Abstract
Background and Purpose. White matter changes (WMCs), or leukoaraiosis (LA), are associated with increased age, hypertension, diabetes mellitus, and history of stroke. Although several lines of evidence suggest a role of atherosclerosis in atherothrombotic vascular events, their involvement in LA remains to be determined. Our study examines this association in ischemic stroke patients. Methods. One hundred and seventy consecutive ischemic stroke or transient ischemic attack (TIA) patients were included. All patients underwent brain computed tomography (CT) with assessment of the extension and severity of WMCs, carotid arteries duplex scan with measurements of intima-media thickness (IMT) and plaques. Results. Seventy-two patients (42.4%) were found to have white matter lesions, of whom 28.8% had advanced LA. Mean IMT was significantly higher in patients with LA and with advanced LA (P = 0.002, P = 0.003, resp.). In addition, LA and LA severity were associated with existence of carotid plaque (P = 0.007, P = 0.004, resp.). In multivariate logistic regression analysis, including all vascular risk factors, LA was found to be associated with age and IMT. Conclusion. This study reinforces the tight association between LA and carotid atherosclerosis in ischemic stroke patients. We conclude that a chronic atherosclerotic disease underlies the pathophysiology of leukoaraiosis and its progression.
1. Objectives
Cerebral white matter changes, also known as leukoaraiosis [1] (LA), are frequently observed on computed tomography (CT) and magnetic resonance imaging (MRI) brain scans of elderly individuals [2–4]. These changes are seen on CT as bilateral patchy or diffuse areas of hypodensity with ill-defined margins or hyperintensities on T2-weighted MRI scans involving the periventricular and centrum semiovale white matter [5]. Although the pathogenesis of LA is not completely elucidated, previous studies have shown that white matter lesions are more common with increasing age, hypertension, diabetes mellitus, and where there is a history of stroke [6]. Moreover, in a large cohort of consecutive patients hospitalized for stroke or transient ischemic attack (TIA), LA predicted poor functional recovery during the first year following stroke onset [7].
Possible contributory factors include occlusive extra-cranial arterial disease, small vessel arteriosclerosis, transient decreases in local perfusion because of autoregulatory dysfunction, and microembolic disease [6]. While carotid atherosclerosis has been demonstrated to have strong relationship with stroke and small-vessel disease, its association with LA is controversial [8, 9]. Inflammatory processes are associated with the pathogenesis of atherosclerosis and cerebral small-vessel disease [10, 11] and also play a role in the response to ischemic events that result from atherosclerosis [11].
In the current study, we examined the association of carotid atherosclerosis, defined by intima-media thickness (IMT) and prevalence of plaques, with LA in ischemic stroke patients.
2. Patients and Methods
2.1. Patients
One hundred and seventy patients admitted consecutively to the Department of Emergency at the Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel, with a documented event of acute ischemic stroke or transient ischemic attack (TIA) between January 2003 and April 2005 were included in the study. Stroke was diagnosed by an experienced vascular neurologist and supported by a CT scan. Exclusion criteria were stroke resulting from trauma or an invasive procedure, cerebral hemorrhage, history of malignant tumor, autoimmune disease, and coagulation disorders.
A written informed consent was obtained from all patients who were enrolled and signed by the patients or a first-degree relative in case of aphasia, as approved by the local Institutional Ethics Committee.
The stroke subtypes were classified according to the TOAST classification: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology [12]. Neurological impairment was assessed using the National Institutes of Health Stroke Scale (NIHSS) [13].
2.2. Diagnosis of Leukoaraiosis
Brain computed tomography (CT) scans were performed on all the patients within 24 hours of admission. Scanning was carried out without contrast enhancement and with 10 mm continuous slices. A single trained neurologist who was blinded to the patients' clinical status evaluated the existence, location, and size of brain infarcts on CT images. White matter lesions were defined as periventricular or subcortical, areas of decreased attenuation below that expected for normal white matter. The changes were always diffusely distributed within the white matter.
Leukoaraiosis was defined as ill-defined and moderately hypodense areas of >5 mm in any CT scan dimension [14]. Extension and severity of CT white matter changes (WMCs) were rated according to the score of Blennow et al. [15]. For the purpose of this study, we classified scans as advanced LA for Blennow score above 1 (therefore, score 0 represents no lesion, 1 is mild LA in one region-frontal, parietal, occipital; scores 2 and 3 are advanced LA in two or more regions).
