Abstract
Patients expect to receive quality medical care by relying on the concepts of evidence-based medicine. This quality care is expected to be provided at decreased costs for payors, some of whom have stopped reimbursement for cases involving “reasonably preventable” surgical complications. The purpose of this paper is to introduce root cause analysis as a tool for identifying the causes of surgical complications. We also discuss preventive measures such as improved communication, checklists, reporting systems, and the use of evidence-based medicine that have been implemented to decrease surgical complications. These preventive measures can be used alone or together to decrease complications and improve overall patient care.
Keywords: Surgery, complications, root cause, safety
The quality of medical care has recently come under fire. Not only is quality care expected using evidence-based practice, but it is constrained under cost-containment measures. Programs such as the National Surgical Quality Improvement Program have been formed to measure the quality of surgical care and empower hospitals to develop quality initiatives.1 Education in quality improvement and patient safety has also been integrated into surgical residents’ training when they are expected to identify causes of a systems error and initiate measures to change subsequent practice.2 Both hospitals and payors have an incentive to reduce surgical complications because higher complication rates result in smaller profit margins per case for hospitals, and the expenditure for cases with complications increase by 54–137% over those same uncomplicated cases.3 Data suggest that at least half of all surgical complications are avoidable.4; 5 Thus, payors such as Medicare have stopped providing reimbursement for complications that they deem to be “reasonably preventable” with medical intervention.6; 7 These “preventable” complications as stipulated by Medicare are foreign objects left in patients after surgery, catheter-associated urinary tract infections, central line-associated bloodstream infections, administration of incompatible blood products, air embolism, patient falls or trauma, mediastinitis after cardiac surgery, pressure ulcers, surgical site infection following bariatric surgery, surgical site infection following certain orthopedic procedures, manifestations of poor glycemic control, and deep vein thrombosis/pulmonary embolism following certain orthopedic procedures.8
Improved quality and decreased cost are incentives to reduce surgical complications. However, there is no consistent definition of a surgical complication9 as it is defined specifically for a research study. The difference between an error and a complication can be difficult to distinguish when patient comorbidities mask whether the outcome is due to error or underlying disease.10 The Institute of Medicine defines an adverse event as one that results in “unintended harm to the patient by an act of omission rather than by the underlying disease or condition of the patient.”11 Sokol and Wilson define a surgical complication as “any undesirable, unintended, and direct result of an operation affecting the patient, which would not have occurred had the operation gone as well as could reasonably be hoped.”12 However, this definition captures both negligent and non-negligent complications.
The purpose of this paper is to introduce root cause analysis as a tool for identifying the causes of adverse medical events and to discuss preventive measures that have been implemented to decrease surgical complications.
Root Cause Analysis
In 1966, Avedis Donabedian, the noted Professor of Public Health from the University of Michigan, presented the concept of evaluating the quality of medical care.13 He introduced three approaches to the assessment of medical care: outcomes, process, and structure (Figure 1). Outcomes measure what is accomplished for patients. Although many factors other than medical care can influence outcome, “outcomes, by and large, remain the ultimate validators of the effectiveness and quality of medical care.” Process evaluates whether “good” medical care has been properly practiced by following a structured sequence of activities that is considered as ideal practice. Structure assesses the settings in which medical care takes place, such as facilities, equipment, the qualifications of medical staff, and the administrative structure of an institution providing medical care (physical and organizational properties).13; 14
Figure 1.
