Abstract
Background
One in four women has moderate to severe symptoms of at least one pelvic floor disorder. Lifetime physical activity, a modifiable risk factor, may theoretically predispose women to, or protect them from, developing pelvic floor disorders. It is neither feasible nor ethical to test this association using the most rigorous (level I) study design.
Purpose
The aim of this manuscript is to describe the methods for the PHysical ACtivity Study (PHACTS), which encompasses two case-control studies and the development of a registry, and to describe challenges and solutions to study progress to date. For each of the case-control studies, the primary aims are to determine, compared to controls with neither pelvic organ prolapse nor urinary incontinence, whether 1) pelvic organ prolapse or 2) stress urinary incontinence is associated with a) increased or decreased current leisure activity or b) increased or decreased overall lifetime activity (including leisure, household, outdoor, and occupational) measured in MET-hours per week, as well as in strenuous hours per week.
Methods
To obtain 175 pelvic organ prolapse cases, 175 stress urinary incontinence cases, and an equal number of age, body mass index and recruitment site matched controls, we plan to enroll 1500 women from about 20 primary care level clinics.
Results
We have encountered various challenges leading to lessons learned about minimizing bias, recruitment from community clinics, the lifetime physical activity instrument used, and data management.
Conclusions
Our experiences can help guide future investigators studying risk factors, particularly physical activity, and pelvic floor disorders.
Keywords: physical activity, pelvic floor disorders, urinary incontinence, pelvic organ prolapse, case-control study
INTRODUCTION
“Pelvic floor disorders”, a term used to describe conditions related to changes in anatomy or functioning of the pelvic organs, include urinary incontinence, pelvic organ prolapsed (when one or more of the pelvic organs fall into the vagina) and fecal incontinence. One in four American women has moderate to severe symptoms of at least one pelvic floor disorder.1 Up to one in seven undergoes surgery for pelvic organ prolapse and/or urinary incontinence in her lifetime.2, 3, 4 Advancing age, childbirth, obesity, and race are associated with both pelvic organ prolapse and urinary incontinence.5,6,7,8,9,10 Other risk factors, such as hysterectomy, hormone therapy and family history, have been less well explored. The rate of pelvic floor disorders is expected to increase dramatically with the aging of the American population.11 Understanding modifiable risk factors is crucial. Preventing 25% of women from developing pelvic floor disorders would save 90,000 women per year from experiencing prolapse or incontinence.12
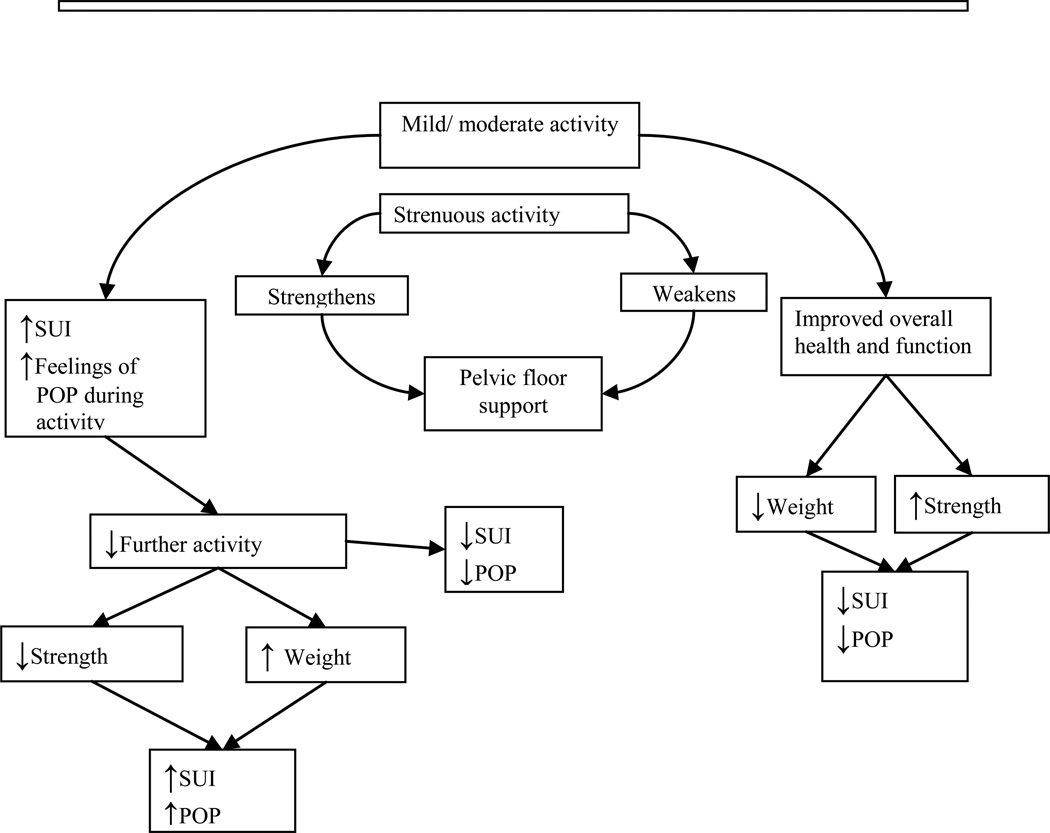
Many believe that strenuous activity predisposes women to stress urinary incontinence and pelvic organ prolapse. Indeed, the patient portal of the American Urogynecologic Society website currently recommends women avoid “increased pressure inside the abdomen”, heavy lifting and repetitive strenuous activities to prevent pelvic floor disorders. (Take the floor; voices for PFD [Internet].Washington DC: American Urogynecologic Society; [Cited 2012 Jan 13]. Available from: http://www.voicesforpfd.org/p/cm/ld/fid=25). Physical activity has numerous health benefits and women should be encouraged to be active. However, there may be a threshold for the pelvic floor at which physical activity’s benefit is negated. Mild or moderate activity and strenuous activity may impact pelvic floor disorders differently, and in a bidirectional manner (Figure 1). Regular low impact activity, like walking, is associated with a lower prevalence of stress incontinence. 13,14 However, many young women report stress urinary incontinence during high-impact, vigorous intensity activities: 28% of college varsity athletes, 41% of elite athletes and 43% of women participating in club sports. 15, 16,17 Some particularly strenuous activities may damage the pelvic floor. Nulliparous military women that completed paratrooper training were more likely to have stage II pelvic organ prolapse than women undergoing regular summer training. 18 Women may stop exercising because of urinary incontinence or fear that such activity will promote pelvic floor disorders. 19,20
Fig. 1.