2.3. Evaluation of Carotid Atherosclerosis
Duplex carotid ultrasonography was performed to evaluate the severity of carotid atherosclerosis. All ultrasound examinations were performed with a 128XP/10 Acuson equipped with a 7.5-MHz linear-array transducer, focus depth of 40 mm, and frame rate of 15 Hz. The intima-media thickness (IMT) was defined as the distance between the intimal-luminal interface and the medial-adventitial interface. We calculated the mean carotid artery IMT (mean IMT) by averaging the thickness at 4 sites at the far walls of both the right and left distal common carotid artery [16, 17]. The subjects were also classified according to the presence of well-defined atherosclerotic plaques. Plaques were defined as a focal structure that encroached more than 0.5 mm into the arterial lumen, or 50% of the surrounding intima-media thickness (IMT) value, or demonstrated a thickness of >1.5 mm as measured from the media-adventitia interface to the intima-lumen interface [17]. The ultrasound examination and assessments were performed by expert technician blinded to clinical and CT features.
2.4. Definitions of Risk Factors
Diabetes mellitus was defined as a fasting blood glucose of >126 mg/dL or the use of insulin or oral hypoglycemic agents; hypertension as intermittent blood pressure measurements of >140/90 mmHg or the use of antihypertensive medications. For individuals without a fasting lipid profile, hyperlipidemia was recorded if the diagnosis of hyperlipidemia was included in their medical records or if they were receiving lipid-lowering medication (HMG-CoA reductase inhibitors or fibrates). For individuals with valid lipid profiles, it was defined by the low-density lipoprotein (LDL) or non-high-density lipoprotein (non-HDL) cholesterol concentrations (in individuals displaying elevated triglyceride concentrations of ≥2.26 mmol/L) above the recommended levels according to the risk profile defined by the updated ATP III recommendations [18] or the intake of lipid-lowering medication; current smokers as those smoking at least 5 cigarettes per day.
2.5. Statistical Analysis
All continuous data were summarized and displayed as mean ± SD. Since IMT values have a non-normal distribution, a logarithmic transformation was employed, and all results expressing IMT values were back-transformed to geometric means ± SD. Demographic and clinical data between stroke patients with and without LA were compared by the χ 2 test and Student's t-test. Multivariate logistic regression analyses were used to assess the relationship of LA or LA severity with the following variables: IMT (bellow and above median = 0.65 mm), age, gender, smoking, diabetes mellitus, hypertension, hyperlipidemia, and medications. P < 0.05 was considered statistically significant. SPSS/WIN (version 15.0, SPSS INC, Chicago, IL, USA) software was used to carry out all statistical analyses.
3. Results
3.1. Patient Characteristics
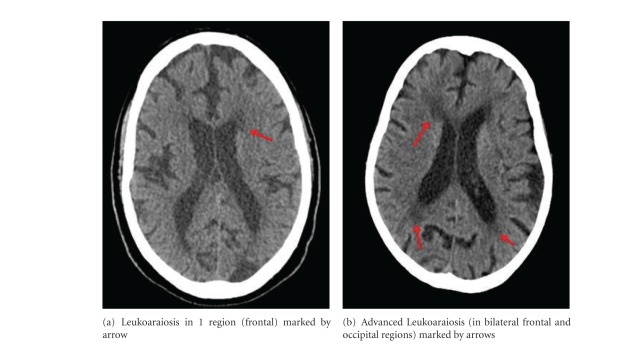
Of the 170 acute stroke patients enrolled (mean ± SD age 66.4 ± 13.4 years, 104 men and 66 women), 94 had lacunar stroke, 32 had large-artery atherosclerotic stroke, 12 had cardioembolic stroke, 1 had stroke of undetermined etiology, and 31 had transient ischemic attack (TIA). In addition to stroke, 28.2% of the patients had diabetes mellitus, 46.7% had hyperlipidemia, 62.7% had hypertension, and 26.2% are current smokers. Seventy-two patients (42.4%) were found to have 1 or more white-matter lesions or leukoaraiosis (LA) on CT images located in frontal, parietal, or occipital region. Of the acute stroke patients, 49 patients (28.8%) were recorded to have advanced LA. Figure 1 illustrates examples of an image from a patient suffering from LA in one region (Figure 1(a)) and a patient suffering from advanced LA (Figure 1(b)). The characteristics of stroke patients with and without LA are summarized in Table 1. Stroke patients with LA differed significantly from stroke patients without LA in their age (71.4 ± 11.3 versus 62.6 ± 13.7 years, P < 0.001) and the prevalence of hypertension (74.6% versus 54.1%, P = 0.006) and diabetes mellitus (36.1% versus 22.4%, P = 0.051) (Table 1).