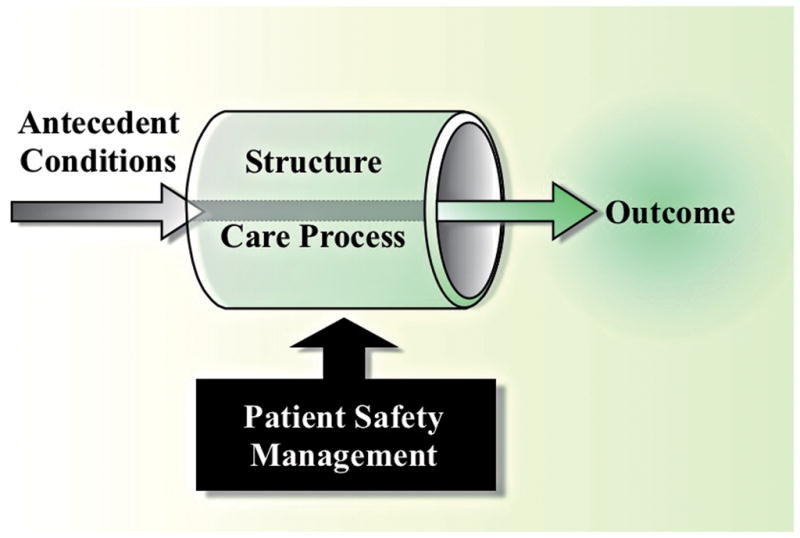
Donabedian model to evaluate the quality of medical care (Adapted from14)
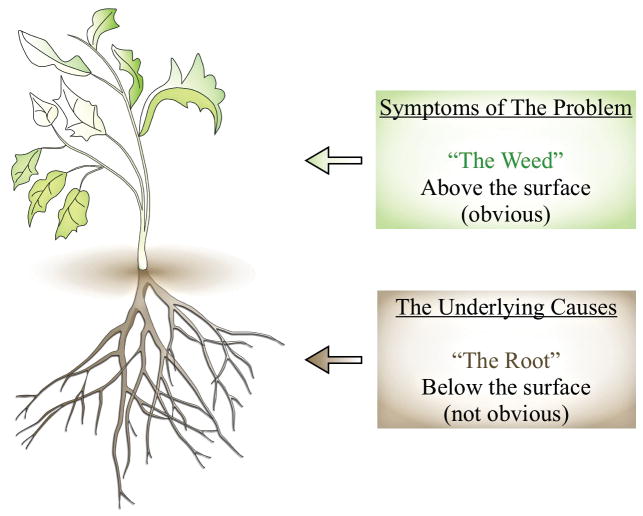
The concepts of process and structure, introduced by Donabedian, are used as part of root cause analysis. Root cause analysis is an approach to identifying the root cause or causes of a problem (instead of the symptoms) in the process or structure of an organization and determining prevention strategies (Figure 2). It is a technique developed by industries that take a system’s approach15 rather than blaming individuals when an error occurs. The goals are to find out 1) what happened; 2) why did it happen; 3) what to do to prevent it from happening again.16 Potential contributing factors to adverse events include: 1) human factors: scheduling/fatigue; 2) human factors: communication; 3) human factors: training; 4) environment/equipment; 5) rules, policies, and procedures; and 6) barriers.17 Root cause analysis involves four steps 1) collect data; 2) create a cause-and-effect diagram; 3) identify the root cause(s); 4) generate and implement recommendations.18
Figure 2.
Root cause analysis (Adapted from41)
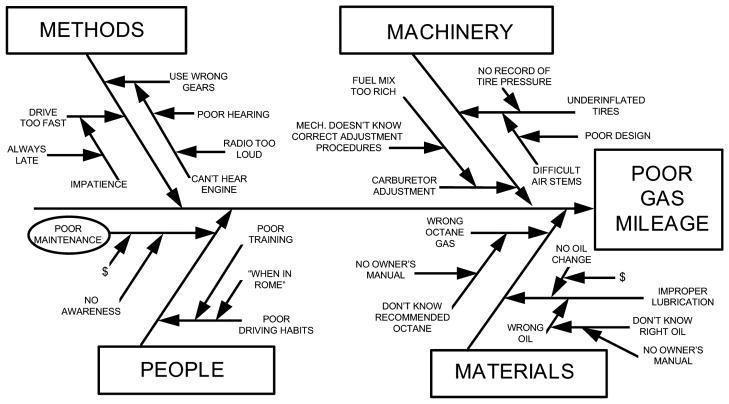
When conducting a root cause analysis, data collection takes the majority of time and continues until the cause-and-effect chart is complete.19 The second step is to create a cause-and-effect diagram (Figure 3), which is a tool that “helps identify, sort, and display possible causes of a specific problem or quality characteristic.” It was proposed by Kaoru Ishikawa, a Professor from the University of Tokyo who is known for his concept in quality improvement of Japanese industries after World War II, and is sometimes referred to as an “Ishikawa diagram” or a “fishbone diagram” because of its structure. This diagram can help to determine the root causes of a problem. The steps are to: 1) identify and define the effect; 2) identify the main causes contributing to the effect; 3) identify factors that may contribute to the main causes (becoming more detailed as you identify more factors)20 (Figure 4). The major contributors to an incident are causal factors and there is rarely just one causal factor. After all the causal factors have been identified, the root causes can then be identified. Root causes are those over which management has control and those for which effective recommendations can be generated.18
Figure 3.
