Abstract
We aimed to examine the relationship between physical activity (PA) and change in body weight and cardiorespiratory fitness (CRF) in severely obese men and women. Thirty-five subjects (10 men, body mass index 43.2 ± 5.1 kg/m2) who participated in a 10-month lifestyle treatment programme were included. The PA duration correlated only with weight change for men (r = −0.69, P = .027 versus r = −0.19, P = .372 for women). Conversely, the PA intensity correlated only with CRF for women (r = 0.61, P = .003 versus r = 0.39, P = .340 for men). PA explained 55.8 and 5.6% of weight change for men and women, respectively, whereas the corresponding explained variances for CRF were 15.6 and 36.7%. We conclude that PA was associated with change in body weight and CRF; however, there was a trend towards a gender specific effect between severely obese men and women.
1. Introduction
As physical activity (PA) is an important contributor to energy expenditure, it is a widely used weight reduction intervention. However, most studies find limited evidence for a decrease in body weight with physical activity alone (1–3 kg) [1–3] or in combination with dietary interventions (3.5–13 kg) [1–6]. A limitation when reporting such changes on a group level is the large interindividual variation in responses. Because there is a dose-response relationship between PA and weight reduction [7–9], this variation may arise from differences in PA level and other health behaviours. Variation could also be due to gender, as intervention studies suggest that PA may be more effective in reducing weight among men, than among women [10–12]. Because gender may be a moderator of response to PA interventions in obesity treatment, clinical studies in which results are analysed separately for men and women allow for better development of tailored lifestyle intervention programmes [4, 13].
Physical activity is shown to decrease weight in severely obese subjects over both the short term [14–18] and the long term [17, 19–25]. Although some studies suggest that greater weight reduction may be related to a higher PA level [17, 20, 23], studies on the relationship between PA and weight change are scarce in this population. In addition to reducing weight, lifestyle interventions incorporating PA can increase cardiorespiratory fitness (CRF), which is an important risk factor for cardiovascular disease (CVD) and mortality [26]. As far as we know, the relationship between PA and change in CRF has not been studied in severely obese subjects.
Therefore, the objective of this study was to explore the effect of PA on changes in weight and CRF in severely obese men and women participating in a lifestyle intervention programme. We argue that because distinct gender-related differences might exist in response to PA, analysis should be performed separately for men and women. Such an analysis will facilitate the tailoring of treatment interventions that ultimately improve success rates.
2. Materials and Methods
2.1. Participants
Seventy-one severely obese patients who chose lifestyle treatment over bariatric surgery were enrolled at the Red Cross Haugland Rehabilitation Centre (RCHRC) in Norway between August 2006 and May 2009. The inclusion criteria for participation in the programme were age between 18 and 60 years old and body mass index (BMI) > 40 kg/m2 without comorbidity, or a BMI > 35 with comorbidity. The exclusion criteria were pregnancy, heart disease, drug or alcohol abuse, previous bariatric surgery and mental disorders and physical impairment that could reduce the ability to comply with the programme. Written informed consent was obtained from each study participant before inclusion in the study. The study meets the standards of the Declaration of Helsinki and was approved by the Regional Committee for Medical Research Ethics.
2.2. Study Protocol
The programme consisted of intermittent inpatient periods of six weeks, four weeks, and three periods of two weeks over a two-year period, separated by three to five months. The present study presents data from the first to the third inpatient period (i.e., 10 months of followup).
An interdisciplinary team of health professionals (dietician, nurse, medical doctor, physiotherapist, and exercise specialist) were responsible for the programme, which consisted of three main components: diet, PA, and cognitive behaviour therapy. Both theoretical and practical sessions were incorporated. With respect to diet, each subject followed a high-fibre, low-fat, reduced-energy meal plan based on the Nordic Nutrition Recommendations [27] that included three main meals and 2-3 snacks each day. Regarding PA, subjects participated in a supervised and structured exercise programme consisting of 20–30 minutes of brisk walking before breakfast and two 45–60 minute exercise sessions (e.g., swimming, aerobics, ball games, hiking, strength training) five days per week. No specific target regarding intensity was applied. Throughout the stay at RCHRC, this programme amounted to between 110 and 150 minutes of PA per day. Together with a team member, each subject developed an individualised plan for PA at home. The main objective of this plan was to increase PA level and strengthen each patient's mastery of everyday life. As such, the plan was based on opportunities at home and subject preferences.
2.3. Measurements
The patient's body weight, waist circumference (WC), height, and CRF were measured at the beginning of the first and third inpatient period. Subjects were weighed to the nearest 0.1 kg in light clothing before breakfast, using a digital scale (BWB-800, Tanita Corp., Tokyo, Japan). Height was measured to the nearest 0.5 cm using a wall-mounted stadiometer. Waist circumference was measured to the nearest 0.5 cm at the level of the iliac crest at the end of a normal expiration using a nonstretchable tape measure. Cardiorespiratory fitness was estimated using the Åstrand/Ryhming test according to suggested guidelines [28, 29]. Each subjects heart rate was measured between minute five and six at one submaximal work load on a bicycle ergometer (Ergometrics 900, Jaeger GmbH, Wursburg, Germany) while pedalling at a constant rate of 50 rpm. The measured heart rate was used to estimate maximal oxygen consumption (VO2max), with a correction factor for age. Because we used an indirect measure of VO2max that has not been validated in severely obese subjects, validity and reliability were examined in a subsample of our subjects (n = 13). The subjects performed a maximal treadmill test using a modified Balke-protocol on day one and performed three Åstrand/Ryhming tests thereafter (on day three, four and five, resp.). Åstrand/Ryhming test number one was used as a learning trial, test number two was used for validation, and test two and three were used for assessing reliability. Oxygen consumption on the treadmill test was measured using the Metamax I and the Metasoft v. 1.11.05 software (Cortex Biophysic, Leipzig, Germany). A one-point gas calibration using ambient air and a volume calibration using a three-liter syringe (SensorMedics Corporation, CA, USA) were performed between each test. The results were comparable to results in normal-weight subjects [30, 31]. There was no systematic bias compared to direct measurement of VO2max (−0.20 mL/kg/min difference, P = .900). For test-retest reliability, we found a Pearson correlation coefficient of r = 0.92 (P < .001) and a standard error of measurement (calculated as suggested by Hopkins [32]) of 1.13 mL/kg/min. These results show that the Åstrand/Ryhming test has acceptable precision in detecting changes in CRF over time in our subjects.
