic acid polymers (23). This line of investigation led to the serendipitous identification of a polymerase (D4) with enhanced RNA polymerase activity, which is the starting point of the work described herein (Fig. S1A). D4 derives from a variant of the replicative DNA polymerase of the hyperthermophilic archaeon T. gorgonarius (Tgo) bearing additional mutations to disable uracil-stalling [V93Q (24)] and 3′-5′ exonuclease (D141A and E143A) functions as well as the “Therminator” (25) mutation (A485L), known to enhance incorporation of unnatural substrates. This mutant polymerase (henceforth termed TgoT) does not display RNA polymerase activity above background levels (Fig. 1 and Fig. S1B): RNA synthesis by TgoT stalls after six to seven incorporations from a DNA primer. TgoT is also unable to extend an RNA primer using NTPs. In contrast, D4 extends both DNA and RNA primers synthesizing RNAs of 20 nt under identical conditions. This gain of function in D4 is attributable to nine additional mutations, comprising a cluster of eight mutations (P657T, E658Q, K659H, Y663H, E664Q, D669A, K671N, and T676I) in the thumb subdomain and a single mutation (L403P) in the A-motif (Fig. S1D).
Fig. 1.
Mutations enabling RNA synthesis. (A) Sequence locations of mutations present in D4, the engineered variant TGK (TgoT: Y409G E664K), and intermediate TGE and TYK polymerases are shown together with the parent TgoT. (B) TGK mutations are mapped onto the structure of a secondary complex of the closely related Pfu polymerase [Protein Data Bank (PDB) ID code 4AIL]. The steric-gate mutation and second-gate mutation are shown in red, secondary mutations present in the starting polymerase TgoT (uracil stalling function and Therminator mutation) are shown in orange, and exonuclease mutations are shown in yellow. (C) RNA polymerase activity of polymerases TgoT, TGE, TYK, and TGK (from an RNA primer). (D) Time course of synthesis of E. coli tRNATyr from RNA primers, showing the appearance of the full-length product (+74 nt) within 30 s for TGK.
Having identified mutations that enhance RNA polymerase activity, we sought to dissect their contribution to the phenotype in the context of a more permissive active site for RNA synthesis. Previous work on the polB-family polymerases had identified a conserved tyrosine (Tgo: Y409) as the steric-gate residue. Mutation of Y409 to a smaller side-chain amino acid was found to reduce NTP/dNTP discrimination by more than 103-fold, yet it is not sufficient to enable RNA synthesis beyond short termination products (6–7 nt) (7–9). We initially mutated Y409 to medium-sized side chains (Y409S, L, and N), of which the Y409N mutation (D4: Y409N, henceforth called D4N) yielded an improved RNA polymerase (Fig. S1B) capable of synthesizing an Escherichia coli supF tRNATyr gene of 74 nt in under 20 min (Fig. S2), superior to the previous best example of engineered RNA polymerase activity (14). The same mutation introduced into the “WT” polymerase TgoT: Y409N (TNE) only marginally improved RNA polymerase activity (Fig. S1B).
Single Mutation Is Critical for RNA Synthesis.
Nine mutations in D4, together with the Y409N steric-gate mutation, enabled efficient RNA synthesis (Figs. S1 and S2). To understand the contributions of individual mutations in D4N better, we reverted each mutation to WT and analyzed its effect on RNA synthesis, revealing a striking pattern: although reversion of seven of the eight thumb mutations (D4N: T657P, Q658E, H659K, H663Y, A669D, N671K, and I676T) did not affect RNA polymerase activity, reversion of one specific residue (D4N: Q664E) all but abolished RNA synthesis (Fig. S1C). Indeed, the reversion mutant D4N: Q664E displayed essentially the same level of RNA polymerase activity as the parent polymerase TgoT. This also indicated that the single mutation in D4 in the A-motif, L403P, did not contribute to RNA polymerase activity (Fig. S1D).
To confirm that RNA polymerase activity in D4N was mainly conferred by E664Q, we introduced the E664Q mutation de novo into the TgoT framework, together with the steric-gate mutation Y409N. The resulting double-mutant TNQ (TgoT: Y409N and E664Q) displayed superior RNA polymerase activity, enabling the synthesis of the 74-nt supF tRNA gene in under 10 min, twice as fast as D4N (Fig. S2C). We concluded that mutation of E664 is both necessary and sufficient for efficient RNA synthesis in conjunction with a steric-gate mutation.
Having established a key role for E664 in enabling RNA synthesis, we sought to identify optimal mutations of this residue for RNA synthesis. We randomized position 664 in TNE and screened for enhanced RNA polymerase activity using our high-throughput polymerase activity assay [PAA (23)]. The PAA is based on capture of primer extension products and their quantification via hybridization to a specific antisense probe (Fig. S3). The PAA screen identified E664K as the most effective mutation.