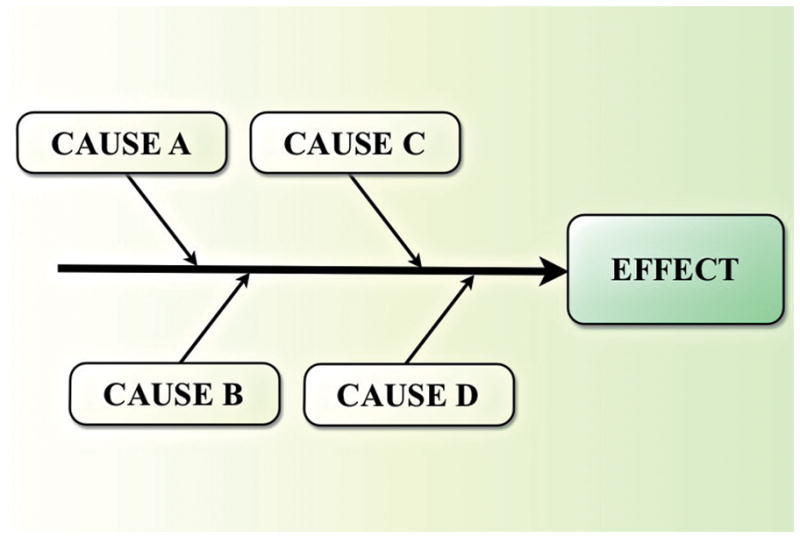
Basic cause-and-effect diagram (Adapted from20)
Figure 4.
Adding detail to the cause-and-effect diagram to determine root causes20
Root cause analysis has been used by several studies including the prevention of wrong site surgeries. In one study,21 a medical center experienced 8 wrong site/procedure/patient events over a period of less than two years. In the root cause analysis, causal maps were first developed and reviewed. In the category of “rules, policies, and procedures,” 22 failure modes were identified. One example is that in 6 out of the 8 events, a consent form was not available, did not denote laterality or was not obtained by the surgeon involved. Human factors, such as scheduling/fatigue, communication, and training accounted for a combined total of 28 failure modes. An example is that in 6 out of the 8 events, there was a lack of standardization (workflow, responsibilities, information flow). Preventive steps included revising the safe surgical checklist policy to require the attending surgeon or physician to call a “time out” prior to incision, and a revision of the consent forms to include a legend (right, left, and bilateral) next to where the practitioner writes the name of the procedure to be performed.
Root cause analysis has been criticized for its varying quality and the lack of literature regarding its effectiveness in reducing risk, especially when the same problem reoccurs after a root cause analysis has recently been completed.19 However, many root cause analyses are performed incorrectly or incompletely,19 and the analysts do not understand the true root causes of the event and therefore do not know how to prevent it from reoccurring.18 Repeat events can also be caused by the incomplete adoption of recommendations. In one study of healthcare professionals who conducted root cause analysis in their workplace, the majority (51%) felt that their recommendations were only partly implemented.22 A study at the Veterans Health Administration found that clinical changes at the bedside as well as high levels of support from management and front-line staff had higher rates of implementation than actions focusing on policy or staff education.23 For example, an action that was found to have one of the biggest impacts in reducing patient falls was when the admission nurse sent an e-mail to the care team to identify at-risk patients and initiated an interim “fall care plan.”23 In general, passive actions, such as disseminating educational materials, are less likely to work than active, collaborative, and multifaceted interventions.24 Furthermore, if events are common across hospitals, action needs to be taken at the health system level rather than the individual hospital level.19 For example, administering the wrong dose of medication may require changes to packaging by the pharmaceutical company rather than attempting to train all of the employees in each hospital who may administer that medication.
The following sections provide examples of preventive measures that have been used by various studies to reduce surgical complications, although formal root cause analyses were generally not performed prior to the institution of the actions used.