Subjects self-monitored PA using simple training diaries. The mode, duration, and PA intensity were recorded. Intensity was reported as rate of perceived exertion (RPE) on the Borg 6–20 scale [33], which has acceptable validity [34]. Because of the overwhelming amount of data in these diaries, the first week in each month was extracted for analysis. The duration of PA per week as well as the duration for specific modes of PA was calculated. Intensity was calculated as the mean RPE for all training sessions per week. Both measures were averaged over the number of valid months. Because most diaries contained information only about modes of PA, such as strength training, bicycling and walking, activities such as housework and gardening were excluded from the analysis on the assumption that these activities would be performed by most subjects and therefore would be equally distributed. Inclusion criteria for the analysis were valid data on both the duration and PA intensity for at least seven out of the ten months of followup. The energy expenditure (EE) was calculated using reported duration and PA intensity, age, baseline BMI and CRF and the regression equation suggested by Jakicic et al. [35].
2.4. Statistical Analyses
Data are presented as mean ± standard deviation (SD). Differences between groups were analysed with a one-way ANOVA and the Bonferroni post-hoc test. Where the assumptions of normality or homogeneity of variances were violated, differences were tested using the Kruskall-Wallis test with the Mann-Whitney test and Bonferroni adjustment for multiple comparisons. Differences over time were tested using a one-sample t-test and tested against a value of 0. Correlation between pairs of variables was analysed using Pearson's correlation coefficients as variables were found to approximate normality. Ninety-five percent confidence intervals (CIs) for correlations were obtained using 10 000 bootstrap samples. Regression analysis was performed using the partial-least-squares- (PLSs-) method allowing for multivariate modeling in small samples. Changes in weight and CRF were used as dependent variables. Four models were established for each dependent variable for men and women separately. Variables to be included were selected on the basis of the bivariate associations with the dependent variables. In model 1, age, baseline BMI, and baseline CRF were used as independent variables. Model 2 included the bivariate relationship between the PA duration and weight change as well as the bivariate relationship between PA intensity and the change in CRF. In model 3, PA duration and intensity were included. Model 4 contained the variables from models 2 and 3. In all PLS analyses, independent variables were standardised to variance because several different units of measurement were used. In order to assess the robustness of the findings, all models were cross-validated excluding every fourth subject. PLS analysis and the cross-validation were performed using Sirius v. 8.0 (Pattern Recognition Systems, Bergen, Norway), while all other analyses were performed using SPSS v. 17.0 (SPSS Inc., Chicago, USA). A two-sided P < .05 indicated significant differences.
3. Results
3.1. Analysis of Attrition
A total of 71 subjects were included in the lifestyle treatment programme. Of these, nine subjects did not consent to take part in this study and six quit during the course of the programme: one to undergo bariatric surgery, one having reached his weight goal, and four for unknown reasons. This left 56 subjects for analysis, among whom 35 subjects (10 men) had valid training diaries. Subjects without valid training diaries were younger than subjects with valid training diaries (39.3 ± 11.1 versus 47.9 ± 8.8 years, P = .005). Dropouts were taller than subjects with valid training diaries (179.8 ± 8.4 versus 168.8 ± 9.5 cm, P = .029). All other baseline- and change values were similar between groups. Further results are based on the group with valid training diaries.
3.2. Baseline Characteristics and Outcomes at the Group Level
Table 1 show that the men were taller, heavier and had better CRF (L/min) than the women at baseline. Changes in weight, WC, and CRF were similar between genders. Results regarding changes over time are therefore collapsed. All variables changed significantly over time (P < .001), except for CRF expressed as an absolute value (L/min) (P = .061). The weight and WC changes were −8.7 ± 5.8% (95% CI −10.7 to −6.7%) and −7.2 ± 5.5% (CI −9.3 to −5.1%), respectively. VO2max increased 9.7 ± 21.1% (CI 1.8 to 17.5%) and 21.1 ± 25.8% (CI 11.4 to 30.7%) when expressed as absolute and relative values, respectively. Taken together, subjects spent about 58 minutes on PA per day during the intervention, which corresponds to an EE of about 536 kcal/day. The duration and volume of PA were significantly higher for men than for women, whereas the intensity was similar between genders. With regard to the mode of PA, the pattern was similar between men and women, except for “other activities,” which was reported more often by women.
Table 1.