A theoretical model of effects of mild/moderate vs strenuous physical activity on pelvic floor disorders.
SUI= Stress Urinary Incontinence
POP= Pelvic Organ Prolapse
Of 60% of women that are employed, 9.8% are engaged in repeated strenuous physical activity for four or more hours each day. 21 Some data indicate that women with prolapse or incontinence are more likely to report strenuous jobs than women without such disorders. 22, 23,24,25,26 However, these studies are variably limited by poorly defined occupational and activity histories, non-standardized outcome assessment, and lack of consideration for confounders.
Attempts to delineate the association between physical activity over a lifetime and pelvic floor disorders face a major obstacle: level I randomized trial research methods cannot be employed to study this association, and lifelong prospective cohort studies are not practical. Therefore, to explore the associations between lifetime physical activity and current pelvic floor disorders, we developed an umbrella research project containing two case-control studies and a registry. Herein, we describe the methods for this research, the actions we took apriori to minimize limitations and bias, and the challenges we faced with study design and implementation.
METHODS
Overview
While many women have both stress incontinence and pelvic organ prolapse and there is overlap between some risk factors for these conditions, we cannot assume that physical activity affects each condition similarly. Therefore, we developed two case-control studies; in one study cases are women with stress urinary incontinence study without pelvic organ prolapse, and in the other, cases are women with pelvic organ prolapse without stress urinary incontinence. To maximize efficiency and minimize cost, we conducted both studies in parallel. We created one larger control group from which we will draw frequency matched controls for each of the case-control studies. All participants were asked to complete all study instruments as case/control determination requires both physical examination and completion of certain questionnaires. All participants were invited to join a registry, for which they can be contacted for future research, regardless of whether they were ultimately eligible for the case-control studies.
Aims
For each of the studies, the primary aims are to determine, compared to controls with neither pelvic organ prolapse nor urinary incontinence, whether 1) pelvic organ prolapse or 2) stress urinary incontinence is associated with a) increased or decreased current leisure activity or b) increased or decreased overall lifetime activity (including leisure, household, outdoor and occupational) measured in MET-hours per weekand in strenuous hours per week.
Defining pelvic floor disorders
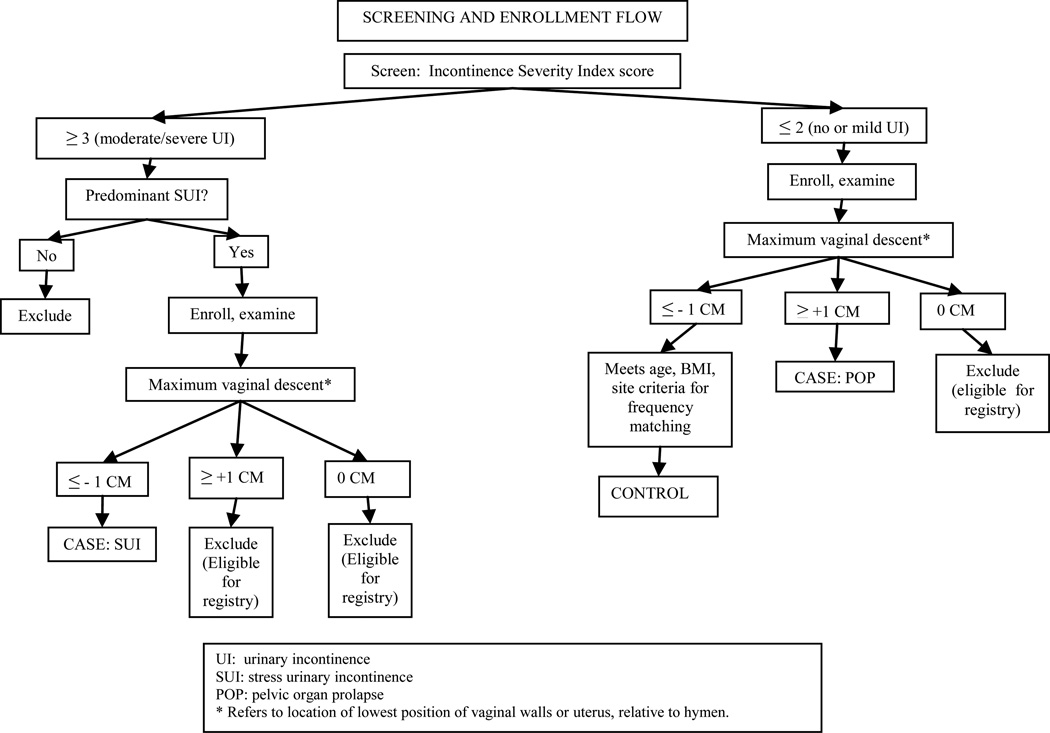
Unlike conditions such as hypertension or diabetes, no numerical cut-point for either urinary incontinence or pelvic organ prolapse exists to dichotomize “disease” and “normal”. Using the official definition of urinary incontinence endorsed by the International Continence Society, “the complaint of any involuntary leakage of urine”, half of young women would be considered “incontinent”. 27 To limit the diagnosis to women with more severe or bothersome symptoms, rather than including women that ever leak a drop of urine, we operationalize incontinence using the validated Incontinence Severity Index , in which a score is obtained by multiplying results of two questions regarding frequency and amount of leakage. This index demonstrates good reliability and responsiveness28, and is valid compared to pad testing 29,30,31. Consistent with definitions used in population-based research, we defined insignificant (none or mild) urinary incontinence as a score of ≤ 2 and moderate/severe incontinence as a score of ≥ 3.32, 33, 1
To further define stress urinary incontinence, we chose the “3IQ”, a simple 3-item questionnaire, which has good test characteristics when compared with the gold standard of an extended evaluation by incontinence specialists. 34 Stress/stress predominant incontinence is defined when women respond that in the last 3 months they most often leak urine with physical activity, such as coughing, sneezing, lifting or exercise.