Following the success of this strategy, we randomized the steric gate (position 409) in the polymerase TNK (TgoT: Y409N and E664K) and performed an analogous PAA screen, identifying Y409G as the most effective steric-gate mutation. Finally, we combined mutations from both screens to yield the TgoT double-mutant TGK (TgoT: Y409G E664K), which could synthesize our benchmark 74-nt supF tRNA in under 30 s (Fig. 1), nearly 100 times faster than the parent polymerase D4N (Fig. S2C).
Synthesis of Protein Coding and Functionalized mRNAs.
Encouraged by this gain in activity, we challenged TGK to generate much longer RNAs. We first tested synthesis of the 748-nt mRNA-encoding GFP. Indeed, TGK was able to synthesize a full-length GFP RNA (primed from an RNA primer) in under 10 min as determined by denaturing agarose gel electrophoresis, RT-PCR, and sequencing (Fig. 2A) as well as the synthesis of a correctly sized 26.8-kDa protein product in an in vitro translation extract (Fig. 2B). We also examined synthesis of a much longer 1,691-nt mRNA encoding firefly (Photinus pyralis) luciferase. Again, TGK generated a full-length luciferase RNA (in 1–2 h) (Fig. 2C). We determined the fidelity of RNA synthesis by TGK through sequencing the 1.7-kb luciferase RNA, yielding a fidelity of 1.03 × 10−3, which is approximately fivefold lower than the fidelity of T7 RNA polymerase on the same RNA (2.1 × 10−4) reported previously (26) but superior to the fidelity of DNA synthesis by both the parent polymerase TgoT (8.3 × 10−3) and TGK itself (3.3 × 10−3).
Fig. 2.
Long-range RNA synthesis by TGK. (A) Synthesis of mRNA encoding GFP (0.8 kb) by TGK as resolved by denaturing agarose electrophoresis (Left) and RT-PCR (Right) after 10 min (M, 100-bp ladder). (B) In vitro translation of the RNA synthesized in A yielding GFP protein (26 kDa; arrow). (C) Synthesis of RNA encoding firefly luciferase (1.7 kb) by TGK as resolved by native agarose electrophoresis (Left) and RT-PCR (Right) after 1 h.
Unlike T7 RNA polymerase or other RNA polymerases, TGK efficiently initiates primer-dependent RNA synthesis from a range of chemistries, including DNA, RNA, 2′-O,4′-C-methylene-β-d-ribonucleic acids [locked nucleic acids (LNA)], and 2′O-methyl (2′OMe)–DNA, thus allowing a free choice of 5′ modification (e.g., fluorophores, biotin) or 5′-end chemistries (DNA, RNA, LNA, and 2′OMe) and RNA 5′ base (G/A/U/C). From these primers, TGK is also able to synthesize modified nucleic acid polymers, for example, fully 2′-azido (2′-N3) or 2′-fluoro (2′-F) substituted 57-nt RNAs in under 10 min or fully 5-methyl-C (5meC) and pseudouridine (Ψ) substituted 1.7-kb RNAs (Fig. S4). Furthermore, we found that the E664K mutation enhances translesion synthesis (TLS) across abasic sites or cyclobutane pyrimidine dimers (CPDs) (Fig. S5).
Auxiliary Mutations.
Our starting polymerase (TgoT) contained four mutations from the Tgo WT sequence (V93Q, D141A, E143A, and A485L). These mutations by themselves do not enable extended RNA synthesis, because both TgoT and TGE (TgoT: Y409G), the variant with the most effective steric-gate mutation, are unable to synthesize RNAs longer than a few nucleotides. Nevertheless, we sought to disentangle the contributions (if any) of the different mutations to RNA synthesis. Because an active exonuclease was unlikely to contribute to RNA synthesis (and is indeed lacking in extant RNA polymerases), we did not consider reversions of the D141A and E143A mutations. In contrast, both the V93Q and A485L mutations have been described to influence polymerase substrate specificity either through enabling template uracil bypass (24) or by means of a generic increase in substrate promiscuity (25). We therefore reverted both of these mutations in the parent polymerase TgoT in TGK (TgoT: Y409G and E664K) and in the intermediate variants TGE (TgoT: Y409G) and TYK (TgoT: E664K), yielding reversion mutants Tgo exo-, TRGK, TRGE, and TRYK, respectively. We found that although reversion reduced overall RNA polymerase or TLS activity, it increased the differential between polymerases with and without the E664K mutation. Where we had previously observed weak RNA synthesis for TGE and TYK (Fig. 1C), the reversion mutants showed none (Fig. S6).
We conclude that although V93Q and A485L contribute to RNA synthesis and TLS in a generic way (presumably by generally increasing polymerase substrate promiscuity), they do not enable it. The key positions controlling RNA polymerization are Y409 and E664 (Fig. 1). Mutation of both is necessary (and sufficient) for synthesis of extended RNA molecules, whereas bypass of DNA damage (TLS) is enhanced by the E664K mutation alone (Fig. S6), despite its being located over 25 Å away from the template lesion. E664K therefore defines a second key checkpoint for RNA synthesis and TLS distinct from the steric gate, and we will refer to it henceforth as the “second gate”.
