Abstract
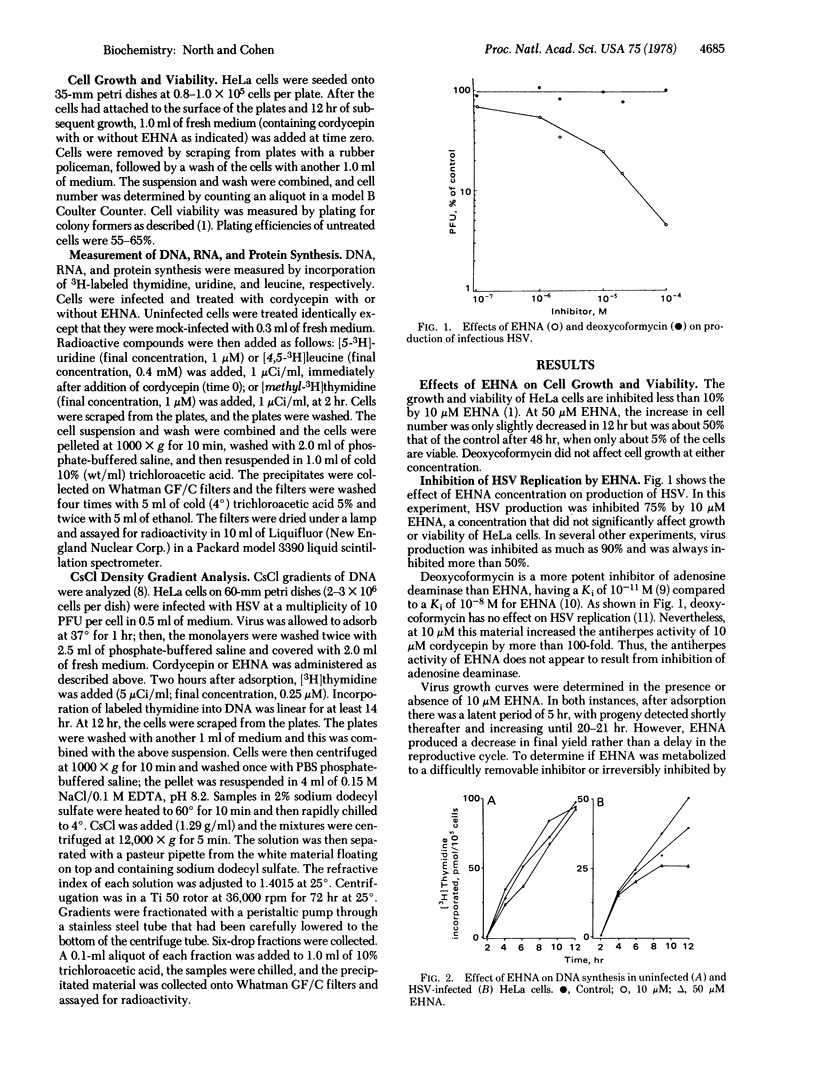
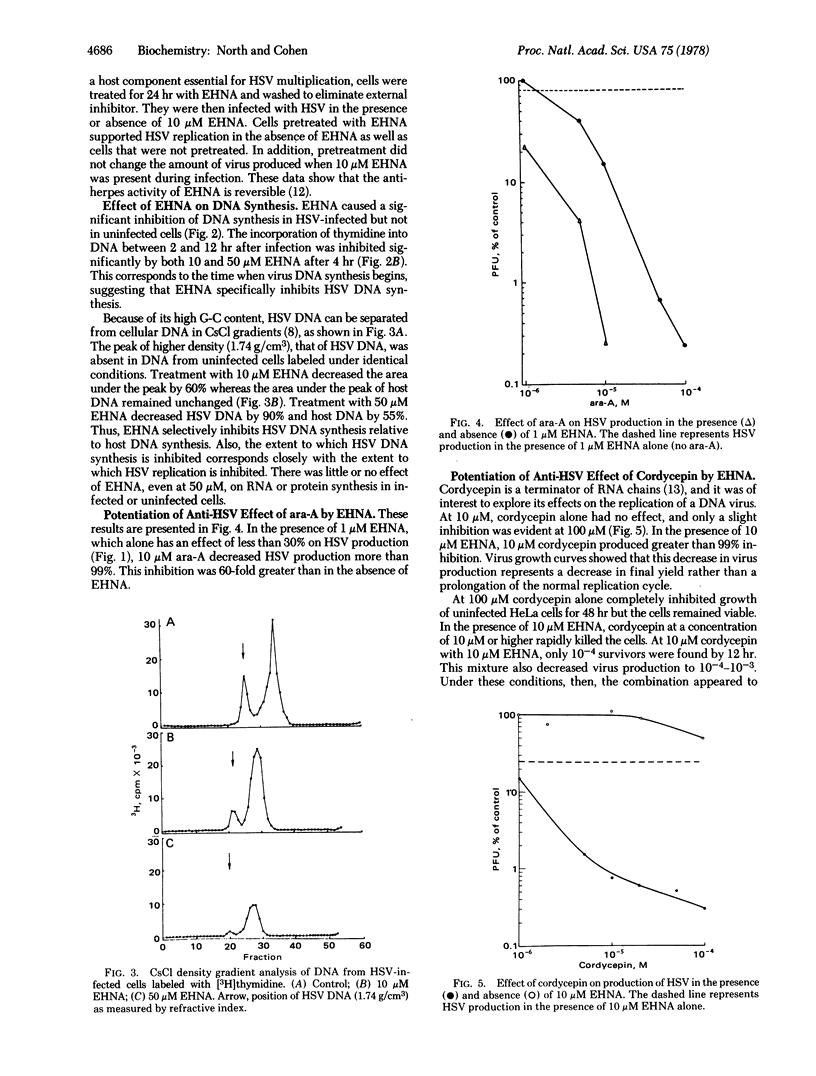
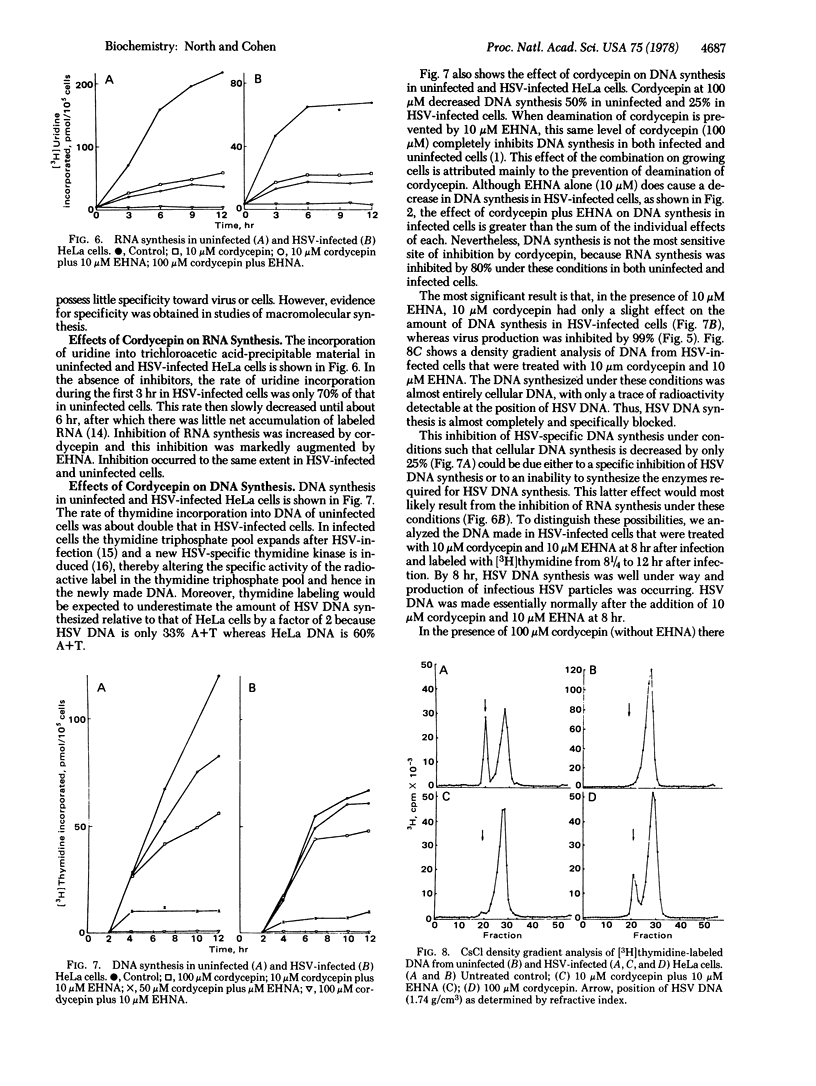
Erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA; erythro-9-[3-(hydroxynonyl)]adenine), a reversible inhibitor of adenosine deaminase, significantly inhibits replication of herpes simplex virus (HSV), whereas the more active inhibitor of the deaminase, 2'-deoxycoformycin, does not. At 10 micron EHNA, which does not affect viability, growth, or DNA synthesis of uninfected HeLa cells, production of HSV and HSV-specific DNA is inhibited 75-90% and 60%, respectively. HSV multiplies normally in cells pretreated with EHNA and washed to remove this inhibitor. EHNA (10 micron) also markedly potentiates the toxicity of adenine arabinonucleoside and of cordycepin (3'-deoxyadenosine) against HeLa cells and against the production of HSV in those cells. Cordycepin alone (10 micron) does not inhibit HSV replication whereas in combination with 10 micron EHNA there is a greater than 99% inhibition of virus production. Under these conditions, RNA synthesis is inhibited by more than 80% whereas protein and DNA synthesis are inhibited to a lesser extent; in this system, virtually all of the DNA synthesis in infected cells is that of host DNA. Thus, EHNA appears to affect the synthesis of HSV DNA specifically in two different ways, depending on whether it is used alone or in the presence of cordycepin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Spector