Abstract
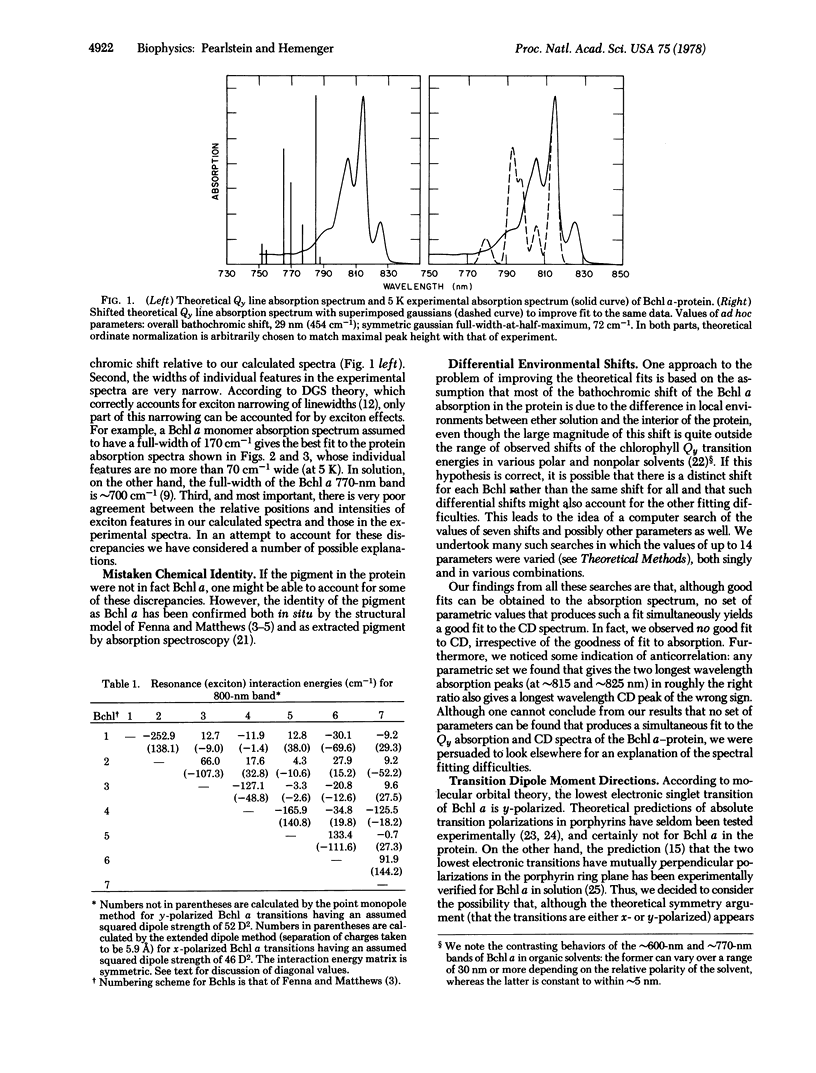
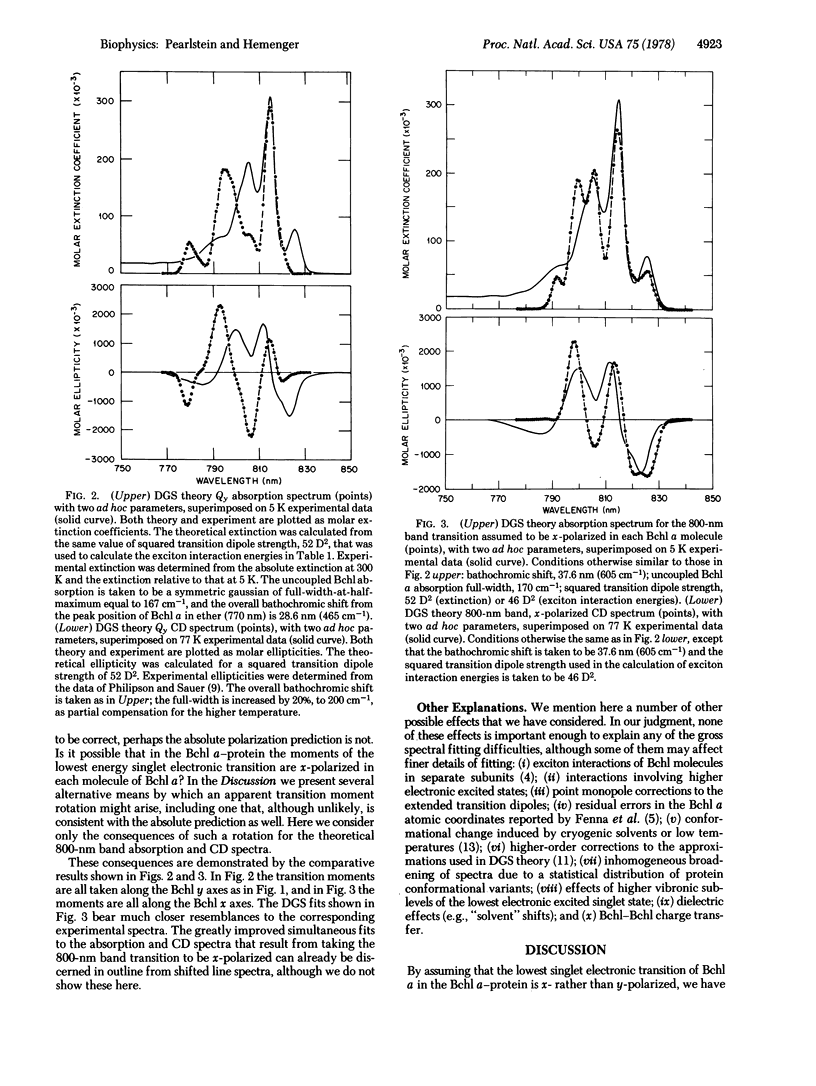
The low-temperature 800-nm band absorption and circular dichroism spectra of the bacteriochlorophyll (Bchl) a-protein from Prosthecochloris aestuarii strain 2K are analyzed theoretically. These spectra show considerable structure that is attributed primarily to resonance (exciton) interactions among the lowest singlet transitions of the Bchl a molecules contained in each protein. We calculate these spectra from the known arrangement of the Bchl molecules in the protein. With the conventional assignment of the lowest singlet transition of Bchl a as Qy (y-polarized), agreement of calculated spectra with experiment is poor. All of our attempts, based on this conventional assignment, to improve the theoretical fits to absorption and circular dichroism spectra simultaneously are unsuccessful. However, by making the simple but unconventional assumption that the lowest singlet transition in each of the Bchl a molecules in each protein is x-polarized rather than y-polarized, we find good agreement between calculated and observed spectra. If these results are not fortuitous, they indicate that there is a systematic error in the protein structural model, that the conventional assignments of Bchl a transitions are incorrect, or that the protein environment provides a sufficiently strong perturbation to rotate the lowest singlet transition moment direction by ∼90°, presumably by changing the order of certain of the Bchl a orbitals.
Keywords: circular dichroism, cyrogenic spectroscopy, excitons, molecular orbital theory, photosynthesis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fenna R. E., Eyck L. F., Matthews B. W. Atomic coordinates for the chlorophyll core of a bacteriochlorophyll a-protein from green photosynthetic bacterial. Biochem Biophys Res Commun. 1977 Apr 11;75(3):751–756. doi: 10.1016/0006-291x(77)91536-4. [DOI] [PubMed] [Google Scholar]