Polymerase Functional Parameters.
To gain a better understanding of the mechanistic aspects of the second-gate mutation E664K on RNA synthesis by TGK, we measured steady-state NTP incorporation kinetics, single-hit extension, and termination probabilities as a measure of polymerase processivity (27) and primer/template affinities for the parent polymerase TgoT, for TGK, and for the intermediate variants TGE and TYK.
The catalytic efficiency [Vmax/Km(f)] of dATP incorporation (fdATP) is essentially identical for all polymerases, but there are differences of four orders of magnitude in the catalytic efficiency of ATP incorporation (fATP). Indeed, the fATP of polymerase variants with the Y409G steric-gate mutation (TGE and TGK) is only slightly worse than their fdATP, whereas in polymerases with a WT Y409 steric gate (TgoT and TYK), fATP is 2 × 104-fold and 2 × 102-fold, respectively, lower than their fdATP (Table 1 and Table S1), mostly attributable to a higher Km. Surprisingly, polymerases with the E664K mutation display a significantly lower Km(ATP), suggesting that the second-gate mutation exerts an effect on substrate utilization. E664K modulates the apparent Km for NTPs despite being located more than 25 Å from the active site, presumably by promoting tertiary complex stability (below). Indeed, fATP of TGK polymerase from a DNA–RNA6 chimeric primer was reduced more than 20-fold and over 200-fold for an all-RNA primer (Table S1), indicating a striking interdependence of the nature of the primer/template duplex and both apparent affinity for substrate (Km) and catalytic efficiency (f) in RNA synthesis.
Table 1.
Steady-state kinetic parameters of engineered polymerases
| dATP |
ATP |
||||||||
| Polymerase | Vmax, % min−1 | Km × 10−2, μM | f, % mM−1⋅min−1 | Vmax, % min−1 | Km, μM | f, % mM−1⋅min−1 | Vmax dATP/Vmax ATP | Km ATP/Km dATP | fdATP/fATP |
| TgoT | 1.04 ± 0.07 | 3.1 ± 0.03 | 33 ± 2 | 0.12 ± 0.03 | 99 ± 9 | 1.2 × 10−3 ± 0.2 × 10−3 | 15 | 3 × 103 | 4.7 × 104 |
| TGE | 0.82 ± 0.02 | 2.4 ± 0.4 | 35 ± 4 | 0.61 ± 0.06 | 0.10 ± 0.01 | 6.4 ± 0.2 | 1.4 | 3.9 | 5.5 |
| TYK | 0.83 ± 0.03 | 1.9 ± 0.1 | 44 ± 6 | 0.07 ± 0.007 | 0.15 ± 0.01 | 0.43 ± 0.06 | 13 | 8.1 | 102 |
| TGK | 0.56 ± 0.01 | 1.4 ± 0.2 | 39 ± 4 | 0.46 ± 0.09 | 0.035 ± 0.004 | 13 ± 1.8 | 1.2 | 2.5 | 3 |
Kinetic parameters were measured as described in Materials and Methods. Data are averages (±SD) from n = 3–6 experiments.
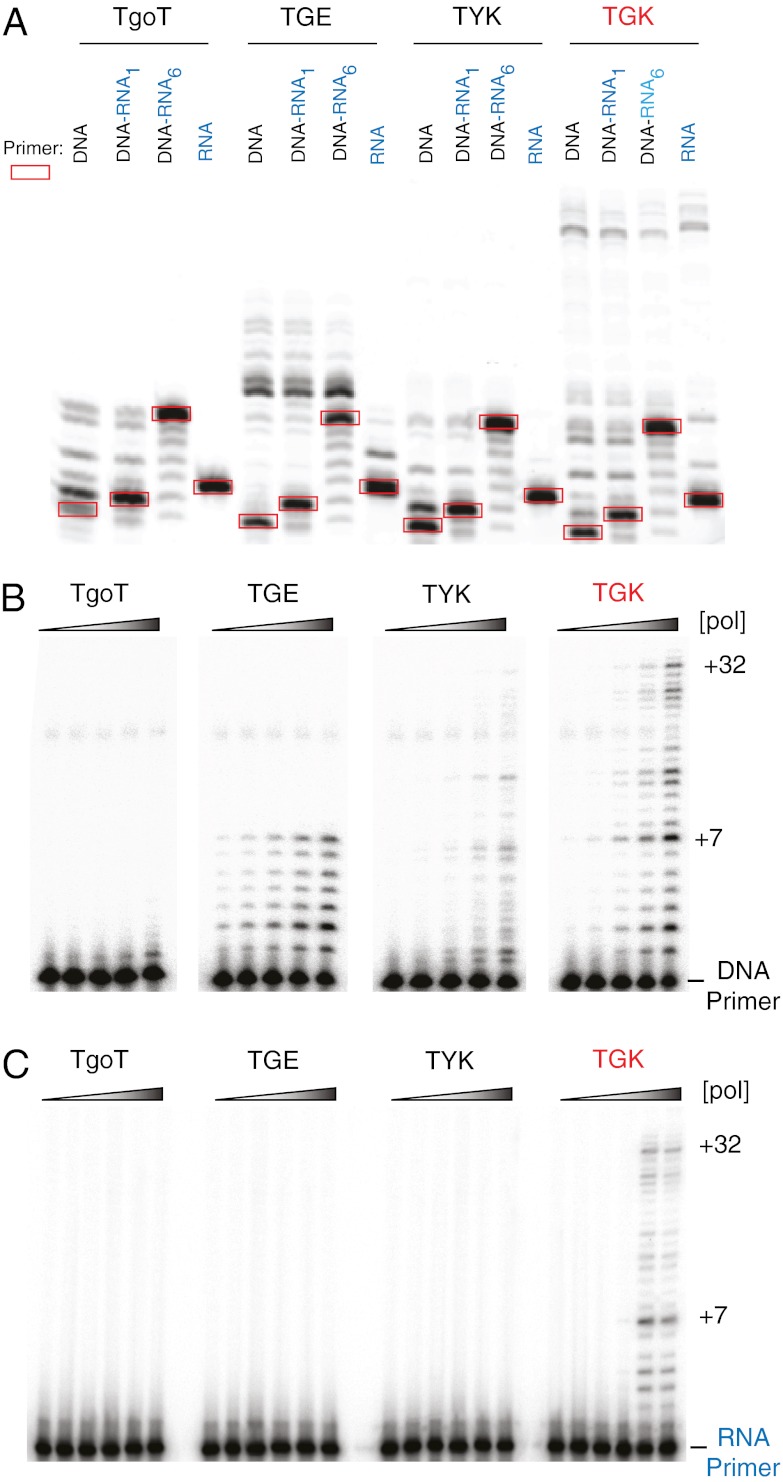