T., Parks R. E., Jr Tight-binding inhibitors--IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977 Mar 1;26(5):359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Prusoff W. H. Deoxyribonucleotide metabolism in Herpes simplex virus infected HeLa cells. Biochim Biophys Acta. 1975 May 16;390(3):253–263. doi: 10.1016/0005-2787(75)90346-9. [DOI] [PubMed] [Google Scholar]
- DE Clercq E., Descamps J., DE Somer P., Holyacute A. (S)-9-(2,3-Dihydroxypropyl)adenine: An Aliphatic Nucleoside Analog with Broad-Spectrum Antiviral Activity. Science. 1978 May 5;200(4341):563–565. doi: 10.1126/science.200.4341.563. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Timbury M. C. Temperature-sensitive mutants of herpes simplex virus type 2: description of three new complementation groups and studies on the inhibition of host cell DNA synthesis. J Gen Virol. 1976 Feb;30(2):207–221. doi: 10.1099/0022-1317-30-2-207. [DOI] [PubMed] [Google Scholar]
- Johns D. G., Adamson R. H. Enhancement of the biological activity of cordycepin (3'-deoxyadenosine) by the adenosine deaminase inhibitor 2'-deoxycoformycin. Biochem Pharmacol. 1976 Jun 15;25(12):1441–1444. doi: 10.1016/0006-2952(76)90121-0. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- Lapi L., Cohen S. S. Toxicities of adenosine and 2'-deoxyadrenosine in L cells treated with inhibitors of adenosine deaminase. Biochem Pharmacol. 1977 Jan 1;26(1):71–76. doi: 10.1016/0006-2952(77)90135-6. [DOI] [PubMed] [Google Scholar]