Prevention
Communication
Most people are likely familiar with the interventions that other industries have taken to improve safety, such as in the airline industry, where training in effective teamwork has been associated with improvements in safety.25 Surveys have also been used to collect data on pilot attitudes about safety and interpersonal interactions and to aid in the development of interventions.26 The field of Surgery shares many of the basic elements of the airline industry where people are working together in a high-technology and high-risk environment.25 Christian et al. found that there was a “generalized vulnerability of the operating room system to information loss. Information loss led to delays, overuse of staff and resources, uncertainty in clinical decision making and planning, and oversights in patient preparation.” “Hand offs,” where responsibility and caregiving is completely transferred from one provider to another, were linked to many instances of information loss in surgical cases.27 Mazzocco et al. found that patients whose surgical teams exhibited less teamwork were at a higher risk for death or complications.25 Because nurses are generally involved in more continuous monitoring than physicians, it is desirable for them to be included in verbal briefings so that they can bridge information gaps.28 A preoperative briefing involving surgeons, nurses, and anesthesiologists including a 1-page checklist of patient information and procedural issues was found to significantly decrease the number of communication failures (p<0.001). The majority of respondents (81%) felt that the briefings were worthwhile overall to identify and resolve problems as well as guard against mistakes.29
One of the four core strategies of the National Patient Safety Foundation at the American Medical Association is to foster communication to enhance patient safety. They feel that “early identification of risk is the key to preventing patient injuries, and this depends on maintaining a culture of trust, honesty, integrity, and open communication among patients and providers in the health care system.”30
Checklists
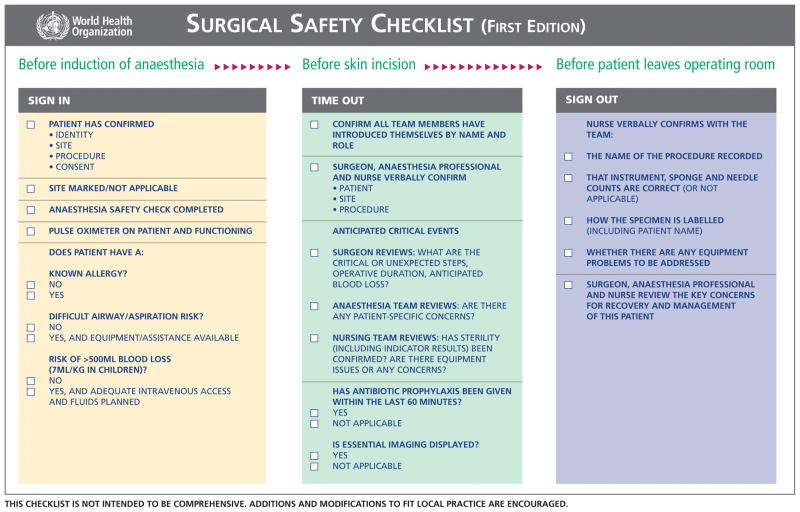
A surgical checklist is an intervention that has been suggested to reduce the rate of surgical complications. Figure 5 is an example of a Surgical Safety Checklist created by the World Health Organization (WHO).31 After implementing this 19-item checklist and studying its results in 3955 patients at 8 sites, the rate of surgical site infection significantly dropped from 6.2% at baseline to 3.4% after the introduction of the checklist (p<0.001).31 The WHO checklist is endorsed by the noted author and surgeon, Dr. Atul Gawande. He states that under conditions of complexity, “not only are checklists a help, they are required for success”32 especially when they are used in conjunction with teamwork. Another study evaluated the effect of the Surgical Pathway Safety System checklist, which is a multidisciplinary checklist that follows the surgical pathway from admission to discharge. The checklist was used for a period of 9 months at 6 hospitals in the Netherlands. Data were compared to 5 control hospitals. All complications that arose prior to discharge were recorded. These authors found that the total number of complications decreased from 27.3 per 100 patients to 16.7 per 100 patients (p<0.001) and inhospital mortality decreased from 1.5% to 0.8% (p=0.003).33 These two studies show great improvement in complication rates with the use of checklists. The reasons for the effectiveness of the checklists include helping surgical teams to avoid simple oversights and/or reducing distractions in the operating room. Some have questioned the “staying power” of checklists and whether their benefits could wane as the checklist becomes one more component of a patient’s care. Furthermore, without a control group, it is difficult to determine whether outcomes improved simply because surgeons and staff know that they are being evaluated.34
Figure 5.