Although TGK efficiently extends both DNA and RNA primers with NTPs, TgoT cannot extend RNA primers (Fig. 3A). When using DNA–RNA chimeric primers comprising one (or more) 3′-terminal ribonucleotides, we again observed a strong termination once a stretch of 6 nt is reached [extension of a primer with 1 terminal ribonucleotide (DNA–RNA1) stalls after incorporation of 5–6 nt, whereas a primer with 6 terminal ribonucleotides (DNA–RNA6) is not extended at all by TgoT] (Fig. 3A). Even with the steric-gate mutation (Y409G), RNA synthesis by TGE terminates at n + 6/7 (100% termination probability) (Fig. 3A and Fig. S7), suggesting a fundamental block to extension beyond 6–7 ribonucleotides. RNA synthesis by TYK, which lacks the steric-gate mutation but harbors the second-gate mutation (E664K), is weaker but does not display the n + 6 termination. In contrast, TGK efficiently extends both DNA and RNA primers beyond n + 6 and to the end of the template with termination probabilities less than 50%, indicating an increase in processivity of RNA synthesis.
Fig. 3.
Second-gate impact on processivity of RNA synthesis. (A) RNA synthesis from DNA (black), RNA (blue), and DNA–RNAx chimeric primers by polymerases TgoT, TGE (TYE: Y409G), TYK (TYE: E664K), and TGK (TYE: Y409G E664K). Red boxes highlight the unextended primers. Processivity of RNA synthesis as determined by single hit extension from a DNA primer (B) and an RNA primer (C).
We also performed RNA synthesis under single-hit conditions, which provides another measure of polymerase processivity, because each extension product is the result of a single polymerase binding event (28). Under conditions in which DNA synthesis is comparable between all four variants (TgoT, TGE, TYK, and TGK), RNA synthesis terminates by the seventh incorporation for both TgoT and TGE (Fig. 3B and Fig. S7), further confirming standard extension experiments. In contrast, TYK and TGK (both with the E664K mutation) can extend beyond that synthetic block to over 30 incorporations from a DNA primer. TGK is the only enzyme that can synthesize RNA from an RNA primer with similar processivity to extension from a DNA primer (Fig. 3C). Examination of termination probabilities in RNA synthesis as a measure of polymerase processivity (27) revealed a clear decrease (Fig. S7) in processivity by polymerases without the E664K mutation, particularly with respect to the strong termination at n + 6/7.