- LePage G. A., Worth L. S., Kimball A. P. Enhancement of the antitumor activity of arabinofuranosyladenine of 2'-deoxycoformycin. Cancer Res. 1976 Apr;36(4):1481–1485. [PubMed] [Google Scholar]
- Plunkett W., Cohen S. S. Two approaches that increase the activity of analogs of adenine nucleosides in animal cells. Cancer Res. 1975 Jun;35(6):1547–1554. [PubMed] [Google Scholar]
- Plunkett W., Lapi L., Ortiz P. J., Cohen S. S. Penetration of mouse fibroblasts by the 5'-phosphate of 9-beta-D-arabinofuranosyladenine and incorporation of the nucleotide into DNA. Proc Natl Acad Sci U S A. 1974 Jan;71(1):73–77. doi: 10.1073/pnas.71.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Borman G. S., Rousta M. K. Macromolecular synthesis in cells infected with herpes simplex virus. Nature. 1965 Jun 26;206(991):1374–1375. doi: 10.1038/2061374a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Gvogel D., Vince R. Enzyme inhibitors. 8. Studies on the mode of binding of some 6-substituted 9-(hydroxyalkyl)purines to adenosine deaminase. J Med Chem. 1965 Jul;8(4):502–506. doi: 10.1021/jm00328a020. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Sloan B. J., Kielty J. K., Miller F. A. Effect of a novel adenosine deaminase inhibitor (co-vidarabine, co-V) upon the antiviral activity in vitro and in vivo of vidarabine (Vira-Atm) for DNA virus replication. Ann N Y Acad Sci. 1977 Mar 4;284:60–80. doi: 10.1111/j.1749-6632.1977.tb21937.x. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]