Contrary to the symptom-based diagnosis of urinary incontinence, diagnosing pelvic organ prolapse based on symptoms is fraught with error. Compared to examination, the sensitivity of the question, “Do you usually have a bulge or something falling out that you can see or feel in your vaginal area?” is only 35% in a low-probability community population.35 Therefore, we define prolapse based on the Pelvic Organ Prolapse Quantification (POP-Q) system36, a reproducible method for assessing vaginal descent37,38. In this system, the lowest level of vaginal descent (that is, maximal vaginal descent) during Valsalva in a supine position is measured relative to its distance in cm from the hymen; points above the hymen are negative while those below are positive.
Up to one-half of middle-aged women have vaginal descent at the level of the hymen, and there is no consensus over whether this represents “normal” or a “condition”. 39, 40 Therefore, women with descent at the hymen are excluded (screened out) from either case-control study to create the clearest delineation between prolapse and “normal”. We define prolapse as present when the maximal vaginal descent is ≥ +1 cm (that is, 1 cm below the hymen or lower) and as absent when the maximal vaginal descent is ≤ − 1 cm (that is, 1cm above the hymen or higher).41,25 Women with vaginal descent at the level of the hymen may remain in the registry.
Symptoms of pelvic floor disorders
To assess symptoms of pelvic floor disorders, we use the Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ), a psychometrically valid screening instrument that identifies women at high risk of having pelvic floor disorders.42 This instrument was validated in populations of women both seeking and not seeking care for pelvic floor disorders and includes questions about urinary incontinence, fecal incontinence, pelvic organ prolapse, and sexual function.
Defining cases and controls
Stress urinary incontinence cases are women without prolapse, with a score of ≥ 3 on the Incontinence Severity Index and with stress or stress predominant incontinence on the 3-IQ. Prolapse cases are women with prolapse and with a score of ≤ 2 on the Incontinence Severity Index. Potential controls for both studies are women without prolapse and with a score of ≤2 on the Incontinence Severity Index.
Exclusion criteria
Women are excluded who are pregnant or within six months postpartum, < 39 or > 65 years, have a body mass index (BMI) < 18.5 kg/m2 or ≥ 40 kg/m2, have had prior surgical treatment for prolapse or incontinence, are not able to walk independently, had major medical problems precluding exercise for the last 12 months, had medical conditions associated with incontinence or low physical activity (diabetes, neurologic disorders such as multiple sclerosis, spinal cord injury, or stroke, rheumatoid arthritis, blind, radical hysterectomy or pelvic irradiation), are currently undergoing treatment for cancer, or are unable to complete questionnaires. We chose the age range 39–65 years to reflect the population of women included in the validation of the physical activity instrument (described below), and that is likely to have developed pelvic organ prolapse and still be of an age likely to engage in physical activity.
Assessing physical activity in women
Many instruments have been described which assess physical activity. Most were designed and tested for men, focus on paid labor and/or exercise and sports, and collect recent activity data only. 43,44,45 However, much of activity that women do is in household areas. After reviewing available instruments validated in women that capture lifetime activity, including household activity, we chose the self-administered Lifetime Physical Activity Questionnaire (LPAQ). 46 The questionnaire assesses weekly hours of physical activity for the reported number of months per year in leisure, outdoor and household activities over four age periods, menarche to age 21, 22–34, 35–50, and 51–65 years. The LPAQ was reproducible in women ages 39 to 65, similar to the population with pelvic floor disorders, and demonstrated validity, compared to four weekly activity logs, in assessing past year physical activity.47 The LPAQ lists common activities, helpful for recall, and allows for inclusion of additional items.
The LPAQ does not assess occupational activity. To assess this domain, we chose the Occupation Questionnaire, a component of the Lifetime Total Physical Activity Questionnaire (LTPAQ), which has demonstrated reliability, particularly in assessing heavy work.48 For each individual job listed, total duration in years, months per year, days per week, hours per day and intensity category are reported. We opted against using this questionnaire for household and leisure activities as it does not list specific activities, therefore making it less conducive to self-administration, and also less feasible for use with the Compendium of Physical Activities (see scoring, below).
Scoring the LPAQ and Occupation Questionnaire
For each activity reported on the LPAQ we assigned a MET value from the Compendium of Physical Activities.49 The MET score is multiplied by the reported number of hours per week, the fraction of months in a year, and the fraction of years per age period to calculate MET hours per week. A similar method is used to calculate MET hours per week for the Occupation Questionnaire, however, the MET values are assigned into categories according to reported occupational activity intensity: 1=sedentary: 1 MET, jobs which are more reflective of sedentary behavior than of physical activity and are not used in the calculation of overall lifetime activity; 2=light activity: 2.5 METs, jobs requiring minimal physical effort such as slow walking with no cardiovascular or metabolic overload; 3=moderate activity: 4.0 METs, jobs requiring carrying light loads (5–10 lbs) and prolonged walking, that slightly increase cardiovascular or metabolic demand but occur indoors; and 4=heavy activity: 6.0 METs, jobs requiring carrying heavy loads (>10 lb) and fast walking, that significantly increase cardiovascular or metabolic demand. Data from occupations reported as light, moderate or heavy on the Occupation Questionnaire are combined with the LPAQ data to arrive at an overall lifetime activity score in MET hours per week.