Having established that the E664K mutation imparts a substantial increase in the processivity of RNA synthesis as judged from product length and termination probabilities, we sought to identify the mechanistic basis of this effect. We determined equilibrium binding affinities (Kd) of DNA/DNA and RNA/DNA primer/template duplexes to polymerase variants by fluorescence polarization (FP). These measurements revealed more than a 25-fold affinity increase in polymerase variants comprising the E664K mutation (TYK and TGK) for a noncognate RNA/DNA primer template duplex compared with TgoT (Table 2). Although E664K also increased affinity for a canonical DNA/DNA duplex (ca. 5-fold), its effect on binding the noncanonical RNA/DNA heteroduplex is much greater. Indeed, TGK binds an RNA/DNA duplex with a slightly higher affinity than the parent polymerase TgoT binds a DNA/DNA duplex.
Table 2.
Kd for primer/template duplexes of engineered polymerases
| Kd, nM (primer/template) | TgoT | TGE | TYK | TGK |
| DNA/DNA | 179.0 ± 8.7 | 280.8 ± 12.6 | 30.5 ± 4.9 | 36.7 ± 4.7 |
| RNA/DNA | 2,504.0 ± 379.3 | 1,764.0 ± 195.1 | 65.32 ± 17.9 | 104.3 ± 10.8 |
Affinities were measured by FP, as described in Materials and Methods. Data are averages (±SD) of n = 3 experiments for DNA/DNA and n = 6 experiments for RNA/DNA primer/template duplexes.
Together, the kinetic, processivity and affinity data reveal the molecular determinants for the strikingly increased efficiency and processivity of RNA synthesis by TGK. The steric-gate mutation allows efficient incorporation of NTPs through lowering Km (substrate utilization), and the second-gate mutation enables tight binding of the nascent RNA/DNA heteroduplex through lowering Kd, and promotes a further decrease in Km.
Discussion
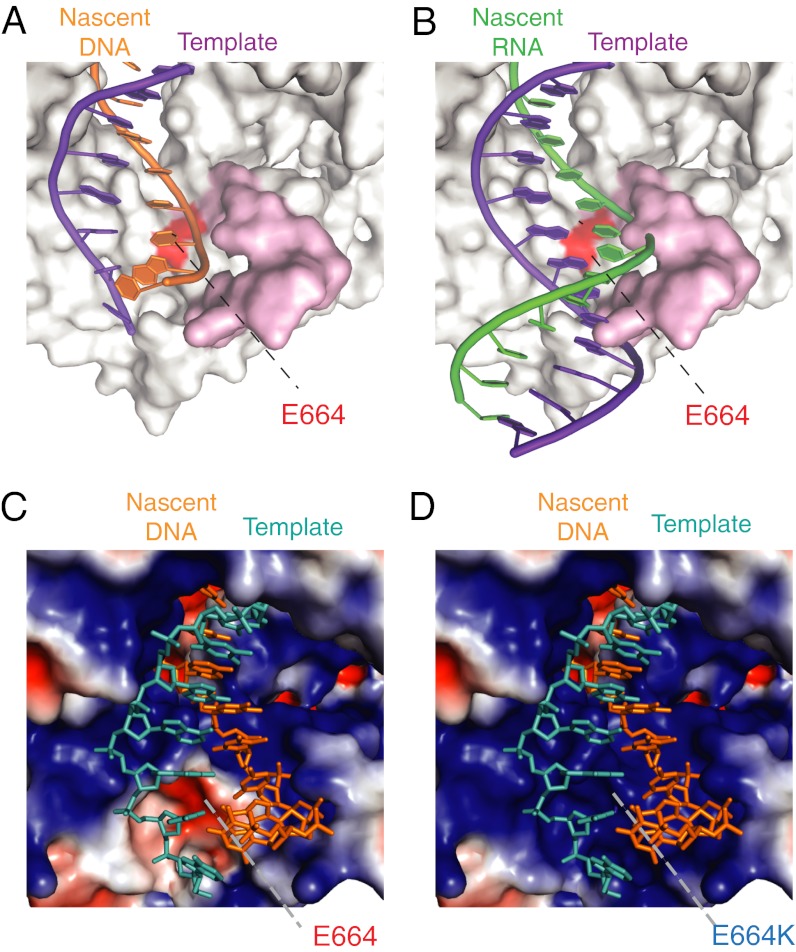
The existence of a second checkpoint for RNA synthesis located in the thumb subdomain had been predicted by us and others (6, 10, 16, 17) on the basis of the phenotype of steric-gate mutants and their premature termination of RNA synthesis, typically at n + 6/7. Indeed, eight of the nine mutations in the starting D4 polymerase (TgoT: L403P, P657T, E658Q, K659H, Y663H, E664Q, D669A, K671N, and T676I) with weak RNA polymerase activity are located in a sequence segment (P657–T676) in the center of the thumb subdomain and in close contact with the nascent strand around n + 6 (Fig. 4 A and B). By dissecting the contributions of different residues to the phenotype, we identified the critical functional determinant to be a single residue (E664), mutation of which proved both necessary and sufficient for efficient RNA synthesis in the context of a steric-gate mutation. Hence, we called this residue the second gate. Although auxiliary mutations (V93Q, D141A, E143A, and A485L) were found to contribute to the enhanced processivity of RNA synthesis, they did not by themselves enable RNA synthesis (Fig. 1). Indeed, the phenotype of Q93V and L485A reversion variants highlighted the gain of function imparted by the steric-gate and second-gate mutations Y409G and E664K (Fig. S6).