Surgical Safety Checklist created by the World Health Organization31
Reporting System
In the airline industry, the Aviation Safety Reporting System is a voluntary, confidential incident reporting system that is used to identify and mitigate deficiencies.35 Medical studies have evaluated the use of a reporting system, either anonymous or non-anonymous. Van Wagtendonk et al.36 conducted a study in ten surgical units of hospitals in the Netherlands. Healthcare providers in the unit were asked to report all unintended events (defined as all events that were unintended and could have or did harm a patient) directly after the event had occurred or was discovered. The data were then analyzed to determine the root causes of the events. The vast majority of the unintended events (72.3%) were caused by human factors. With voluntary reporting that is not anonymous, it is possible that events are underreported because of fear of embarrassment or condemnation. The discussion of complications with peers in a weekly Morbidity and Mortality Conference may assist in detecting underreporting when the complication rate of one surgeon or division is atypically lower compared to others. Input from peers in such a conference is available to decrease high complication rates and there is the opportunity to disseminate prevention measures based on the experience derived from when complication rates are low.37
Evidence-Based Medicine
Evidence-based medicine is being pushed to standardize care and cut costs. Accordingly, some researchers have studied whether evidence-based measures can reduce surgical complications. In a study by Serra-Aracil et al.,38 a protocol of evidence-based preventive measures was applied to control surgical site infection (SSI) after colon and rectal cancer resections. This was a prospective, observational, multi-center study in 19 Spanish hospitals with a follow-up of 30 days. Preoperative preventive measures included shower, and perioperative measures included prophylactic antibiotics administered 30 minutes prior to the surgical incision. These authors found that the incidence of SSI with the preventive measures was higher than expected when compared to the incidence of SSI reported in the literature. Because patients were administered all of the preventive measures at once, as a “bundle,” there was no way to assess the efficacy of each preventive measure. Similarly, Anthony et al.39 conducted a randomized controlled trial in which subjects either received a bundle of 5 evidence-based practices to reduce SSI after transabdominal colorectal surgery or subjects were treated according to the standard practice. This study found that patients who were allocated to the treatment arm had a 45% rate of SSI compared to a 24% rate in the control arm (p=0.003). Again, the authors were unable to determine if one or all of the preventive measures led to the increased rate of SSI. They hypothesized that in trying to adhere to multiple interventions, surgeon attention may have been diverted away from other aspects that prevent SSI. Of course, evidence-based practices can still be useful but they may need to be introduced one at a time or in combination with one of the aforementioned interventions, such as checklists, to ensure that they are being properly instituted.
A study to decrease catheter-related bloodstream infections incorporated several of the previously mentioned interventions.40 A daily goals sheet was used to improve clinician-to-clinician communication, a checklist was used to ensure infection control practices, clinician meetings provided feedback on the number of bloodstream infections, and evidence-based procedures were implemented. A total of 103 Michigan intensive care units reported data. The outcome was the quarterly rate of catheter-related bloodstream infection which decreased from an overall median rate of 2.7 infections per 1000 catheter-days at baseline to 0 after 3 months of implementing the study interventions (p≤0.002). The outcome was sustained at 0 infections after 18 months of follow-up. Although the authors were unable to evaluate the importance of each of the interventions, the goal was to maximally improve patient safety which was accomplished with the implementation of several interventions as a study program.
Conclusion
Root cause analysis is a valuable tool for identifying the root causes of adverse medical events and providing recommendations for their prevention. Preventive measures such as improved communication between providers, safety checklists, reporting systems, and the use of evidence-based medicine when used alone or in conjunction can greatly improve the quality of patient care. The prevention of complications is not easy, requiring teamwork and a systems-wide approach to achieve the highest quality of care that patients expect and deserve.
Acknowledgments
Supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging (R01 AR062066) and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR047328) and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to Dr. Kevin C. Chung).
We acknowledge the fine drawings produced by Dr. Shimpei Ono for this manuscript.
Footnotes
Disclosure and Conflict of interest - none
References
- 1.American College of Surgeons. [Accessed October 27, 2011];National Surgical Quality Improvement Program. Available at: http://www.acsnsqip.org.