Strenuous activity
The LPAQ, as with most activity questionnaires, measures metabolic exertion and is not specifically designed to assess the processes that are believed to influence urinary incontinence and pelvic organ prolapse: abdominal loading and impact. To examine separately the effects of high-impact and high-loading activities on pelvic floor disorders, we developed a preliminary Strenuous Activity Index. We used results from an unpublished pilot study, in which 18 clinicians specializing in pelvic floor disorders identified activities that they believed might cause, promote or hasten recurrence of pelvic floor disorders, and from previous work of intra-abdominal pressures during various activities.50 We assigned all activities listed on the LPAQ a categorical value from 1 to 4 using methods established in the validation and scoring of the Occupation Questionnaire. A score of “1” indicated no relationship, “2” probably no, “3” possibly, and “4” a highly likely relationship with promoting pelvic floor disorders (based on the expert panel). Activities associated with a known increase in intra-abdominal pressure of 100cm H2O or more were automatically assigned a “4”, as were activities strongly associated with urinary leakage, such as gymnastics. “Strenuous activity” is the sum of time spent in leisure, household, outdoor and occupational activities categorized as 3 or 4, since activities categorized as 1 or 2 are not thought to make significant contributions to the development of pelvic floor disorders (and in any case, cannot be avoided).
Summary of physical activity variables to be used in analyses
We will analyze three primary variables: 1) weighted average MET-hours per week of overall lifetime activity (leisure + household + outdoor +occupational activity in each timespan, weighted by years lived in each timespan), 2) average MET-hours per week of current leisure activity (leisure activity over the past one year), 3) and weighted average strenuous-hours per week of lifetime activity (leisure + household + outdoor + occupational activity in each timespan, weighted by years lived in each timespan).
Quality Control
While the LPAQ was designed to be self-administered, we employed a graduate research assistant with expertise in assessing physical activity to review each LPAQ and Occupation Questionnaire and verbally clarify missing or inconsistent responses with participants. This proved more difficult than anticipated, as discussed below.
Measurement variability sub-studies
In planning this study, we designed a limited test-retest sub-study to obtain data that would provide us with intraclass correlations and other measures to use during data analysis to address the issue of bias due to measurement errors.51,52. Before initiating the study, we added two additional sub-studies to assess the inter-method reliability of web vs. paper versions of the LPAQ and the EPIQ, as well as their individual test-retest reliability. Women willing to complete the instruments twice do so within 3–6 weeks and complete either: 1) paper/paper, 2) web/web, or 3) paper/web or web/paper (the latter two categories randomly assigned using randomizations in sealed envelopes provided by the study statistician).
Observer Objectivity
Study personnel who performed pelvic exams were masked to survey responses. Study personnel who reviewed the surveys with participants were masked to case/control status.
Collection of potential risk factors
We collected self-reported information about the following potential risk factors for pelvic floor disorders: age, ethnicity/race, education, smoking, caffeine intake, childbirth (number of vaginal vs cesarean deliveries), hysterectomy, hormonal therapy, medical conditions, overall health status, BMI and pelvic muscle function. Because of the inaccuracy of recall of obstetric events, other than type of delivery, we did not ask more focused questions about childbirth history.53 Because women with incontinence may decrease their activity because of incontinence, we added questions at the end of each age epoch in the LPAQ pertaining to whether incontinence resulted in a change in their physical activity behavior.
Recruitment
Participants (cases and controls) are being primarily recruited from over 20 primary care level gynecologic and family medicine clinics located across the Salt Lake Valley and from community advertising. In addition, we were concerned about the feasibility of recruiting sufficient women with pelvic organ prolapse from primary care clinics, and so also recruited these cases from a tertiary care level urogynecology clinic, and planned to match controls from a tertiary care level dermatology clinic. Because case or control designation cannot be accomplished until after the pelvic examination, we collected complete study data in all women. All women that met the basic inclusion criteria were eligible to be in the study; case vs control status was designated by the study statistician after all data collection and quality review were completed. All participants were also offered enrollment into a registry, allowing us to contact them in the future for studies about pelvic floor disorders. The schema for screening and enrollment is summarized in Figure 2.
Fig. 2.
Screening and enrollment flow diagram
Sample size calculation
Our proposed sample size of 175 per case group provides over 80% power to detect the following scenarios at the 2-sided 5% significance level54,55: 1) In the scenario in which the exposure odds ratio is nonlinear in quintiles of physical activity based on the control group, with the distribution of cases from lowest to highest quintile: 25%, 25%, 30%, 10%, 10%, or more extreme, using as an approximation an analysis of a 2×5 contingency table using Person’s chi-squared (NQUERY v6 software.); 2) Considering physical activity as a continuous variable with errors in measurements:
| Odds ratio for a 1 SD increase in the true exposure to physical activity | .295 | .455 |
| Correlation between true and observed exposure | 0.7 | 0.7 |
| Prevalence of the outcome at the mean for the exposure and covariate | 0.06 | 0.5 |
| Odds ratio for a 1 SD increase in the covariate | .295 | .455 |
| Correlation between exposure and covariate | 0.3 | 0.3 |
Data Analysis
The control group will be frequency matched to cases to ensure a similar distribution of age (39–49, 50–60, 61–65 years), BMI (18.5– 24.9, 25–29.9 and 30–30.9 kg/m2) and recruitment site (primary care clinics, community advertising and tertiary care clinic). While the control group for the stress urinary incontinence analysis will have some overlap with the control group for the pelvic organ prolapse analysis, the anticipated older average age of women with pelvic organ prolapse suggests that these control groups will not be identical. Each case group and its corresponding control group will be analyzed in separate analyses.
Each group will be characterized in terms of demographics and risk factors. Physical activity variables (lifetime MET-hours/week, lifetime strenuous hours/week, current MET-hours/week, current strenuous hours/week) will be characterized descriptively by group.