Fig. 4.
Mechanistic model of second-gate function. (A) DNA/DNA duplex (nascent DNA strand shown in orange, DNA template shown in purple) as observed. (B) RNA/DNA hybrid duplex [PDB ID code 1EFS (36)] (nascent RNA strand green, DNA template purple) modeled into the secondary complex of Pfu (PDB ID code 4AIL). Modeling illustrates the likely steric clash with the thumb subdomain (Pfu: Y654–I678; pink) in proximity to the second-gate residue (E664; red). Electrostatic potential of the primer/template binding surface of Pfu (C) and its change on the E664K equivalent mutation (D). The second-gate mutation creates a continuous positively charged binding surface.
The second-gate residue, E664, is most effectively mutated to lysine (E664K), but mutation to the uncharged glutamine (E664Q) already provided significant RNA polymerase activity (Fig. S1), suggesting that the positioning of a repulsive negative charge in proximity to the nascent strand phosphate backbone may be an important mechanistic aspect of second-gate function. Indeed, mapping of the polymerase surface electrostatic potential of the primer/template duplex binding interface reveals a significant change on E664K mutation, providing a continuous highly positively charged surface to interact with the nascent duplex (Fig. 4 C and D). This change in electrostatic potential is reflected by the increase in affinity for the noncognate RNA/DNA heteroduplex (Table 2) and the increased processivity of RNA synthesis, allowing bypass of the synthetic block at n + 6/7 (Fig. 3 and Fig. S7).
There are natural precedents for enhanced processivity mediated by either increased affinity for the primer/template duplex or, indeed, the presence of a continuous positively charged surface in the polymerase thumb subdomain (29, 30). For example, extensive interactions between the continuous highly positively charged DNA binding surface and primer/template duplex have been observed in the structure of human Pol η, where such an interface is thought to act as a “molecular splint” reshaping the distorted CPD DNA structure into a canonical B-form to allow processive TLS (29). By analogy, we propose that the continuous positively charged surface generated by the E664K mutation may not only increase the affinity (Table 2) but may reshape the nascent RNA/DNA duplex from its preferred A-form (31) into a cognate B-form conformation to allow efficient RNA synthesis and TLS (Fig. 3 and Fig. S5). By implication, the function of the negatively charged second-gate residue (E664) in the WT Tgo polymerase may be to decrease affinity for primer/template, thus enhancing discrimination against noncognate duplexes.
Although highly conserved among members of the Thermococcales, the identity of the second-gate residue 664 (or equivalent) varies among more distantly related members of the polB family (23) (Table S2). The second gate may therefore be a specific adaptation to prevent the deleterious consequences of NTP misincorporation or replication of genomic lesions at high temperature (32). Conversely, despite significant structural variability in the elaboration of the thumb subdomain, extension checkpoints have been observed in different polymerase families, including polA, where similar n + 6 termination of RNA synthesis is observed in steric-gate mutants (16, 17). In analogy to the diverse nature of steric-gate residues, the second gate may therefore be elaborated in different ways in the context of different thumb domain structures.
The unique TGK polymerase described herein has clear utility in biotechnology because it differs in useful ways from the standard ssRNAPs [e.g., T7 RNA polymerase (T7RP)], including thermostability and, most importantly, a capacity for primer-dependent RNA synthesis, which is not commonly observed in nature. Primer-dependent RNA synthesis obviates the need to initiate transcription with 5′-(pp)pG (as with T7RP) and allows free choice of the 5′-end or 5′-UTR chemistry: TGK is capable of extending a wide variety of primers, including those bearing 5′ groups, such as fluorescent dyes and biotin, facilitating synthesis and characterization of RNAs with unusual 5′ groups (33) as well as all DNA, RNA, LNA, and 2′OMe-DNA primers or chimeras thereof (Fig. S4). Furthermore, TGK enables the synthesis of fully 2′-F– or 2′-N3–substituted RNAs with applications in the aptamer field as well as RNAs in which C and U are completely replaced by 5meC and Ψ (Fig. S4), with utility in gene therapy and stem cell reprogramming (34).
In conclusion, we show that the evolutionary path from DNA to RNA polymerases is surprisingly short, comprising just two critical mutations: the classic steric-gate mutation in the active site (Y409G) (6, 10) and the herein described second-gate mutation (E664K) in the thumb subdomain. Together, these enable efficient synthesis of both long RNAs and highly modified nucleic acid polymers through modification of key aspects of the molecular interaction of the polymerase with nascent duplex and substrate NTP. The second gate thus pinpoints a previously undescribed postsynthetic determinant of polymerase substrate specificity with implications for the development of strategies for the enzymatic synthesis and evolution of unnatural nucleic acid polymers (23).