- Fenna R. E., Matthews B. W. Structure of a bacteriochlorophyll a-protein from Prosthecochloris aestuarii. Brookhaven Symp Biol. 1976 Jun 7;(28):170–182. [PubMed] [Google Scholar]
- Fowler C. F., Gray B. H., Nugent N. A., Fuller R. C. Absorbance and fluorescence properties of the bacteriochlorophyll a reaction center complex and bacteriochlorophyll a protein in green bacteria. Biochim Biophys Acta. 1973 Apr 5;292(3):692–699. doi: 10.1016/0005-2728(73)90017-0. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Giddings T. H., Jr, Shaw E. K. An enriched reaction center preparation from green photosynthetic bacteria. Biochim Biophys Acta. 1976 Nov 9;449(2):197–208. doi: 10.1016/0005-2728(76)90133-x. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Ke B., Thompson K. H. Exciton interaction among chlorophyll molecules in bacteriochlorophyllaproteins and bacteriochlorophyllareaction center complexes from green bacteria. Biochim Biophys Acta. 1976 Jun 8;430(3):524–537. doi: 10.1016/0005-2728(76)90028-1. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Sauer K. Exciton interaction in a bacteriochlorophyll--protein from Chloropseudomonas ethylica. Absorption and circular dichroism at 77 degrees K. Biochemistry. 1972 May 9;11(10):1880–1885. doi: 10.1021/bi00760a024. [DOI] [PubMed] [Google Scholar]
- Whitten W. B., Nairn J. A., Pearlstein R. M. Derivative absorption spectroscopy from 5--300 K of Prosthecochloris aestuarii. Biochim Biophys Acta. 1978 Aug 8;503(2):251–262. doi: 10.1016/0005-2728(78)90186-x. [DOI] [PubMed] [Google Scholar]