Baseline characteristics and change values for the group with valid training diaries. (Mean ± SD n; when different from the total in each group). WC: waist circumference; BMI: body mass index; VO2max: estimated maximal oxygen consumption; EE: energy expenditure.
| Valid training diaries | Men | Women | P between sexes | |
|---|---|---|---|---|
| Baseline | ||||
| N | 35 | 10 | 25 | |
| Age | 47.9 ± 8.8 | 48.8 ± 8.6 | 47.5 ± 9.0 | .694 |
| Height (cm) | 168.8 ± 9.5 | 178.8 ± 8.1 | 164.8 ± 6.8 | .000 |
| Weight (kg) | 123.4 ± 20.9 | 134.9 ± 22.3 | 118.7 ± 18.8 | .036 |
| WC (cm) | 125.8 ± 13.4 [n = 30] | 134.6 ± 17.1 [n = 8] | 122.6 ± 10.5 [n = 22] | .056 |
| BMI (kg/m2) | 43.2 ± 5.1 | 42.0 ± 4.5 | 43.6 ± 5.3 | .400 |
| VO2max (L/min) | 2.37 ± 0.57 | 2.81 ± 0.68 | 2.18 ± 0.41 | .004 |
| VO2max (mL/kg/min) | 19.5 ± 5.0 | 21.5 ± 5.8 | 18.7 ± 4.6 | .171 |
| Change to 10 months | ||||
| Weight | −10.73 ± 7.05 | −10.67 ± 7.52 | −10.76 ± 7.01 | .975 |
| WC | −9.22 ± 7.18 [n = 28] | −7.71 ± 7.29 [n = 7] | −9.72 ± 7.25 [n = 21] | .531 |
| VO2max (L/min) | 0.17 ± 0.48 [n = 30] | 0.20 ± 0.62 [n = 8] | 0.16 ± 0.44 [n = 22] | .855 |
| VO2max (mL/kg/min) | 3.54 ± 4.54 [n = 30] | 3.54 ± 4.54 [n = 8] | 3.41 ± 4.52 [n = 22] | .800 |
| Physical activity | ||||
| Duration (min/week) | 403 ± 171 | 506 ± 198 | 361 ± 143 | .022 |
| Intensity (RPE) | 13.9 ± 1.4 | 13.8 ± 0.9 | 13.9 ± 1.6 | .855 |
| EE (kcal/week) | 3751 ± 1928 | 5313 ± 2191 | 3183 ± 1506 | .005 |
| Mode of activity | ||||
| Walking (%) | 57.7 ± 23.2 | 59.7 ± 28.5 | 57.0 ± 21.3 | .759 |
| Bicycling (%) | 11.7 ± 19.2 | 19.1 ± 26.9 | 8.7 ± 14.8 | .483 |
| Swimming (%) | 13.2 ± 9.8 | 10.9 ± 8.6 | 14.1 ± 10.3 | .398 |
| Strength training (%) | 12.2 ± 11.3 | 8.7 ± 7.1 | 13.6 ± 12.4 | .250 |
| Other (%) | 5.3 ± 6.9 | 1.7 ± 2.8 | 6.7 ± 7.5 | .031 |
3.3. Relationships between PA and Change in Weight and CRF
Figure 1 shows individual changes in weight and CRF. The figure clearly shows large individual variation in both outcome variables. Most subjects lost weight and displayed increases in CRF; however, the opposite was also seen. Changes in the two outcomes were not related (r = −0.16, P = .401, n = 30).
Figure 1.
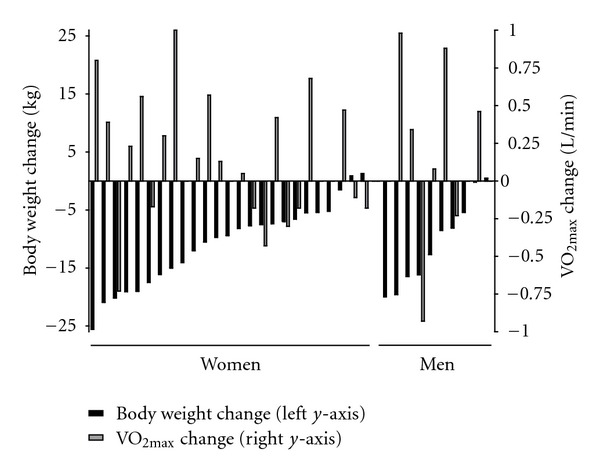
Individual changes in body weight and cardiorespiratory fitness (n = 22 for women; n = 8 for men). Subjects are sorted within gender with respect to body weight change.
Correlation between the PA duration and weight change was r = −0.33 (P = .050); the correlation between the PA intensity and weight change was r = −0.17 (P = .336). The correlation between the PA duration and the change in CRF was r = −0.04 (P = .853, n = 30); the correlation between the PA intensity and the change in CRF was r = 0.51 (P = .004, n = 30). The relationship between the PA duration and weight change was much stronger for men than for women (r = −0.69, CI −0.88 to −0.41, P = .027 versus r = −0.19, CI −0.60 to 0.26, P = .372). Conversely, the relationship between the PA intensity and change in CRF was stronger for women than for men (r = 0.61, CI 0.10 to 0.85, P = .003 (n = 22) versus r = 0.39, CI −0.29 to 0.86, P = .340 (n = 8)). Scatterplots of PA duration versus change in weight and PA intensity versus change in CRF are shown in Figures 2(a) and 2(b), respectively. There were no relationship between PA duration and WC (r = −0.10, P = .624, n = 28), nor between PA intensity and WC (r = −0.01, P = .967, n = 28).
Figure 2.
