Abstract
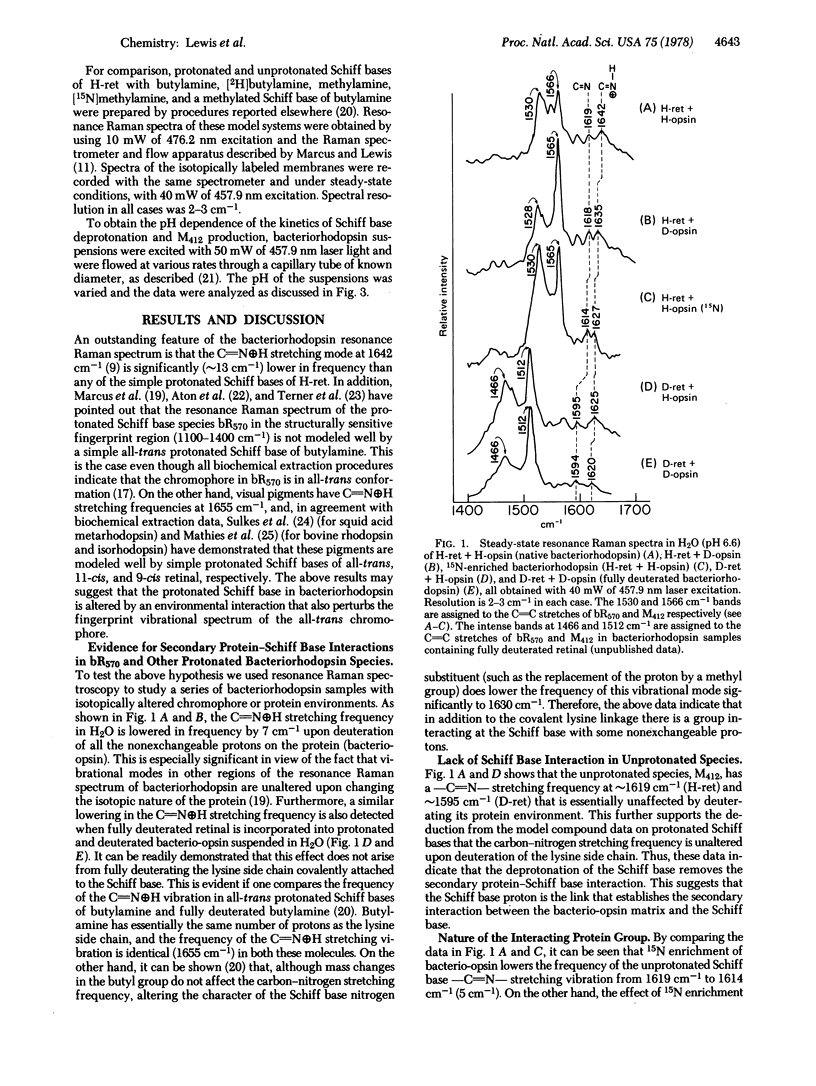
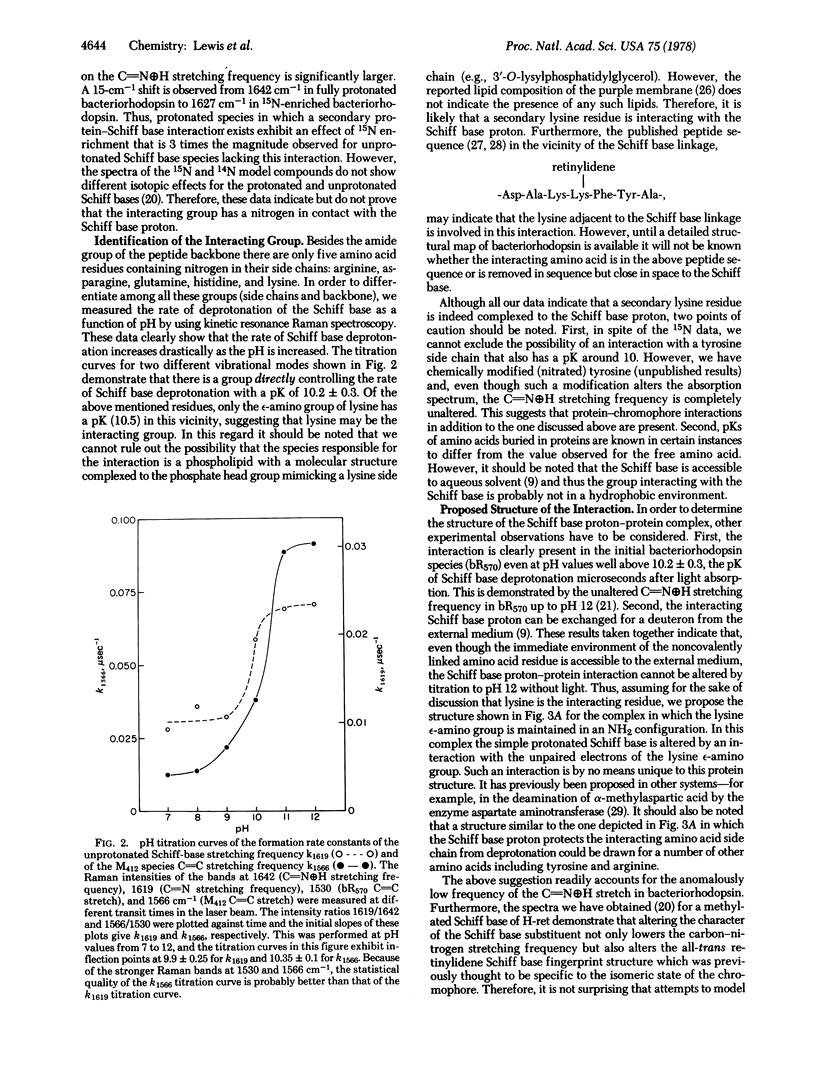
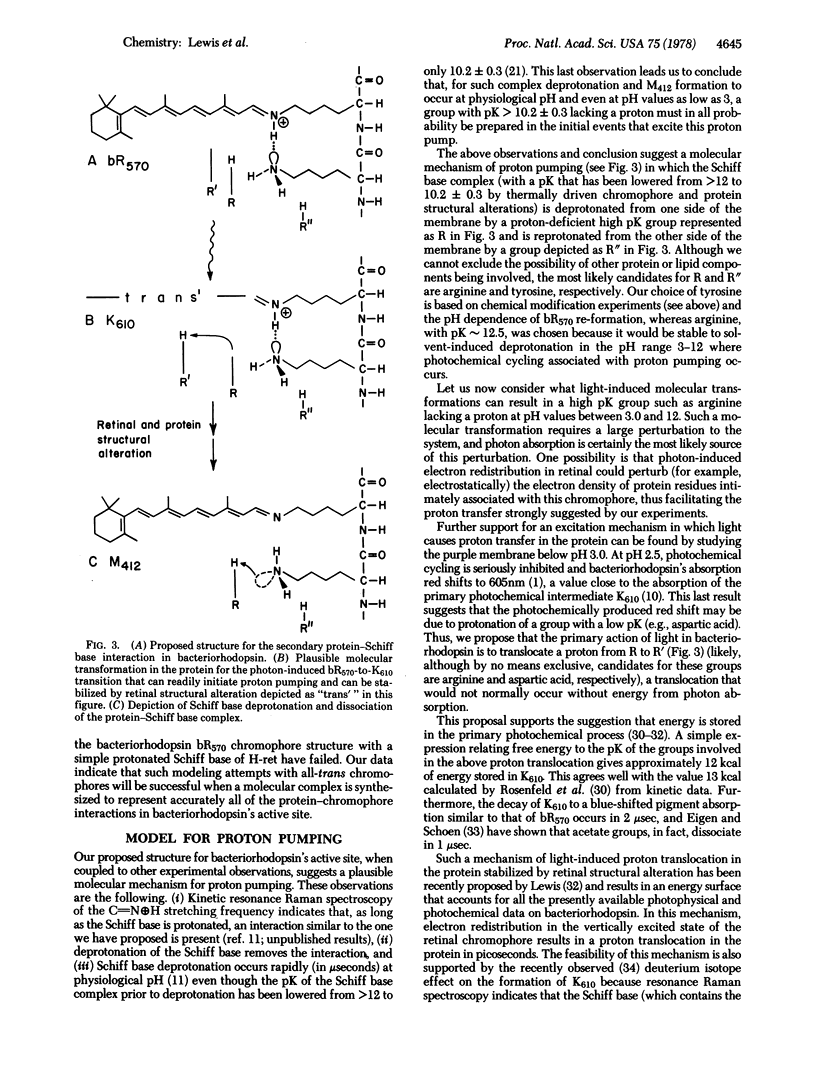
Resonance Raman spectroscopy of the retinylidene chromophore in various isotopically labeled membrane environments together with spectra of isotopically labeled model compounds demonstrates that a secondary protein interaction is present at the protonated Schiff base linkage in bacteriorhodopsin. The data indicate that although the interaction is present in all protonated bacteriorhodopsin species it is absent in unprotonated intermediates. Furthermore, kinetic resonance Raman spectroscopy has been used to monitor the dynamics of Schiff base deprotonation as a function of pH. All our results are consistent with lysine as the interacting group. A structure for the interaction is proposed in which the interacting protein group in an unprotonated configuration is complexed through the Schiff base proton to the Schiff base nitrogen. These data suggest a molecular mechanism for proton pumping and ion gate molecular regulation. In this mechanism, light causes electron redistribution in the retinylidene chromophore, which results in the deprotonation of an amino acid side chain with pK >10.2 ± 0.3 (e.g., arginine). This induces subsequent retinal and protein conformational transitions which eventually lower the pK of the Schiff base complex from >12 before light absorption to 10.2 ± 0.3 in microseconds after photon absorption. Finally, in this low pK state the complex can reprotonate the proton-deficient high pK group generated by light, and the complex is then reprotonated from the opposite side of the membrane.