Figure 1.
Two examples illustrating degrees of LA in CT scan.
Table 1.
Risk factors and clinical characteristics of stroke patients with and without leukoaraiosis.
| No Leukoaraiosis | Leukoaraiosis | ||
| Index | (n = 98) | (n = 72) | P-value |
|
| |||
| Age, years | 62.6 ± 13.7 | 71.4 ± 11.3 | <0.001 |
| Male gender, % | 62.0 | 59.7 | 0.770 |
| BMI, kg/m2 | 27 ± 4.5 | 26.8 ± 4.5 | 0.700 |
| Diabetes mellitus, % | 22.4 | 36.1 | 0.051 |
| Hypertension, % | 54.1 | 74.6 | 0.006 |
| Hyperlipidemia, % | 45.9 | 47.9 | 0.800 |
| Current smoker, % | 29.8 | 21.4 | 0.230 |
| Ever smoker, % | 56.8 | 53.5 | 0.670 |
| Mean IMT*, mm | 0.81 ± 1.12 | 0.86 ± 1.13 | 0.002 |
| Median IMT, (IQR), mm | 0.60 (0.55–0.74) | 0.70 (0.6–0.84) | 0.002 |
| Carotid plaque/s, % | 63.3 | 82.6 | 0.007 |
|
| |||
| NIHSS | 3.9 ± 4.4 | 4.3 ± 3.4 | 0.580 |
|
| |||
| Medication use | |||
|
| |||
| Acetylsalicylic acid, % | 33.7 | 50.0 | 0.032 |
| Clopidogrel, % | 1.0 | 6.9 | 0.039 |
| Beta Blockers, % | 21.4 | 33.3 | 0.082 |
| Calcium blockers, % | 14.3 | 29.2 | 0.018 |
| ACE Inhibitors, % | 30.6 | 45.8 | 0.042 |
| ARBs, % | 1.0 | 4.2 | 0.181 |
| HMG-CoA reductaseinhibitors, % | 21.6 | 25.0 | 0.609 |
| Within lacunar strokes, % | 50.0 | 50.0 | 0.121 |
BMI indicates body mass index; IMT: intima-media thickness; NIHSS: National Institutes of Health Stroke Scale; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blockers; HMG-CoA, 3-hydroxy-3-methylglutaryl-Coenzyme A.
*Data were logarithmically transformed before analysis, presented is the geometric mean (SD).
3.1.1. Relationship between LA and Carotid Atherosclerosis
We observed significantly increased mean IMT measures in stroke patients with LA compared to those without it (P = 0.002). Also, LA was associated with carotid plaque existence (χ 2 = 7.151, P = 0.013), (Table 1).
We employed a logistic regression model in a stepwise forward manner. This encompassed age, gender, body mass index, use of acetylsalicylic acid, clopidogrel, beta blockers, calcium blockers, ACE inhibitors, ARBs, HMG-CoA reductase inhibitors, vascular risk factors, stroke history, stroke etiology, and mean IMT. The only two variables retained in the model for LA occurrence were IMT above 0.65 mm and age (O.R. 1.043, 95% CI 1.012–1.075, P = 0.006; O.R. 2.503, 95% CI 1.181–5.302, P = 0.017, resp.), (Table 2(a), (Figure 2).
Table 2.
(a) OR (95% CI) for incidence of leukoaraiosis. (b) OR (95% CI) for severity of white-matter lesions.
| Multivariate logistic regression analysis | ||
|---|---|---|
| (a) OR (95% CI) | (b) OR (95% CI) | |
| Adjusted for gender, BMI, ever smoking, hypertension, diabetes mellitus, hyperlipidemia, medication use, acetylsalicylic acid, clopidogrel, beta blockers, calcium blockers, ACE inhibitors, ARBs, HMG-CoA reductase inhibitors, stroke history, stroke etiology | for Leukoaraiosis occurrence | for severity of white matter lesions |
| Age, years | 1.043 (1.012–1.075), P = 0.006 | 1.065 (1.027–1.103), P = 0.001 |
| IMT above median (0.65 mm) | 2.503 (1.181–5.302), P = 0.017 | 3.012 (1.315–6.897), P = 0.009 |
OR indicates odds ratio; CI: confidence interval; BMI: body mass index; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blockers; HMG-CoA, 3-hydroxy-3-methylglutaryl-Coenzyme A; IMT: intima-media thickness.