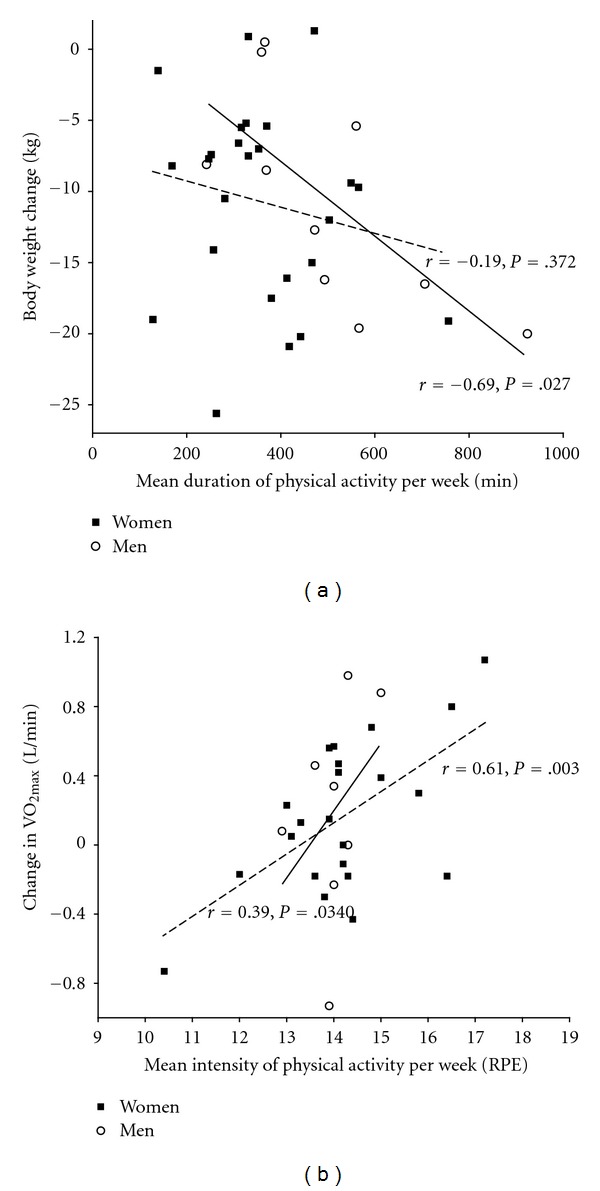
(a) Scatterplot showing weight change versus duration of physical activity for men (full regression line) and women (dotted regression line). (b) Scatterplot showing change in cardiorespiratory fitness versus intensity of PA for men (full regression line) and women (dotted regression line).
3.4. Regression Analysis
Tables 2(a) and 2(b) show the PLS regression analysis with respect to changes in weight and CRF, respectively. A correlation matrix including independent and dependent variables is shown in Table 3. For weight change, model 1 (baseline variables) was quite similar for both genders, explaining about 20% of the variance. Model 2 (PA duration) yielded remarkably different results between genders, explaining 47.8% of the variance for men compared to 4% for women. The PA variables together (model 3) explained 55.8% of the weight change for men, whereas 5.6% of the weight change was explained for women. Together, baseline characteristics and PA (model 4) explained about two-thirds of the weight change for men, whereas only one-third of the change for women was explained.
Table 2.
(a) Regression equations, standard errors, and explained variance in change in weight. All models are cross-validated excluding every fourth subject. Independent variables that have a significant contribution (P < .05) to the model are labeled†. SEE: standard error of the estimate; SECV: standard error of the cross-validation. (b) Regression equations, standard errors, and explained variance in change in cardiorespiratory fitness. All models are cross-validated excluding every fourth subject. Independent variables that have a significant contribution (P < .05) to the model are labeled†. SEE: standard error of the estimate; SECV: standard error of the cross-validation.
(a)
| Regression equation (standardized) | Regression equation (actual) | SEE | SECV | R2 | Adj R2 | |
|---|---|---|---|---|---|---|
| Men | ||||||
| Model 1 | 21.60 − 1.17 ∗ Age − 2.28 ∗ Baseline BMI − 1.10 ∗ Baseline VO2max | 21.60 − 0.14 ∗ Age − 0.50 ∗ Baseline BMI − 1.62 ∗ Baseline VO2max | 7.17 | 8.68 | 0.193 | 0.092 |
| Model 2 | 2.62 − 0.03 ∗ Duration | 5.76 | 5.62 | 0.478 | 0.412 | |
| Model 3 | 41.30 − 4.15 ∗ Duration† − 2.82 ∗ Intensity† | 41.30 − 0.02 ∗ Duration − 3.00 ∗ Intensity | 5.30 | 5.45 | 0.558 | 0.502 |
| Model 4 | 68.40 − 1.18 ∗ Age† − 2.29 ∗ Baseline BMI† − 1.11 ∗ Baseline VO2max − 3.72 ∗ Duration† − 2.53 ∗ Intensity† | 68.40 − 0.14 ∗ Age − 0.50 ∗ Baseline BMI − 1.62 ∗ Baseline VO2max − 0.02 ∗ Duration − 2.69 ∗ Intensity | 4.41 | 5.86 | 0.694 | 0.656 |
| Women | ||||||
| Model 1 | 10.60 − 0.11 ∗ Age − 3.19 ∗ Baseline BMI† + 0.99 ∗ Baseline VO2max | 10.60 − 0.01 ∗ Age − 0.60 ∗ Baseline BMI + 2.40 ∗ Baseline VO2max | 6.38 | 7.85 | 0.208 | 0.173 |
| Model 2 | −7.43 − 0.01 ∗ Duration | 6.88 | 7.04 | 0.040 | 0.000 | |
| Model 3 | 0.95 − 1.57 ∗ Duration† − 0.89 ∗ Intensity | 0.95 − 0.01 ∗ Duration − 0.56 ∗ Intensity | 6.96 | 7.38 | 0.056 | 0.015 |
| Model 4 | 33.40 − 0.96 ∗ Age − 3.97 ∗ Baseline BMI† + 2.00 ∗ Baseline VO2max − 2.84 ∗ Duration† − 1.17 ∗ Intensity | 33.40 − 0.11 ∗ Age − 0.74 ∗ Baseline BMI + 4.84 ∗ Baseline VO2max − 0.02 ∗ Duration − 0.72∗ Intensity | 5.83 | 8.19 | 0.368 | 0.310 |
(b)
| Regression equation (standardized) | Regression equation (actual) | SEE | SECV | R2 | Adj R2 | |
|---|---|---|---|---|---|---|
| Men | ||||||