Keywords: resonance Raman spectroscopy, isotopically labeled purple membrane, pH titration of M412 kinetics, active transport, proton switch-ion gate molecular regulation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg B., Lewis A. The pK of Schiff base deprotonation in bacteriorhodopsin. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1154–1159. doi: 10.1016/0006-291x(78)90307-8. [DOI] [PubMed] [Google Scholar]
- Fasella P., Giartosio A., Hammes G. G. The interaction of aspartate aminotransferase with alpha-methylaspartic acid. Biochemistry. 1966 Jan;5(1):197–202. doi: 10.1021/bi00865a026. [DOI] [PubMed] [Google Scholar]
- Ippen E. P., Shank C. V., Lewis A., Marcus M. A. Subpicosecond spectroscopy of bacteriorhodopsin. Science. 1978 Jun 16;200(4347):1279–1281. doi: 10.1126/science.663607. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Racker E. Light-dependent proton and rubidium translocation in membrane vesicles from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1054–1061. doi: 10.1016/0006-291x(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Rentzepis P. M., Stoeckenius W., Lewis A. Primary photochemical processes in bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1109–1115. doi: 10.1016/0006-291x(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Stoeckenius W. Comparison of purple membrane from Halobacterium cutirubrum and Halobacterium halabium. Biochim Biophys Acta. 1976 Apr 5;426(4):703–710. doi: 10.1016/0005-2736(76)90135-8. [DOI] [PubMed] [Google Scholar]
- Lewis A. Primary photophysical and photochemical processes in visual excitation. Biophys Struct Mech. 1977 Jun 29;3(2):97–100. doi: 10.1007/BF00535800. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A., Crespi H. Physiological and structural investigations of bacteriorhodopsin analogs. Biochem Biophys Res Commun. 1977 Sep 23;78(2):669–675. doi: 10.1016/0006-291x(77)90231-5. [DOI] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Kinetic resonance Raman spectroscopy: dynamics of deprotonation of the Schiff base of bacteriorhodopsin. Science. 1977 Mar 25;195(4284):1328–1330. doi: 10.1126/science.841330. [DOI] [PubMed] [Google Scholar]
- Mathies R., Freedman T. B., Stryer L. Resonance Raman studies of the conformation of retinal in rhodopsin and isorhodopsin. J Mol Biol. 1977 Jan 15;109(2):367–372. doi: 10.1016/s0022-2836(77)80040-5. [DOI] [PubMed] [Google Scholar]
- Mathies R., Stryer L. Retinal has a highly dipolar vertically excited singlet state: implications for vision. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L., Gruber H. Light-dependent reaction of bacteriorhodopsin with hydroxylamine in cell suspensions of Halobacterium halobium: demonstration of an apo-membrane. FEBS Lett. 1974 Aug 30;44(3):257–261. doi: 10.1016/0014-5793(74)81152-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L. Reconstitution of bacteriorhodopsin. FEBS Lett. 1974 Aug 30;44(3):262–265. doi: 10.1016/0014-5793(74)81153-1. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Salem L., Bruckmann P. Conversion of a photon to an electrical signal by sudden polarisation in the N-retinylidene visual chromophore. Nature. 1975 Dec 11;258(5535):526–528. doi: 10.1038/258526a0. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Gaber B. P. Laser Raman scattering as a probe of protein structure. Annu Rev Biochem. 1977;46:553–572. doi: 10.1146/annurev.bi.46.070177.003005. [DOI] [PubMed] [Google Scholar]
- Sulkes M., Lewis A., Lemley A. T., Cookingham R. Modeling the resonance Raman spectrum of a metarhodopsin: implications for the color of visual pigments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4266–4270. doi: 10.1073/pnas.73.12.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner J., Campion A., El-Sayed M. A. Time-resolved resonance Raman spectroscopy of bacteriorhodopsin on the millisecond timescale. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5212–5216. doi: 10.1073/pnas.74.12.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]