Materials and Methods
Primer Extension.
Primer extension was typically performed in 3- to 5-μL reactions containing 1–10 pmol of primer with twofold template excess. Usually, primer FD (either DNA or RNA) was used with DNA template TempN in 1× Thermopol buffer (New England Biolabs) with 0.25–0.75 mM each NTP and supplemented with MgSO4. A typical extension protocol was two cycles of 10 s at 94 °C, 1 min at 50 °C, and 5 min at 65 °C. Details of the tRNA time courses, GFP and luciferase RNA syntheses, and in vitro translation (IVT) are described in SI Materials and Methods 1.2 and 1.3.
Kinetics, Lesion Bypass, Single-Hit Extension, and Affinity Assays.
Steady-state kinetic parameters Km and Vmax for dATP and ATP incorporation were measured in standing start reactions as described previously (35) and in SI Materials and Methods 1.5. Lesion bypass and single-hit extension assays are described in SI Materials and Methods 1.6. Termination probabilities were determined as previously described (27). Polymerase primer/template affinities were assayed using FP as described in SI Materials and Methods 1.7. FP measurements were analyzed using GraphPad Prism v5.0d for Mac OS X. Raw data (Y) were fit to the equation:
 |
where Max is the FP saturation, Min is the free ligand polarization, a is the fixed ligand concentration, b is ligand + X + Kd, c is ligand⋅X, and X is the polymerase concentration.
Supplementary Material
Acknowledgments
This work was supported by Medical Research Council Grant U105178804 (to C.C., V.B.P., and P.H.), European Union Grant FP6-STREP029092 NEST (to V.B.P. and P.H.), and the National Institute of Child Health and Human Development/National Institutes of Health Intramural Research Program (A.V. and R.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120964109/-/DCSupplemental.
References
- 1.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 2.Nick McElhinny SA, et al. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc Natl Acad Sci USA. 2010;107:4949–4954. doi: 10.1073/pnas.0914857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shewach DS, Reynolds KK, Hertel L. Nucleotide specificity of human deoxycytidine kinase. Mol Pharmacol. 1992;42:518–524. [PubMed] [Google Scholar]
- 4.Nick McElhinny SA, et al. Genome instability due to ribonucleotide incorporation into DNA. Nat Chem Biol. 2010;6:774–781. doi: 10.1038/nchembio.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Breaker RR. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J Am Chem Soc. 1999;121:5364–5372. [Google Scholar]
- 6.Brown JA, Suo Z. Unlocking the sugar “steric gate” of DNA polymerases. Biochemistry. 2011;50:1135–1142. doi: 10.1021/bi101915z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnin A, Lázaro JM, Blanco L, Salas M. A single tyrosine prevents insertion of ribonucleotides in the eukaryotic-type phi29 DNA polymerase. J Mol Biol. 1999;290:241–251. doi: 10.1006/jmbi.1999.2900. [DOI] [PubMed] [Google Scholar]
- 8.Gardner AF, Jack WE. Determinants of nucleotide sugar recognition in an archaeon DNA polymerase. Nucleic Acids Res. 1999;27:2545–2553. doi: 10.1093/nar/27.12.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Franklin M, Li J, Lin TC, Konigsberg W. A conserved Tyr residue is required for sugar selectivity in a Pol alpha DNA polymerase. Biochemistry. 2002;41:10256–10261. doi: 10.1021/bi0202171. [DOI] [PubMed] [Google Scholar]
- 10.Astatke M, Ng K, Grindley ND, Joyce CM. A single side chain prevents Escherichia coli DNA polymerase I (Klenow fragment) from incorporating ribonucleotides. Proc Natl Acad Sci USA. 1998;95:3402–3407. doi: 10.1073/pnas.95.7.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JA, et al. A novel mechanism of sugar selection utilized by a human X-family DNA polymerase. J Mol Biol. 2010;395:282–290. doi: 10.1016/j.jmb.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLucia AM, Grindley ND, Joyce CM. An error-prone family Y DNA polymerase (DinB homolog from Sulfolobus solfataricus) uses a ‘steric gate’ residue for discrimination against ribonucleotides. Nucleic Acids Res. 2003;31:4129–4137. doi: 10.1093/nar/gkg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G, Orlova M, Georgiadis MM, Hendrickson WA, Goff SP. Conferring RNA polymerase activity to a DNA polymerase: A single residue in reverse transcriptase controls substrate selection. Proc Natl Acad Sci USA. 1997;94:407–411. doi: 10.1073/pnas.94.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staiger N, Marx A. A DNA polymerase with increased reactivity for ribonucleotides and C5-modified deoxyribonucleotides. ChemBioChem. 2010;11:1963–1966. doi: 10.1002/cbic.201000384. [DOI] [PubMed] [Google Scholar]