- 2.Dunnington GL, Williams RG. Addressing the new competencies for residents’ surgical training. Acad Med. 2003;78:14–21. doi: 10.1097/00001888-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Weeks WB, Karia RJ, Das S, Campbell DA., Jr Who pays for poor surgical quality? Building a business case for quality improvement. J Am Coll Surg. 2006;202:933–937. doi: 10.1016/j.jamcollsurg.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Gawande AA, Thomas EJ, Zinner MJ, Brennan TA. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery. 1999;126:66–75. doi: 10.1067/msy.1999.98664. [DOI] [PubMed] [Google Scholar]
- 5.Kable AK, Gibberd RW, Spigelman AD. Adverse events in surgical patients in Australia. Int J Qual Health Care. 2002;14:269–276. doi: 10.1093/intqhc/14.4.269. [DOI] [PubMed] [Google Scholar]
- 6.Pronovost PJ, Goeschel CA, Wachter RM. The wisdom and justice of not paying for “preventable complications”. JAMA. 2008;299:2197–2199. doi: 10.1001/jama.299.18.2197. [DOI] [PubMed] [Google Scholar]
- 7.Wald HL, Kramer AM. Nonpayment for harms resulting from medical care: catheter-associated urinary tract infections. JAMA. 2007;298:2782–2784. doi: 10.1001/jama.298.23.2782. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. [Accessed December 1, 2011];Hospital-acquired conditions. Available at: http://www.cms.gov/HospitalAcqCond/06_Hospital-Acquired_Conditions.asp#TopOfPage.
- 9.Rampersaud YR, Moro ER, Neary MA, White K, Lewis SJ, Massicotte EM, Fehlings MG. Intraoperative adverse events and related postoperative complications in spine surgery: implications for enhancing patient safety founded on evidence-based protocols. Spine (Phila Pa 1976) 2006;31:1503–1510. doi: 10.1097/01.brs.0000220652.39970.c2. [DOI] [PubMed] [Google Scholar]
- 10.Brennan TA, Gawande A, Thomas E, Studdert D. Accidental deaths, saved lives, and improved quality. N Engl J Med. 2005;353:1405–1409. doi: 10.1056/NEJMsb051157. [DOI] [PubMed] [Google Scholar]
- 11.Aspden P, Wolcott J, Bootman J, Cronenwett L, editors. Institute of Medicine of the National Academies. Preventing Medication Errors: Quality Chasm Series. National Academies Press; Washington, DC: 2007. [Google Scholar]
- 12.Sokol DK, Wilson J. What is a surgical complication? World J Surg. 2008;32:942–944. doi: 10.1007/s00268-008-9471-6. [DOI] [PubMed] [Google Scholar]
- 13.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44:166–206. [PubMed] [Google Scholar]
- 14.Donabedian A. The Definition of Quality and Approaches to its Assessment. I. Health Administration Press; Ann Arbor, MI: 1980. Explorations in Quality Assessment and Monitoring. [Google Scholar]
- 15.Longo DR, Hewett JE, Ge B, Schubert S. The long road to patient safety: a status report on patient safety systems. JAMA. 2005;294:2858–2865. doi: 10.1001/jama.294.22.2858. [DOI] [PubMed] [Google Scholar]
- 16.United States Department of Affairs. [Accessed October 27, 2011];Root cause analysis (RCA) Available at: http://www.patientsafety.gov/rca.html.
- 17.US Department of Veterans Affairs National Center for Patient Safety (NCPS) Triggering and Triage Cards (Version 10/01) US Department of Veterans Affairs; Washington, DC: 2001. [Google Scholar]
- 18.Rooney J, Vanden Heuvel L. [Accessed December 1, 2011];Root cause analysis for beginners. Available at: http://webspace.utexas.edu/mae548/www/research/digital%20forensics/qp0705rooney.pdf.
- 19.Wu AW, Lipshutz AK, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299:685–687. doi: 10.1001/jama.299.6.685. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed October 27, 2011];Basic tools for process improvement. Available at: http://saferpak.com/cause_effect_articles/howto_cause_effect.pdf.