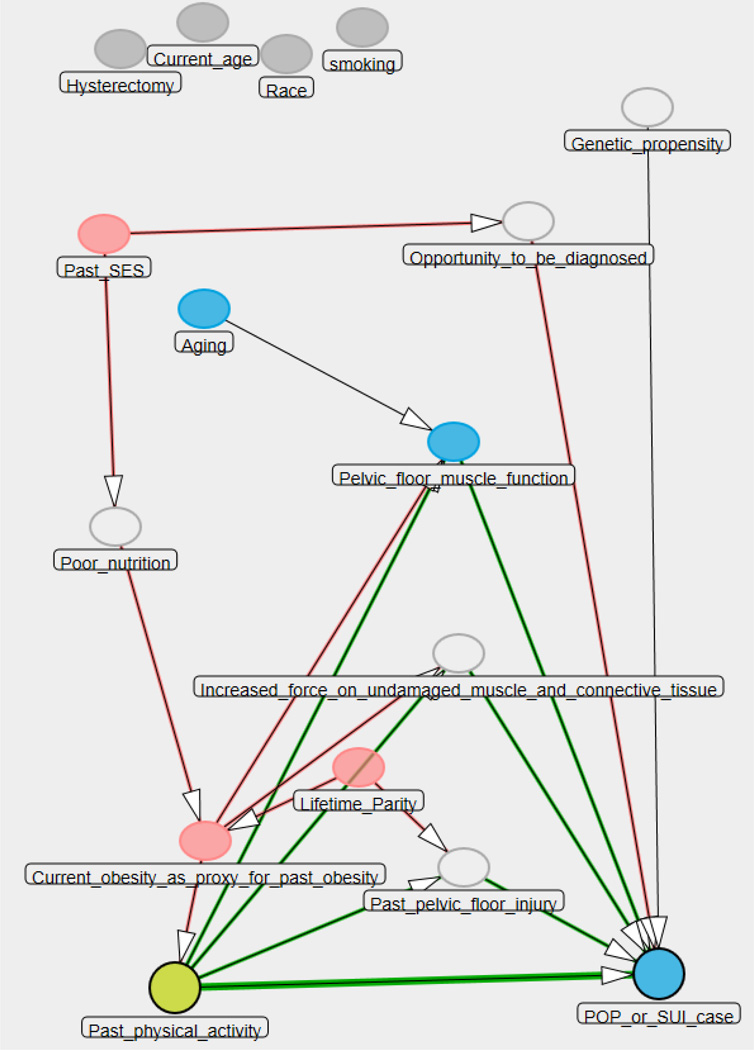
Physical activity variables will be divided into quintiles based on the distribution in the control group. Odds ratios relative to the lowest quintile of physical activity will be estimated in a crude analysis; adjusted one-by-one for potential confounders; and adjusted for multiple potential confounders in further modeling via logistic regression, based on a causal diagram. (Proposed directed acrylic graph (DAG) summarized in Figure 3.) Models will be checked for effect modification. Logistic regression models will also be checked for adequacy of the model, multicollinearity, influential observations, etc., using standard regression diagnostics. Nominal p-values and 95% confidence intervals for the odds ratio will be reported. Final models will utilize multiple imputation for missing values, which has been found to be more unbiased and precise than older imputation methods.56 Multiple imputation software continues to improve. We will most likely use sequential regression multivariate imputation57 (SRMI) , using, e.g., IVEWARE or the expanded mi command in Stata 12. All tests will be two-sided at the conventional 5% significance level. Analysis will be performed in SAS or Stata.
Fig. 3.
Directed acrylic graph (DAG) illustrating effect of past physical activity on pelvic organ prolapse or stress urinary incontinence.61
KEY (colors are noted for black and white printing; if figure would be in color, we will change key):
Open oval: unmeasured confounders in PHACTS study
Light grey oval: additional confounders
Dark grey circles: exposure of interest (physical activity) and outcome (POP/ SUI).
Adjustment by current obesity as a proxy for past obesity is sufficient to adjust for all confounding in this DAG.
Genetic propensity is not a confounder, so it is the exception to the above.
Secondary analyses will include logistic regression models incorporating several measures of physical activity, graphical displays of time trends and subcategories of physical activity; and sensitivity analyses to examine and correct for errors in measurement where the data permit. Odds ratios will be adjusted for errors in measurements using the SIMEX method.58 Alternative analyses using causal methods will be explored.
Under the assumption of nondifferential errors (variability) in measurement of current leisure physical activity in the case vs. control group, the bias induced in the odds ratio by measurement error would be anticipated to be in the direction of the null hypothesis. 59 Thus, if a statistically significant exposure odds ratio of important magnitude for current leisure physical activity and stress urinary incontinence is observed, it will have overcome any bias anticipated by the statistical theory of errors in measurements. If confounders also incur nondifferential errors in measurement, the direction of the bias is unpredictable.
Human subjects approval
The Institutional Review Boards of the University of Utah and Intermountain Healthcare approved the overarching study. All participants completed an informed consent process.
DISCUSSION
While several longitudinal studies query both physical activity and urinary incontinence in women, the existing literature has limitations. Activity is questioned only during study years with no assessment of past activity. The full spectrum of activity is not addressed (that is, occupational and household, as well as leisure). Strenuous loading and impact types of activity are not specified. Pelvic organ prolapse cannot be addressed without examination. Pertinent confounders are not uniformly assessed. Our study, despite the imperfections inherent in any case-control study, attempts to overcome these limitations. We evaluate historical as well as current activity, use a reproducible instrument designed for studying activity in women, assess lifetime occupational activity, analyze the effect of strenuous (high magnitude load and impact) activities separately, assess outcomes using validated measures, recruit subjects from primary care level clinics, and adjust for known risk factors for pelvic floor disorders. We did not study women with primarily urge incontinence; while this is likely to decrease current activity, it is less plausible that lifetime strenuous activity impacts bladder contractions. Some women may have other pelvic symptoms associated with physical activity, such as urgency and frequency or a symptom of a bulge in the absence of one on exam and our study is not powered to compare activity in these types of sub-groups.