| Model 1 | 0.88 + 0.56 ∗ Age† − 0.14 ∗ Baseline BMI − 0.55 ∗ Baseline VO2max† | 0.55 + 0.04 ∗ Age − 0.02 ∗ Baseline BMI − 0.49 ∗ Baseline VO2max | 0.56 | 0.58 | 0.404 | 0.165 |
| Model 2 | −5.24 + 0.39 ∗ Intensity | 0.56 | 0.62 | 0.145 | 0.002 | |
| Model 3 | −8.85 + 0.10 ∗ Duration + 0.38 ∗ Intensity† | −5.47 + 0.00 ∗ Duration + 0.39 ∗ Intensity | 0.61 | 0.59 | 0.156 | 0.015 |
| Model 4 | −19.00 + 0.73 ∗ Age† + 0.33 ∗ Baseline BMI† − 1.01 ∗ Baseline VO2max† + 0.09 ∗ Duration + 0.71 ∗ Intensity† | −11.7 + 0.05 ∗ Age + 0.04 ∗ Baseline BMI − 0.90 ∗ Baseline VO2max + 0.00 ∗ Duration + 0.72 ∗ Intensity | 0.35 | 0.62 | 0.816 | 0.677 |
| Women | ||||||
| Model 1 | 3.46 − 0.15 ∗ Age† + 0.14 ∗ Baseline BMI − 0.63 ∗ Baseline VO2max† | 1.51 − 0.01 ∗ Age + 0.01 ∗ Baseline BMI − 0.66 ∗ Baseline VO2max | 0.36 | 0.41 | 0.342 | 0.310 |
| Model 2 | −2.39 + 0.18 ∗ Intensity | 0.35 | 0.34 | 0.375 | 0.344 | |
| Model 3 | −4.86 − 0.12 ∗ Duration + 0.58 ∗ Intensity† | −2.12 − 0.00 ∗ Duration + 0.17 ∗ Intensity | 0.36 | 0.36 | 0.367 | 0.336 |
| Model 4 | −3.93 − 0.15 ∗ Age + 0.25 ∗ Baseline BMI† − 0.47 ∗ Baseline VO2max† + 0.12 ∗ Duration + 0.56 ∗ Intensity† | −1.71 − 0.01 ∗ Age + 0.02 ∗ Baseline BMI − 0.50 ∗ Baseline VO2max + 0.00 ∗ Duration + 0.16 ∗ Intensity | 0.29 | 0.32 | 0.606 | 0.564 |
Table 3.
Correlation matrix between independent and dependent variables in the regression analyses for men (n = 8–10) in the lower left corner and women (n = 22–25) in the upper right corner (Pearson r (P value)). BMI: body mass index; VO2max: estimated maximal oxygen consumption.
| Age | Baseline BMI | Baseline VO2max | PA duration | PA intensity | Change in weight | Change in VO2max | |
|---|---|---|---|---|---|---|---|
| Age | 1 | −0.15 (.467) | −0.40 (.064) | −0.08 (.696) | −0.48 (.014) | −0.01 (.947) | −0.12 (.605) |
| Baseline BMI | 0.11 (.755) | 1 | 0.14 (.549) | −0.20 (.349) | −0.02 (.945) | −0.42 (.039) | 0.11 (.632) |
| Baseline VO2max | 0.45 (.224) | 0.35 (.350) | 1 | 0.29 (.189) | −0.18 (.416) | 0.14 (.526) | −0.49 (.019) |
| PA duration | 0.32 (.372) | 0.13 (.725) | 0.33 (.384) | 1 | −0.19 (.362) | −0.19 (.372) | −0.12 (.584) |
| PA intensity | −0.22 (.541) | −0.15 (.671) | −0.21 (.589) | 0.26 (.471) | 1 | −0.11 (.605) | 0.61 (.003) |
| Change in weight | −0.22 (.545) | −0.43 (.218) | −0.24 (.532) | −0.69 (.027) | −0.45 (.195) | 1 | −0.24 (.274) |
| Change in VO2max | 0.26 (.527) | −0.21 (.613) | −0.41 (.313) | 0.10 (.809) | 0.39 (.340) | 0.03 (.951) | 1 |
Regarding the change in CRF, model 1 explained 40.4 and 34.2% of the variance for men and women, respectively. The corresponding variances explained by model 2 were 14.5 and 37.5%. For women, the PA variables together (model 3) explained one-third of the change in CRF, whereas 15.6% was explained for men. Taken together, the baseline variables and PA variables explained 81.6% of the change in CRF for men and 60.6% for women (model 4). However, the SEEs for men were generally almost twice as high as for women, reflecting considerable uncertainty in the predictions.
4. Discussion
This study found that severely obese subjects undergoing a 10-month lifestyle intervention showed great individual variation in the change in weight and CRF. Physical activity was related differently to change in weight and CRF in men and women. Duration and intensity of PA explained 55.8% of the weight change in men and only 5.6% in women. For CRF the opposite pattern was seen. While PA explained only 15.6% of the change for men, it explained 36.7% of the change for women.