- 21.Mallett R, Conroy M, Saslaw LZ, Moffatt-Bruce S. Preventing Wrong Site, Procedure, and Patient Events Using a Common Cause Analysis. Am J Med Qual. 2011 Aug 10; doi: 10.1177/1062860611412066. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Braithwaite J, Westbrook MT, Mallock NA, Travaglia JF, Iedema RA. Experiences of health professionals who conducted root cause analyses after undergoing a safety improvement programme. Qual Saf Health Care. 2006;15:393–399. doi: 10.1136/qshc.2005.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills P, Neily J, Luan D, Stalhandske E, Weeks W. Using aggregate root cause analysis to reduce falls and related injuries. Jt Comm J Qual Patient Saf. 2005;31:21–31. doi: 10.1016/s1553-7250(05)31004-x. [DOI] [PubMed] [Google Scholar]
- 24.Grimshaw JM, Eccles MP, Walker AE, Thomas RE. Changing physicians’ behavior: what works and thoughts on getting more things to work. J Contin Educ Health Prof. 2002;22:237–243. doi: 10.1002/chp.1340220408. [DOI] [PubMed] [Google Scholar]
- 25.Mazzocco K, Petitti DB, Fong KT, Bonacum D, Brookey J, Graham S, Lasky RE, Sexton JB, Thomas EJ. Surgical team behaviors and patient outcomes. Am J Surg. 200;197:678–685. doi: 10.1016/j.amjsurg.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Sexton JB, Thomas EJ, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320:745–749. doi: 10.1136/bmj.320.7237.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christian CK, Gustafson ML, Roth EM, Sheridan TB, Gandhi TK, Dwyer K, Zinner MJ, Dierks MM. A prospective study of patient safety in the operating room. Surgery. 2006;139:159–73. doi: 10.1016/j.surg.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Donchin Y, Gopher D, Olin M, Badihi Y, Biesky M, Sprung CL, Pizov R, Cotev S. A look into the nature and causes of human errors in the intensive care unit. 1995. Qual Saf Health Care. 2003;12:143–147. doi: 10.1136/qhc.12.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lingard L, Regehr G, Orser B, Reznick R, Baker GR, Doran D, Espin S, Bohnen J, Whyte S. Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anesthesiologists to reduce failures in communication. Arch Surg. 2008;143:12–17. doi: 10.1001/archsurg.2007.21. [DOI] [PubMed] [Google Scholar]
- 30.Leape LL, Woods DD, Hatlie MJ, Kizer KW, Schroeder SA, Lundberg GD. Promoting patient safety by preventing medical error. JAMA. 1998;280:1444–1447. doi: 10.1001/jama.280.16.1444. [DOI] [PubMed] [Google Scholar]
- 31.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, Herbosa T, Joseph S, Kibatala PL, Lapitan MC, Merry AF, Moorthy K, Reznick RK, Taylor B, Gawande AA. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 32.Peregrin T. Checklists for success inside the OR and beyond: an interview with Atul Gawande, MD, FACS. Bull Am Coll Surg. 2010;95:24–27. [PubMed] [Google Scholar]
- 33.de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, van Helden SH, Schlack WS, van Putten MA, Gouma DJ, Dijkgraaf MG, Smorenburg SM, Boermeester MA. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363:1928–1937. doi: 10.1056/NEJMsa0911535. [DOI] [PubMed] [Google Scholar]
- 34.Birkmeyer NJ, Birkmeyer JD. Strategies for improving surgical quality--should payers reward excellence or effort? N Engl J Med. 2006;354:864–870. doi: 10.1056/NEJMsb053364. [DOI] [PubMed] [Google Scholar]
- 35.Kohn LCJ, MD, editors. Insititute of Medicine. To Err is Human: Building a Safer Health System. National Academy Press; Washington D.C: 2000. [PubMed] [Google Scholar]
- 36.van Wagtendonk I, Smits M, Merten H, Heetveld MJ, Wagner C. Nature, causes and consequences of unintended events in surgical units. Br J Surg. 2010;97:1730–1740. doi: 10.1002/bjs.7201. [DOI] [PubMed] [Google Scholar]
- 37.Healey MA, Shackford SR, Osler TM, Rogers FB, Burns E. Complications in surgical patients. Arch Surg. 2002;l137:611–617. doi: 10.1001/archsurg.137.5.611. [DOI] [PubMed] [Google Scholar]
- 38.Serra-Aracil X, Garcia-Domingo MI, Pares D, Espin-Basany E, Biondo S, Guirao X, Orrego C, Sitges-Serra A. Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Arch Surg. 2011;146:606–612. doi: 10.1001/archsurg.2011.90. [DOI] [PubMed] [Google Scholar]
- 39.Anthony T, Murray BW, Sum-Ping JT, Lenkovsky F, Vornik VD, Parker BJ, McFarlin JE, Hartless K, Huerta S. Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Arch Surg. 2011;146:263–269. doi: 10.1001/archsurg.2010.249. [DOI] [PubMed] [Google Scholar]
- 40.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 41.ThinkReliability. [Accessed December 8, 2011];Root cause analysis. Available at: http://www.thinkreliability.com/Root-Cause-Analysis-CM-Basics.aspx.