In this section, we share some challenges we have encountered.
Recruitment projections, strategy and actual trends to date
Following we share our apriori recruitment estimates based on available literature and best clinical guess, and our actual recruitment trends, calculated after enrollment of the first 669 women: 1) Proportion of participants with stress incontinence eligible to be a stress incontinence case (ie, demonstrating no prolapse on examination): estimate: 50–75%, actual: 76%; 2) Proportion of participants without urinary incontinenceeligible to be a control (ie, demonstrating no prolapse on examination): estimate: 75%, actual: 71% ; 3) Proportion of participants eligible to be a prolapse case (ie, endorsing no/mild urinary incontinence): estimate: 5%, actual: 31%. Our estimate that only 5% of women recruited from the community would meet criteria for a prolapse case would yield only 75 prolapse cases during our study timeframe and therefore we planned to recruit an additional 100 cases from our tertiary care urogynecology clinic. However, most women from the urogynecology clinics were excluded because of previous surgery for pelvic floor disorders or concomitant incontinence. Because the prevalence of prolapse in our community population is higher than estimated, we are now able to exclude prolapse cases recruited through our urogynecology clinics from our analyses, thereby limiting the potential bias inherent in recruiting cases from a tertiary care setting.
We initially estimated that our non-tertiary care level recruitment would be divided evenly between primary care clinics and community. While we continuously blanketed our community with flyers and brochures, gave media interviews, created web-based recruitment information, and attended health fairs, very few women to date have been recruited through these avenues. In contrast, the primary care clinic recruitment strategy, while labor intensive, is successful. The PI and research nurse met various clinic groups, generally during their staff meeting, to explain the study background and logistics. All clinics were willing to facilitate recruitment and most were also willing to provide the research nurse with the intermittent use of an examination room in which to complete screening materials, consent and perform the prolapse quantification measure. In this way, women did not have to return to the university to be examined. Women then completed study instruments at home. Our research nurses were present in person in any given clinic approximately one half day per 2 week period. The time spent by a research nurse to recruit one participant has ranged from 0.8 hours to 9.1 hours; we abandoned recruitment from sites that yielded fewer than one participant in four hours. We also recruited through the Utah Health Research Network, a network of community primary care clinics that facilitates research, by sending out batches of letters to women in the clinics’ databases meeting basic entry criteria, with follow-up telephone calls. Women willing to participate completed the study instruments at home, presented to one of the community clinics for examination, and at the same setting, the graduate exercise science research assistant quality-control checked the instruments. While inefficient up front, in that we telephoned 20 women to obtain one participant, participants’ data were complete in one setting.
Minimizing biases inherent in case-control studies
The strength of association between risk factors and various conditions may differ depending on whether cases are derived from patients in a specialty clinic or people in the community. 60 Women presenting for treatment of pelvic floor disorders may have already changed activity because of the disorder. Therefore, we are recruiting mainly through primary care level clinics and exclude women that have undergone surgery for pelvic floor disorders from both case and control groups. While not as optimal as using a population based sampling approach, the need to do pelvic examinations presented a trade-off between optimal and feasible. Participants are masked to specific study hypotheses and are told that we are investigating the impact of lifetime physical activity on women’s health and pelvic floor. We made no attempt to recruit women more or less likely to be physically active. Participants are not told that they fall into a specific study group and thus have no knowledge of this while completing the LPAQ. The study coordinator is masked to LPAQ results and the exercise science graduate assistant to group assignment. Given these various factors, it is likely that the control group represents a relatively unbiased representation of the exposure distribution amongst relatively healthy 39–65 year old women living in our geographic population.
Limitations of the LPAQ instrument
Establishing concurrent or criterion validity for lifetime physical activity measures is highly impractical as it would require assessing activity prospectively over a lifetime. To date, no instrument validated in this manner exists. However, the LPAQ and Occupation Questionnaire were developed using sound measurement principles. They are most valuable in understanding physical activity in relative terms, and are useful for ranking groups of individuals by level of activity.
Ensuring high-quality data from the LPAQ proved to be a challenge; indeed, the difficulty in assessing the self-report of lifetime physical activity is acknowledged in a recent historical review 59. While designed to be self-administered, we found that the instrument was rarely completed adequately. Common problems included omitting age epochs, providing ranges, rather than the required single number for recording hours, overlapping household and occupational activities (for example, some women that do not work outside the home recorded household labor on both the LPAQ and the Occupational Questionnaire) and exceeding plausible weekly hours spent doing activities. Surprisingly, we were unable to find set parameters for upper limits of weekly MET-hours. We considered that it is largely impossible for someone to do high-MET type activities more than 18 hours per day, but searched the literature and consulted colleagues in the field for a more evidence-based limit. We identified two studies that provided values for MET-hrs/wk, one in Amish women for household and outdoor activity and the other in marathon athletes for leisure activity 60,61. We calculated maximum values by adding the mean + 3 Standard Deviations, which yielded 486 and 186 MET-hrs/ week for household/outdoor and leisure activity, respectively. We then applied this total of 671 MET-hrs/wk as the maximum acceptable value for the combined categories of household, leisure and outdoor activity. If, after quality control, values remain above this cut-point, the LPAQ results are considered not-analyzable.
The graduate student spent about 1.5 hours per questionnaire on quality control; this included often multiple attempts to contact participants by telephone, clarifying responses, reentering data, keeping a data log of changes and participating in QC weekly meetings with the Exercise Science principal investigator to review. We developed a manual of operations for LPAQ quality control to ensure that response changes were done in the same way each time.