4.1. Weight Change
We found a decrease in weight of −10.7 kg (−8.7%) over 10 months in both genders. This is in line with other studies investigating lifestyle treatment for severely obese subjects [17, 24, 25]. However, other studies have shown more beneficial results after similar programmes [21] and larger weight losses have been achieved with more severe lifestyle interventions using low-energy or very low-energy diets [22, 23]. Weight losses in the present study seem consistent with results seen in overweight and moderately obese subjects undergoing lifestyle interventions [3–5]. Thus, regarding weight loss, lifestyle treatment programmes seem to work equally well in severely obese and less obese persons.
Few previously published studies of severely obese subjects have reported PA levels. Interestingly, Hofsø et al. [24] reported a median physical activity level of approximately the same magnitude as in the present study (65 minutes per day); the weight losses were also nearly identical. Unfortunately, Goodpaster et al. [25], who recently published the first randomised controlled trial in the field of lifestyle intervention for severely obese subjects, did not report total minutes spent in moderate to vigorous physical activity or EE, although PA was measured both objectively and through training diaries. Comparing the present study results with the results of Maffiuletti et al. [17] is also difficult because PA levels were determined using a scoring system that, while allowing for acceptable internal validity, limits its external validity.
Although reports on dose-response relationships between PA and weight change in severely obese subjects are scarce, some studies have shown convincing results in less obese subjects [7–9]. The most recent study by Wadden et al. [7] showed a consistent increase in one-year weight loss of 4.4, 7.1, 9.0, and 11.9% for increasing quartiles of mean weekly minutes of physical activity (25.9, 84.8, 148.7, and 287.1 min) in 2570 subjects with a baseline BMI of about 36, randomised to lifestyle intervention.
Overall, a correlation of r = 0.41 was found, yielding a 16.1% explained variance in weight change. This relationship is of approximately the same strength as in the present study when the male and female groups are collapsed (r = 0.33). However, we found a marked difference between genders. In men, a moderate-to-high correlation was found (r = −0.69), whereas in women, a nonsignificant association with weight change was found (r = −0.19). Although it should be noted that the CIs overlapped between genders, there was a clear difference in the strength of the relationships. Although our results must be interpreted carefully due to the small samples, the results are in line with previous intervention studies suggesting larger weight losses due to physical activity for men than for women [10–12].
4.2. Change in Cardiorespiratory Fitness
An overall mean increase in CRF of 3.5 mL/kg/min (21.1%) was achieved in the present study. This change parallels other study results for both severely obese and less obese subjects undergoing lifestyle interventions incorporating PA [17, 18, 36]. Such an increase in CRF may reduce the risk of all-cause mortality and the incidence of cardiovascular disease in healthy men and women by 13 and 15%, respectively [26]. As studies consistently show an especially large risk reduction with an increase in CRF beyond some minimal threshold [26, 37], any increase would be beneficial to health for most severely obese subjects, whose CRF is generally low [17, 18, 38, 39]. Moreover, low baseline CRF was a significant predictor for increased CRF in both genders in the present study, showing that subjects with the highest intervention potential benefitted the most. The increase in CRF may also benefit the subjects by allowing them to increase their PA level and thereby lose more weight [4]. This is indicated by the inverse relationship between baseline CRF and PA duration found in the present study (r = 0.40, P = .024, results not shown).
Duration of PA was not correlated with change in CRF in the present study, whereas PA intensity was significantly correlated with increases in CRF in the group as a whole, as well as for women. For men, this relationship was nonsignificant. The importance of PA intensity to increase CRF is well established [40]. The variation in change in CRF explained by PA for women (36.7%) is consistent with nine studies reviewed by Williams [37] in which PA explained 35% of the variation in CRF. The weaker explanation of the variation among men in the present study (15.6%) may be a result of men being a relatively small homogenous group, which resulted in a minimal range in PA intensities.
4.3. Interpretation of the Results
We found that regression models based on both baseline measures and PA variables explained 69.4 and 36.8% of the change in weight for men and women, respectively, with corresponding values for the change in CRF of 81.6 and 60.6%. These values are quite high compared to other prospective studies, where explained variances in weight change are in the range of 12 to 38% [10, 41, 42]. However, the residuals are substantial in all models for both genders, indicated by the SEEs of ∼6 kg for weight and 0.3–0.6 L/min for VO2max. Therefore, care should be taken to ensure the ethical use of such models in clinical settings. Although selection strategies may ensure more effective use of limited resources [43], we believe individuals in need of essential healthcare who are found to have a low probability of success should not be given less attention and followup based on such predictions.
If the dissimilar responses to PA between genders are accurate, tailoring the content and focus of lifestyle treatment programmes to men and women may improve the rate of treatment success. It can be argued that men and women in our sample used different strategies to lose weight because PA explains 55.8 versus 5.6% of the changes in body weight for men and women, respectively, whereas both genders lose the same amount of weight. In other words, factors other than PA may cause the weight reduction in women and might not benefit men equally. Although we have no measure of the diet component of this lifestyle intervention, we can speculate that women relied more on changes in eating to regulate energy balance, than men did. As the literature suggests [44, 45], women may have a closer regulation of appetite than men in short periods of increased energy expenditure, which leads to a partial compensation for the negative energy balance. This would influence weight change in our study and may imply that women should increase their PA level together with having a strict diet. This finding is also in line with results showing an interaction between PA and diet for women such that PA was only beneficial when performed together with changes in diet [10]. Hence, women should be advised to increase their PA level and to make dietary changes. Moreover, PA intensity is important to increase CRF. Thus, we recommend that PA should be an important part of lifestyle treatment programmes for severely obese subjects of both genders.