Figure 2.

Prevalence (%) of leukoaraiosis by median of intima-media thickness (IMT). *P computed by χ 2 analysis.
3.1.2. Relationship between Severity of WMCs and Carotid Atherosclerosis
Stroke patients with advanced WMCs represented 28.8% of the sample. Stroke patients with advanced WMCs differed significantly from stroke patients without advanced WMCs in their age (P < 0.001), and the prevalence of hypertension (P = 0.015), but not in other cardiovascular risk factors (Table 3). Measures of carotid IMT were higher in patients with advanced WMCs (P = 0.003) and WMCs severity was associated with carotid plaque existence (χ 2 = 8.5, P = 0.004), (Table 3).
Table 3.
Risk factors and carotid ultrasonography characteristics by severity of WMCs.
| Advanced WMCs | |||
|---|---|---|---|
| No | Yes | P-value | |
| (n = 121) | (n = 49) | ||
| Age, years | 63.6 ± 13.4 | 73.1 ± 10.8 | <0.001 |
| Male gender | 62.0 | 59.2 | 0.734 |
| BMI, kg/m2 | 27.1 ± 4.6 | 26.4 ± 4.1 | 0.373 |
| Diabetes mellitus, % | 26.4 | 32.7 | 0.415 |
| Hypertension, % | 57.0 | 77.1 | 0.015 |
| Hyperlipidemia, % | 46.3 | 47.9 | 0.848 |
| Current smoker, % | 29.1 | 19.1 | 0.192 |
| Ever smoker, % | 56.8 | 52.1 | 0.581 |
| Mean IMT*, mm | 0.81 ± 1.12 | 0.87 ± 1.13 | 0.003 |
| Carotid plaque/s, % | 64.9 | 87.5 | 0.004 |
| Within Lacunar strokes, % | 68.5 | 31.5 | 0.834 |
WMC indicates white matter changes; IMT: intima-media thickness.
*Data were logarithmically transformed before analysis, presented is the geometric mean (SD).
In logistic regression analysis, including age, gender, body mass index, use of acetylsalicylic acid, clopidogrel, beta blockers, calcium blockers, ACE inhibitors, ARBs, HMG-CoA reductase inhibitors, and all vascular risk factors, severity of WMCs was found to be associated with age and IMT above 0.65 mm (O.R. 1.065, 95% CI 1.027–1.103, P < 0.001; O.R. 3.012, 95% CI 1.315–6.897, P = 0.009; resp.).
4. Discussion
This study demonstrates a significant relation between carotid atherosclerosis, reflected as IMT and existence of carotid plaques, and LA in a cohort of acute ischemic stroke and TIA patients. Previous studies have shown that white matter lesions are related to age, hypertension, diabetes, and stroke history [6]. We report that age and IMT are more significant contributors to LA compared to other cardiovascular risk factors, as seen in multivariate regression analysis, although the prevalence of hypertension and diabetes mellitus was higher in patients with LA. Additionally, stroke patients with carotid plaques tended to have more prevalence of WMCs as well as advanced WMCs than patients free of carotid plaques. Breteler et al. [19] reported that WMCs were related to atherosclerosis, defined by increased common carotid IMT and carotid plaques. Similarly, Manolio et al. [8] found that infarcts defined by MRI and white matter score were strongly associated with carotid IMT and stenosis degree after adjustment for age and sex. Furthermore, Pico et al. [20] confirmed the relationship between carotid IMT and plaques with WMCs in a 4-year follow-up study of healthy subjects.
Our study provided further evidence that the relation between carotid atherosclerosis and WMCs is independent of other vascular risk factors and history of stroke. Moreover, strengthening for those findings received by the multivariate analysis, where the findings that the only significant and independent predictors of advanced LA were age and elevated IMT as a marker of carotid atheroclerosis. It has been suggested that WMCs progress gradually over time with the accumulation of vascular risk factors [21]. Therefore our findings are in agreement with the notion that IMT and carotid plaques are a cumulative marker of cardiovascular risk. It can be assumed that patients suffering from advanced atherosclerosis (reflected as plaque formation) are in increased risk to develop advanced WMCs, based on our analysis of patients with advanced WMCs.