Data management programming
On the LPAQ and Occupation Questionnaire, participants record responses for up to 45 categories of activities for past year and 4 age epochs. Occupational entries are added to this list. MET-hour values are calculated for all and then weekly averages are determined for each activity and various activity and timespan groupings. This results in over 8,000 intermediate and final variables created on hundreds of pages of SAS code. Quality of calculations is ensured by independent calculation and verification by multiple study personnel. Data quality is ensured by creating frozen files of the data every 2 months, and performing regular computer-assisted edit checks and review of data discrepancies, blind to case/control status. Each identified problem then requires complex detective work to identify its source: a questionnaire that despite the QC measures described still has implausible or inconsistent values, a problem with data entry, or a problem with coding and programming. By doing data cleansing as an ongoing activity rather than a massive enterprise after enrollment is completed, we are able to redress some errors while the information is fresh in participants’ minds and to continually improve our methods.
Conclusion
By understanding the long-term effects of different levels of activity on pelvic floor disorders, we can either reassure women that strenuous physical activity appears to have no effect on pelvic floor disorders or warn them that certain physical activities may in some cases be harmful. We will use our results to plan future studies about the role, if any, of long-term activity restrictions after treatments for incontinence or prolapse. The registry established through this research will enable future research about the natural history of pelvic floor disorders in a carefully phenotyped population.
Acknowledgments
Funding acknowledgement
The project described was supported by Grant Number R01HD057895-01 from the Eunice Kennedy Schriver National Institute of Child Health and Human Development. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Other than funding from NIH (noted above), the authors have no financial disclosures or conflicts to report.
Textor J: DAGitty Version 1.1 User Manual , 11/20/2011; dagitty.net
Contributor Information
Ingrid Nygaard, Department of Obstetrics and Gynecology, University of Utah School of Medicine.
Janet Shaw, Department of Exercise and Sport Science, University of Utah College of Health.
Marlene J Egger, Department of Family and Preventive Medicine, University of Utah School of Medicine.
References
- 1.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997 Apr;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096–1100. doi: 10.1097/AOG.0b013e3181f73729. [DOI] [PubMed] [Google Scholar]
- 4.Wiskind AK, Crighton SM, Stanton SL. The incidence of genital prolapse after the Burch colposuspension. Am J Obstet Gynecol. 1992;167:399–405. doi: 10.1016/s0002-9378(11)91419-7. [DOI] [PubMed] [Google Scholar]
- 5.Progetto Menopausa Italia Study Group. Risk factors for genital prolapse in non-hysterectomized women around menopause: results from a large cross-sectional study in menopausal clinics in Italy. Eur J Obstet Gynecol Reprod Biol. 2000;93:135–140. [PubMed] [Google Scholar]
- 6.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107:1253–1260. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 7.Foldspang A, Mommsen S, Lam GW, Elving L. Parity as a correlate of adult female urinary incontinence prevalence. J Epidem Commun Health. 1992;46:595–600. doi: 10.1136/jech.46.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RD, Posner SF. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 9.Nygaard I, Turvey C, Burns TL, Crischilles C, Wallace R. Urinary incontinence and depression in middle-aged United States women. Obstet Gynecol. 2003;101:149–156. doi: 10.1016/s0029-7844(02)02519-x. [DOI] [PubMed] [Google Scholar]
- 10.Sampselle CM, Harlow SD, Skurnick J, Brubaker L, Bondarenko I. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100:1230–1238. doi: 10.1016/s0029-7844(02)02241-x. [DOI] [PubMed] [Google Scholar]
- 11.Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184(7):1496–1501. doi: 10.1067/mob.2001.114868. [DOI] [PubMed] [Google Scholar]
- 12.DeLancey JO. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005;192(5):1488–1495. doi: 10.1016/j.ajog.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109:721–727. doi: 10.1097/01.AOG.0000255973.92450.24. [DOI] [PubMed] [Google Scholar]
- 14.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? BJOG. 2003;110:247–254. [PubMed] [Google Scholar]
- 15.Nygaard IE, Thompson FL, Svengalis SL, Albright JP. Urinary incontinence in elite nulliparous athletes. Obstet Gynecol. 1994;84:183–187. [PubMed] [Google Scholar]
- 16.Bo K, Borgen JS. Prevalence of stress and urge urinary incontinence in elite athletes and controls. Med Sci Sports Exerc. 2001;33:1797–1802. doi: 10.1097/00005768-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Thyssen HH, Clevin L, Olesen S, et al. Urinary incontinence in elite female athletes and dancers. Int Urogynecol J. 2002;13:15–17. doi: 10.1007/s001920200003. [DOI] [PubMed] [Google Scholar]
- 18.Larsen WI, Yavorek T. Pelvic prolapse and urinary incontinence in nulliparous college women in relation to paratrooper training. Inter Urogyn J. 2006 Oct 12; doi: 10.1007/s00192-006-0226-3. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 19.Nygaard I, Handa V, Brubaker L, et al. Physical activity in women planning sacrocolpopexy. Int Urogynecol J. 2007;18:33–37. doi: 10.1007/s00192-006-0116-8. [DOI] [PubMed] [Google Scholar]
- 20.Nygaard I, DeLancey JO, Arnsdorf L, Murphy E. Exercise and incontinence. Obstet Gynecol. 1990;75:848–851. [PubMed] [Google Scholar]
- 21.Wagener DK, Walstedt J, Jenkins L, et al. Women: Work and Health. Vital Health Stat. 1997;3(31):34. [PubMed] [Google Scholar]