4.4. Strengths, Limitations, and Suggestions for Further Research
The present study has several limitations. First, as the sample was small, relationships may be made spurious by low representativeness or by increasing the influence of specific cases or outliers in the data. However, all regression models were cross-validated, with minor changes made to the standard errors. Second, as weight regain following weight loss is very common, the 10-month followup in the present study may not have been long enough to determine the clinical importance of our results. However, PA is consistently seen as a predictor of weight maintenance [46, 47], so its effect could become more important over time. Third, as precise measures are important to reveal true relationships, the measures used could undermine our conclusions. In particular, body weight has low sensitivity to changes in fatness after PA interventions. Because PA stimulates muscle hypertrophy, decreases in fat mass may be masked [48, 49]. Furthermore, self-report measures show large deviations compared to objective measures of PA [50]. However, as no gold standard exists, PA measurement remains a topic for debate. There is no evidence, as far as we know, that these measures have less reliability in obese subjects than in other subjects.
While our use of training diaries may be a limitation as discussed above, it may also be a strength. We calculated PA duration and intensity as the mean for at least seven of the ten months of followup, thereby avoiding variation in PA from week to week and during seasons as a source of error. This variation could be a serious threat to studies where PA levels are measured for single points in time, as illustrated by correlations ranging between r = −0.43 and 0.00 between PA duration for each of the ten months and total weight change in the present study. Finally, the lack of diet as a research measure, one of the two main components determining energy balance, constitutes a limitation for the prediction of changes in weight.
Due to the methodological weaknesses described above, our results are preliminary. Further research examining PA and CRF in severely obese subjects undergoing lifestyle treatment should include objective measures to determine PA level and direct measurements of CRF (i.e., by measuring oxygen consumption and/or by performance tests). Furthermore, results should be reported for men and women separately. Finally, comprehensive studies including both objective and subjective measures of health should be conducted, and relationships with PA and CRF should be determined.
5. Conclusion
This study showed beneficial changes in weight and CRF for severely obese subjects undergoing lifestyle treatment. Individual variation in outcomes was large, and PA was related differently to changes in weight and CRF between men and women. Further research is needed to determine the effects of PA in severely obese subjects. Until then, it is reasonable to state that more PA is better.
Conflict of Interests
The authors declared that they have no conflict of interests.
Acknowledgment
The authors thank colleagues at the Sogn og Fjordane University College for revising the paper.
References
- 1.Shaw K, Gennat H, O’Rourke P, del Mar C. Exercise for overweight or obesity. Cochrane database of systematic reviews. 2006;(4) doi: 10.1002/14651858.CD003817.pub3. Article ID CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. Journal of the American Dietetic Association. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. International Journal of Obesity. 1997;21(10):941–947. doi: 10.1038/sj.ijo.0800499. [DOI] [PubMed] [Google Scholar]
- 4.Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nature Clinical Practice Endocrinology and Metabolism. 2007;3(7):518–529. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galani C, Schneider H. Prevention and treatment of obesity with lifestyle interventions: review and meta-analysis. International Journal of Public Health. 2007;52(6):348–359. doi: 10.1007/s00038-007-7015-8. [DOI] [PubMed] [Google Scholar]
- 6.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis: obesity management. Obesity Reviews. 2009;10(3):313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 7.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the look AHEAD study: factors associated with success. Obesity. 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. Journal of the American Medical Association. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 9.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Archives of Internal Medicine. 2004;164(1):31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Dunn CL, Hannan PJ, Jeffery RW, Sherwood NE, Pronk NP, Boyle R. The comparative and cumulative effects of a dietary restriction and exercise on weight loss. International Journal of Obesity. 2006;30(1):112–121. doi: 10.1038/sj.ijo.0803046. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the midwest exercise trial. Archives of Internal Medicine. 2003;163(11):1343–1350. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 12.Westerterp KR, Meijer GAL, Janssen EME, Saris WHM, Hoor FT. Long-term effect of physical activity on energy balance and body composition. British Journal of Nutrition. 1992;68(1):21–30. doi: 10.1079/bjn19920063. [DOI] [PubMed] [Google Scholar]
- 13.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis: etiology and pathophysiology. Obesity Reviews. 2009;10(2):154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 14.Sartorio A, Lafortuna CL, Conte G, Faglia G, Narici MV. Changes in motor control and muscle performance after a short-term body mass reduction program in obese subjects. Journal of Endocrinological Investigation. 2001;24(6):393–398. doi: 10.1007/BF03351039. [DOI] [PubMed] [Google Scholar]
- 15.Sartorio A, Lafortuna CL, Vangeli V, Tavani A, Bosetti C, la Vecchia C. Short-term changes of cardiovascular risk factors after a non-pharmacological body weight reduction program. European Journal of Clinical Nutrition. 2001;55(10):865–869. doi: 10.1038/sj.ejcn.1601235. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro JR, Stout AL, Musante GJ. ‘Structure-size me:’ weight and health changes in a four week residential program. Eating Behaviors. 2006;7(3):229–234. doi: 10.1016/j.eatbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Maffiuletti NA, Agosti F, Marinone PG, Silvestri G, Lafortuna CL, Sartorio A. Changes in body composition, physical performance and cardiovascular risk factors after a 3-week integrated body weight reduction program and after 1-y follow-up in severely obese men and women. European Journal of Clinical Nutrition. 2005;59(5):685–694. doi: 10.1038/sj.ejcn.1602130. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen JØ, Zimmermann E, Stallknecht BM, et al. Lifestyle intervention in the treatment of severe obesity. Ugeskrift for Laeger. 2006;168(2):167–172. [PubMed] [Google Scholar]