- 15.Patel PH, Loeb LA. Multiple amino acid substitutions allow DNA polymerases to synthesize RNA. J Biol Chem. 2000;275:40266–40272. doi: 10.1074/jbc.M005757200. [DOI] [PubMed] [Google Scholar]
- 16.Xia G, et al. Directed evolution of novel polymerase activities: Mutation of a DNA polymerase into an efficient RNA polymerase. Proc Natl Acad Sci USA. 2002;99:6597–6602. doi: 10.1073/pnas.102577799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong JL, Loakes D, Jaroslawski S, Too K, Holliger P. Directed evolution of DNA polymerase, RNA polymerase and reverse transcriptase activity in a single polypeptide. J Mol Biol. 2006;361:537–550. doi: 10.1016/j.jmb.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 18.McCullum EO, Chaput JC. Transcription of an RNA aptamer by a DNA polymerase. Chem Commun (Camb) 2009;45(20):2938–2940. doi: 10.1039/b820678c. [DOI] [PubMed] [Google Scholar]
- 19.Delarue M, Poch O, Tordo N, Moras D, Argos P. An attempt to unify the structure of polymerases. Protein Eng. 1990;3:461–467. doi: 10.1093/protein/3.6.461. [DOI] [PubMed] [Google Scholar]
- 20.Moras D. Polymerases. Two sisters and their cousin. Nature. 1993;364:572–573. doi: 10.1038/364572a0. [DOI] [PubMed] [Google Scholar]
- 21.Sousa R. Structural and mechanistic relationships between nucleic acid polymerases. Trends Biochem Sci. 1996;21(5):186–190. [PubMed] [Google Scholar]
- 22.Cermakian N, et al. On the evolution of the single-subunit RNA polymerases. J Mol Evol. 1997;45:671–681. doi: 10.1007/pl00006271. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro VB, et al. Synthetic genetic polymers capable of heredity and evolution. Science. 2012;336:341–344. doi: 10.1126/science.1217622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogg MJ, Pearl LH, Connolly BA. Structural basis for uracil recognition by archaeal family B DNA polymerases. Nat Struct Biol. 2002;9:922–927. doi: 10.1038/nsb867. [DOI] [PubMed] [Google Scholar]
- 25.Gardner AF, Jack WE. Acyclic and dideoxy terminator preferences denote divergent sugar recognition by archaeon and Taq DNA polymerases. Nucleic Acids Res. 2002;30:605–613. doi: 10.1093/nar/30.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Brieba LG, Sousa R. Misincorporation by wild-type and mutant T7 RNA polymerases: Identification of interactions that reduce misincorporation rates by stabilizing the catalytically incompetent open conformation. Biochemistry. 2000;39:11571–11580. doi: 10.1021/bi000579d. [DOI] [PubMed] [Google Scholar]
- 27.Kokoska RJ, McCulloch SD, Kunkel TA. The efficiency and specificity of apurinic/apyrimidinic site bypass by human DNA polymerase eta and Sulfolobus solfataricus Dpo4. J Biol Chem. 2003;278:50537–50545. doi: 10.1074/jbc.M308515200. [DOI] [PubMed] [Google Scholar]
- 28.Bambara RA, Fay PJ, Mallaber LM. Methods of analyzing processivity. Methods Enzymol. 1995;262:270–280. doi: 10.1016/0076-6879(95)62023-0. [DOI] [PubMed] [Google Scholar]
- 29.Biertümpfel C, et al. Structure and mechanism of human DNA polymerase eta. Nature. 2010;465:1044–1048. doi: 10.1038/nature09196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber HE, Tabor S, Richardson CC. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987;262:16224–16232. [PubMed] [Google Scholar]
- 31.Noy A, Pérez A, Márquez M, Luque FJ, Orozco M. Structure, recognition properties, and flexibility of the DNA.RNA hybrid. J Am Chem Soc. 2005;127:4910–4920. doi: 10.1021/ja043293v. [DOI] [PubMed] [Google Scholar]
- 32.Drake JW. Avoiding dangerous missense: Thermophiles display especially low mutation rates. PLoS Genet. 2009;5:e1000520. doi: 10.1371/journal.pgen.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowtoniuk WE, Shen Y, Heemstra JM, Agarwal I, Liu DR. A chemical screen for biological small molecule-RNA conjugates reveals CoA-linked RNA. Proc Natl Acad Sci USA. 2009;106:7768–7773. doi: 10.1073/pnas.0900528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Creighton S, Bloom LB, Goodman MF. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- 36.Hantz E, et al. Solution conformation of an RNA–DNA hybrid duplex containing a pyrimidine RNA strand and a purine DNA strand. Int J Biol Macromol. 2001;28:273–284. doi: 10.1016/s0141-8130(01)00123-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.