- 22.Spernol R, Bernaschek G, Schaller A. Entstehungsursachen des deszensus (Factors promoting descensus, in German with English abstract) Geburtsh u Frauenheilk. 1983;43:33–36. doi: 10.1055/s-2008-1037054. [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen S, Hein HO, Gyntelberg F. Heavy lifting at work and risk of genital prolapse and herniated disc in assistant nurses. Occup Med. 1994;44:47–49. doi: 10.1093/occmed/44.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Chiaffarino F, Chatenoud L, Dindelli M, et al. Reproductive factors, family history, occupation and risk of urogenital prolapse. Europ J Obstet Gynecol and Reprod Biol. 1999;82:63–67. doi: 10.1016/s0301-2115(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 25.Woodman PJ, Swift SE, O’Boyle A, et al. Prevalence of severe pelvic organ prolapse in relation to job description and socioeconomic status: a multicenter cross-sectional study. Int Urogynecol J. 2006;17:340–345. doi: 10.1007/s00192-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 26.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 27.Abrams P, Cardozo L, Fall M, et al. The standardization of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 28.Hanley J, Capewell A, Hagen S. Validity study of the severity index, a simple measure of urinary incontinence in women. BMJ. 2001;322:1096–1097. doi: 10.1136/bmj.322.7294.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19:137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Hanley J, Capewell A, Hagen S. Validity study of the severity index, a simple measure of urinary incontinence in women. BMJ. 2001;322:1096–1097. doi: 10.1136/bmj.322.7294.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donovan JL, Ruud Bosch JLH, Gotoh M, Jackson S, Naughton M, Radley S, Valiquette L. Symptom and quality of life assessment. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 3rd International Consultation on Incontinence. Plymouth, UK: Health Publication Ltd.; 2005. pp. 519–584. [Google Scholar]
- 32.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J. 2006;17:520–524. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 33.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J. 2006;17:520–524. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 34.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715–723. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber MD, Neubauer NL, Klein-Olarte V. Can we screen for pelvic organ prolapse without a physical examination in epidemiologic studies? Am J Obstet Gynecol. 2006;195:942–948. doi: 10.1016/j.ajog.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 36.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 37.Kobak WH, Rosenberger K, Walters MD. Interobserver variation in the assessment of pelvic organ prolapse. Int Urogynecol J. 1996;7:121–124. doi: 10.1007/BF01894199. [DOI] [PubMed] [Google Scholar]
- 38.Hall AF, Theofrastous JP, Cundiff GW, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. Am J Obstet Gynecol. 1996;175:1467–1470. doi: 10.1016/s0002-9378(96)70091-1. [DOI] [PubMed] [Google Scholar]
- 39.Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol. 2003;189:377–379. doi: 10.1067/s0002-9378(03)00698-7. [DOI] [PubMed] [Google Scholar]
- 40.Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104:489–497. doi: 10.1097/01.AOG.0000136100.10818.d8. [DOI] [PubMed] [Google Scholar]
- 41.Delancey JOL, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 42.Lukacz ES, Lawrence JM, Buckwalter JG, et al. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:272–284. doi: 10.1007/s00192-005-1314-5. [DOI] [PubMed] [Google Scholar]
- 43.Ainsworth BE. Issues in the assessment of physical activity in women. Research Quart Exer Sport. 2000;71:37–42. doi: 10.1080/02701367.2000.11082784. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs DR, Ainsworth BE, Hartman TH, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exer. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Richardson MT, Ainsworth BE, Wu H, Jacobs DR, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 46.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155(3):282–289. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- 47.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Validity and reproducibility of a physical activity questionnaire in women. Med Sci Sports Exerc. 2002;34(6):987–992. doi: 10.1097/00005768-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30(2):266–274. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 50.Weir LF, Nygaard IE, Wilken J, Brandt D, Janz KF. Postoperative activity restrictions: any evidence? Obstet Gynecol. 2006;107:305–309. doi: 10.1097/01.AOG.0000197069.57873.d6. [DOI] [PubMed] [Google Scholar]
- 51.Carroll R, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models. 2nd ed. Chapman and Hall/CRC Press; 2006. [Google Scholar]
- 52.Carroll R, Hardin J, Schmiediche H. Measurement Error in Nonlinear Models Short Course. Boston: Stata North American Users Group; 2003. Mar 18–19th, ( http://www.stata.com/merror/call4.pdf) [Google Scholar]
- 53.Elkadry E, Kenton K, White P, Creech S, Brubaker L. Do mothers remember key events during labor? Am J Obstet Gynecol. 2003;189:195–200. doi: 10.1067/mob.2003.371. [DOI] [PubMed] [Google Scholar]
- 54.Tosteson TD, Buzas JS, Demidenko E, Karagas M. Power and sample size calculations for generalized regression models with covariate measurement error. Statistics in Medicine. 2003;22:1069–1082. doi: 10.1002/sim.1388. http://biostat.hitchcock.org/MeasurementError/default.asp. [DOI] [PubMed] [Google Scholar]
- 55.NQUERY sample size software, v.6, http://www.statsol.ie/html/nquery/nquery_home.html
- 56.Donders AR, van der Heijden GJ, Stijnen T, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Raghunathan TE, Lepkowski JM, VanHoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 58.Cook J, Stefanski LA. A simulation extrapolation method for parametric measurement error models. J Amer Statistical Assoc. 1995;89:1314–1328. [Google Scholar]
- 59.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 60.Horwitz BJ, Fisher RS. The irritable bowel syndrome. N Engl J Med. 2001;344:1846–1850. doi: 10.1056/NEJM200106143442407. [DOI] [PubMed] [Google Scholar]
- 59.Haskell WL. Physical activity by self-report: A brief history and future issues. J Phys Act Health. 2012;9(Suppl 1):S5–S10. doi: 10.1123/jpah.9.s1.s5. [DOI] [PubMed] [Google Scholar]
- 60.Bassett DR, Schneider PL, Huntington GE. Physical activity in an old order Amish community. Med Sci Sports Exerc. 2004;36:79–85. doi: 10.1249/01.MSS.0000106184.71258.32. [DOI] [PubMed] [Google Scholar]
- 61.Billat VL, Demarle A, Slawinski J, Paiva M, Koralsztein J-P. Physical and training characteristics of top-class marathon runners. Med Sci Sports Exerc. 2001;33:2089–2097. doi: 10.1097/00005768-200112000-00018. [DOI] [PubMed] [Google Scholar]