- 19.Bjorvell H, Rossner S. Short communication: a ten-year follow-up of weight change in severely obese subjects treated in a combined behavioural modification programme. International Journal of Obesity. 1992;16(8):623–625. [PubMed] [Google Scholar]
- 20.Christiansen T, Bruun JM, Madsen EL, Richelsen B. Weight loss maintenance in severely obese adults after an intensive lifestyle intervention: 2- to 4-year follow-up. Obesity. 2007;15(2):413–420. doi: 10.1038/oby.2007.530. [DOI] [PubMed] [Google Scholar]
- 21.Martins C, Strømmen M, Stavne OA, Nossum R, Mårvik R, Kulseng B. Bariatric surgery versus lifestyle interventions for morbid obesity—changes in body weight, risk factors and comorbidities at 1 year. Obesity Surgery. 2011;21(7):841–849. doi: 10.1007/s11695-010-0131-1. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JW, Conley SB, Nicholas AS. One hundred-pound weight losses with an intensive behavioral program: changes in risk factors in 118 patients with long-term follow-up. American Journal of Clinical Nutrition. 2007;86(2):301–307. doi: 10.1093/ajcn/86.2.301. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JW, Grant L, Gotthelf L, Stifler LTP. Weight loss and long-term follow-up of severely obese individuals treated with an intense behavioral program. International Journal of Obesity. 2007;31(3):488–493. doi: 10.1038/sj.ijo.0803423. [DOI] [PubMed] [Google Scholar]
- 24.Hofsø D, Nordstrand N, Johnson LK, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. European Journal of Endocrinology. 2010;163(5):735–745. doi: 10.1530/EJE-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodpaster BH, DeLany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. Journal of the American Medical Association. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. Journal of the American Medical Association. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 27.Nordic Council of Ministers. Nordic Nutrition Recommendations 2004—Integrating Nutrition and Physical Activity. Copenhagen, Denmark: Nordic Publishing; 2004. [Google Scholar]
- 28.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiologica Scandinavica, Supplementum. 1960;49:1–92. [PubMed] [Google Scholar]
- 29.Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. Journal of Applied Physiology. 1954;7:218–221. doi: 10.1152/jappl.1954.7.2.218. [DOI] [PubMed] [Google Scholar]
- 30.Cink RE, Thomas TR. Validity of the Astrand-Ryhming nomogram for predicting maximal oxygen intake. British Journal of Sports Medicine. 1981;15(3):182–185. doi: 10.1136/bjsm.15.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macsween A. The reliability and validity of the Astrand nomogram and linear extrapolation for deriving (V)over-dot-O-2max from submaximal exercise data. Journal of Sports Medicine and Physical Fitness. 2001;41(3):312–317. [PubMed] [Google Scholar]
- 32.Hopkins WG. Measures of reliability in sports medicine and science. Sports Medicine. 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Borg G. Perceived exertion as an indicator of somatic stress. Scandinavian Journal of Rehabilitation Medicine. 1970;2(2):92–98. [PubMed] [Google Scholar]
- 34.Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. Journal of Sports Sciences. 2002;20(11):873–899. doi: 10.1080/026404102320761787. [DOI] [PubMed] [Google Scholar]
- 35.Jakicic JM, Donnelly JE, Pronk NP, Jawad AF, Jacobsen DJ. Prescription of exercise intensity for the obese patient: the relationship between heart rate, VO2 and perceived exertion. International Journal of Obesity. 1995;19(6):382–387. [PubMed] [Google Scholar]
- 36.Kelley GA, Kelley KS, Vu Tran Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. International Journal of Obesity. 2005;29(8):881–893. doi: 10.1038/sj.ijo.0802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Medicine and Science in Sports and Exercise. 2001;33(5):754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulens M, Vansant G, Claessens AL, Lysens R, Muls E. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scandinavian Journal of Medicine and Science in Sports. 2003;13(2):98–105. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 39.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Exercise capacity in lean versus obese women. Scandinavian Journal of Medicine and Science in Sports. 2001;11(5):305–309. doi: 10.1034/j.1600-0838.2001.110509.x. [DOI] [PubMed] [Google Scholar]
- 40.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Medicine. 1986;3(5):346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lavery MA, Loewy JW. Identifying predictive variables for long-term weight change after participation in a weight loss program. Journal of the American Dietetic Association. 1993;93(9):1017–1024. doi: 10.1016/0002-8223(93)92041-u. [DOI] [PubMed] [Google Scholar]
- 42.Klesges RC, Klesges LM, Haddock CK, Eck LH. A longitudinal analysis of the impact of dietary intake and physical activity on weight change in adults. American Journal of Clinical Nutrition. 1992;55(4):818–822. doi: 10.1093/ajcn/55.4.818. [DOI] [PubMed] [Google Scholar]
- 43.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity Reviews. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 44.Stubbs RJ, Sepp A, Hughes DA, et al. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. European Journal of Clinical Nutrition. 2002;56(2):129–140. doi: 10.1038/sj.ejcn.1601295. [DOI] [PubMed] [Google Scholar]
- 45.Stubbs RJ, Sepp A, Hughes DA, et al. The effect of graded levels of exercise on energy intake and balance in free-living women. International Journal of Obesity. 2002;26(6):866–869. doi: 10.1038/sj.ijo.0801874. [DOI] [PubMed] [Google Scholar]
- 46.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? American Journal of Clinical Nutrition. 2007;85(4):954–959. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 47.Wing RR, Phelan S. Long-term weight loss maintenance. The American Journal of Clinical Nutrition. 2005;82(supplement 1):222S–222S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Medicine and Science in Sports and Exercise. 1999;31(9):1320–1329. doi: 10.1097/00005768-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Hunter GR, Byrne NM, Sirikul B, et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity. 2008;16(5):1045–1051. doi: 10.1038/oby.2008.38. [DOI] [PubMed] [Google Scholar]
- 50.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity. 2008;5, article 55 doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]