The present study has a limitation of possible misclassification in the diagnosis of leukoaraiosis as the diagnosis was based on CT scans rather than MR. However, the use of CT scan for the assessment of leukoaraiosis was found to be a reliable diagnostic method. Previous studies have shown a good intrareader agreement in the use of CT scan for the assessment of leukoaraiosis [22, 23]. In fact, although CT is widely recognized to be less sensitive to white-matter changes than MRI, it is still the most easily accessible neuroimaging procedure in many centers and often the only available method for brain imaging.
In summary, this study reinforces the relationship between LA and carotid atherosclerosis in a population of ischemic stroke patients, although it is not possible to suggest the probable causative relation between them. This association emerged over most vascular risk factors, as seen in multivariate regression analysis. Also, severity of WMCs was strongly related to carotid IMT and plaques. Together, these observations support diagnostic measurements of carotid IMT in stroke patients as an additional clinical tool for risk stratification. We suggest that a chronic atherosclerotic disease is probably the basic pathophysiology of leukoaraiosis and its progression.
Authors' Contribution
E. B. Assayag and M. Mijajlovic equally contributed to this work.
Acknowledgment
This study was partly granted (Dr. M. Mijajlovic) by the Ministry of Science and Education of Serbia, Project No. 175022.
References
- 1.Hackinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of Neurology. 1987;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 2.Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report: A review. Stroke. 1995;26(7):1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 3.Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: The ARIC study. Stroke. 1996;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 4.De Leeuw FE, De Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. Journal of Neurology Neurosurgery and Psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantoni L, Simoni M, Pracucci G, Schmidt R, Barkhof F, Inzitari D. Visual rating scales for age-related white matter changes (leukoaraiosis): Can the heterogeneity be reduced? Stroke. 2002;33(12):2827–2833. doi: 10.1161/01.str.0000038424.70926.5e. [DOI] [PubMed] [Google Scholar]
- 6.Patankar T, Widjaja E, Chant H, et al. Relationship of deep white matter hyperintensities and cerebral blood flow in severe carotid artery stenosis. European Journal of Neurology. 2006;13(1):10–16. doi: 10.1111/j.1468-1331.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 7.Koton S, Schwammenthal Y, Merzeliak O, et al. Cerebral leukoaraiosis in patients with stroke or TIA: Clinical correlates and 1-year outcome. European Journal of Neurology. 2009;16(2):218–225. doi: 10.1111/j.1468-1331.2008.02389.x. [DOI] [PubMed] [Google Scholar]
- 8.Manolio TA, Burke GL, O'Leary DH, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults : the Cardiovascular Health Study. CHS Collaborative Research Group. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 9.De Leeuw FE, De Groot JC, Bots ML, et al. Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. Journal of Neurology. 2000;247(4):291–296. doi: 10.1007/s004150050586. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. Atherosclerosis - An inflammatory disease. New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk EJ, Prins ND, Vermeer SE, et al. C-reactive protein and cerebral small-vessel disease: The Rotterdam scan study. Circulation. 2005;112(6):900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 14.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 15.Blennow K, Wallin A, Uhlemann C, Gottfries CG. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurologica Scandinavica. 1991;83(3):187–193. doi: 10.1111/j.1600-0404.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 16.Fox CS, Larson MG, Corey D, et al. Absence of association between polymorphisms in the hemostatic factor pathway genes and carotid intimal medial thickness: the Framingham Heart Study. Stroke; a journal of cerebral circulation. 2004;35(3):e65–67. doi: 10.1161/01.STR.0000117095.96234.A6. [DOI] [PubMed] [Google Scholar]
- 17.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006): an update on behalf of the advisory board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovascular Diseases. 2007;23(1):75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 18.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Breteler MMB, Van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 20.Pico F, Dufouil C, Lévy C, et al. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: The EVA-MRI cohort. Cerebrovascular Diseases. 2002;14(2):109–115. doi: 10.1159/000064741. [DOI] [PubMed] [Google Scholar]
- 21.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986;17(6):1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- 22.Pantoni L, Simoni M, Pracucci G, Schmidt R, Barkhof F, Inzitari D. Visual rating scales for age-related white matter changes (leukoaraiosis): can the heterogeneity be reduced? Stroke. 2002;33(12):2827–2833. doi: 10.1161/01.str.0000038424.70926.5e. [DOI] [PubMed] [Google Scholar]
- 23.Scheltens P, Erkinjunti T, Leys D, et al. White matter changes on CT and MRI: An overview of visual rating scales. European Neurology. 1998;39(2):80–89. doi: 10.1159/000007921. [DOI] [PubMed] [Google Scholar]