Abstract
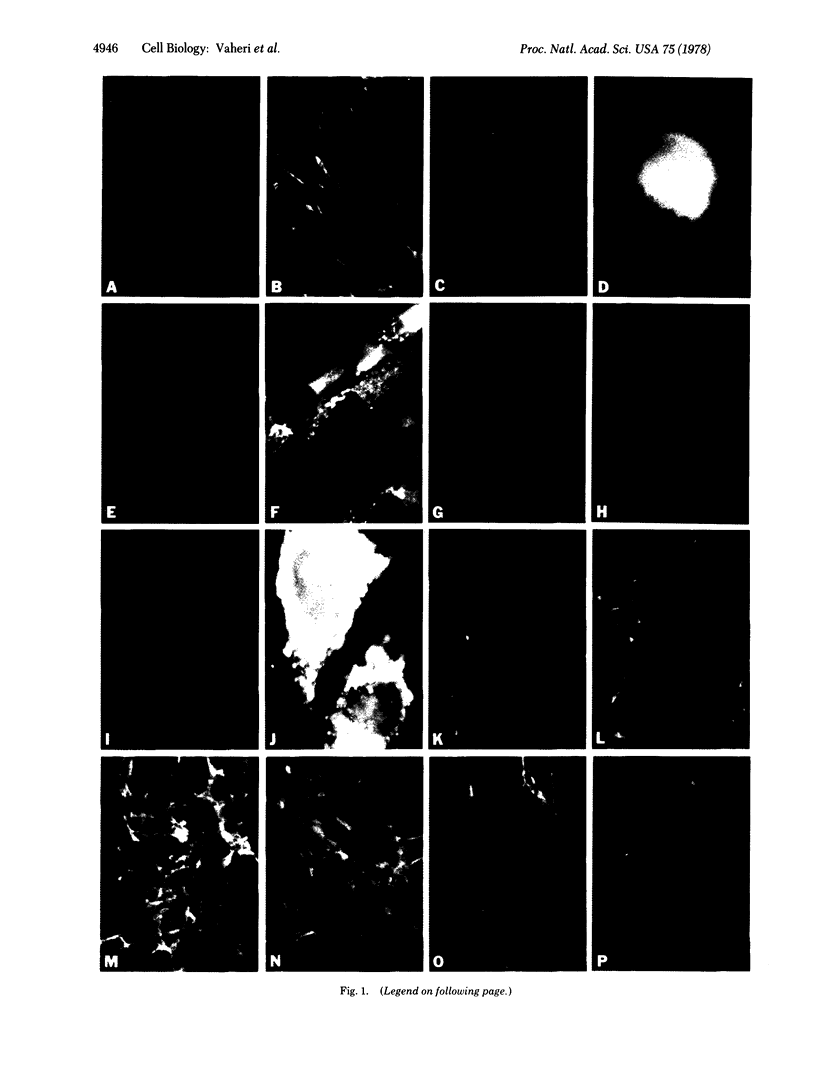
Antibodies to fibronectin and to distinct types of procollagens and collagens were used in immunofluorescent staining to localize these proteins in cell cultures. Normal human skin or lung fibroblasts produced a fibrillar pericellular matrix in which fibronectin and procollagen (types I and III) showed extensive codistribution. Fibronectin and procollagen were synthesized by the same cells as judged by double-stain immunofluorescence. Pericellular procollagen was specifically digested with collagenase without an effect on the fibrillar distribution of matrix fibronectin. Brief treatment with trypsin removed both matrix proteins. The human tumor cell lines HT-1080 (fibrosarcoma) and RD (rhabdomyosarcoma) produced little or no matrix fibronectin or procollagen. At sites of cell contact, simian virus 40-transformed lung fibroblasts (VA13) produced small amounts of pericellular fibrillar matrix fibronectin that codistributed with procollagen type I. Intracellular fibronectin and procollagen were visualized in all of these human sarcoma cell lines. When chicken embryo fibroblasts infected with a T class mutant (NY68) of Rous sarcoma virus temperature-sensitive for transformation were maintained at the nonpermissive temperature (41°) the cells had normal phenotype and a fibrillar matrix containing fibronectin and procollagen was present. At the permissive temperature (35°), the cells showed transformed phenotype and the matrix was lost. The failure to produce a pericellular fibronectin/collagen matrix may account for several phenotypic characteristics of transformed cultured fibroblasts.
Keywords: connective tissue matrix, cell surface, immunofluorescence, malignant transformation, cell adhesion
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast B. W., Yoshimura M., Kefalides N. A., Holtzer H., Kaji A. Failure of cultured chick embryo fibroblasts to incorporate collagen into their extracellular matrix when transformed by Rous sarcoma virus. An effect of transformation but not of virus production. J Biol Chem. 1977 Dec 25;252(24):8863–8868. [PubMed] [Google Scholar]
- Bergquist N. R., Nilsson P. The conjugation of immunoglobulins with tetramethylrhodamine isothiocyanate by utilization of dimethylsulfoxide (DMSO) as a solvent. J Immunol Methods. 1974 Jul;5(2):189–198. doi: 10.1016/0022-1759(74)90009-x. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Ash J. F. Cell surface-associated structural proteins in connective tissue cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2480–2484. doi: 10.1073/pnas.74.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., Adelmann B. C., Timpl R. Identification of the sites in collagen alpha-chains that bind serum anti-gelatin factor (cold-insoluble globulin). Biochem J. 1978 Jan 1;169(1):55–59. doi: 10.1042/bj1690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gay S., Martin G. R., Muller P. K., Timpl R., Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4037–4040. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Kinetics of processing of type I and type III procollagens in fibroblast cultures. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3322–3325. doi: 10.1073/pnas.74.8.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- Hedman K., Vaheri A., Wartiovaara J. External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol. 1978 Mar;76(3):748–760. doi: 10.1083/jcb.76.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Olsen B. R., Prokop D. J. Intracellular collagen and protocollagen from embryonic tendon cells. J Biol Chem. 1973 Jan 25;248(2):720–729. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B. Localization of the cell attachment region in types I and II collagens. Biochem Biophys Res Commun. 1976 Sep 20;72(2):426–432. doi: 10.1016/s0006-291x(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Linder E., Vaheri A., Ruoslahti E., Wartiovaara J. Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med. 1975 Jul 1;142(1):41–49. doi: 10.1084/jem.142.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Keski-Oja J., Vaheri A. Distribution of a major surface-associated glycoprotein, fibronectin, in cultures of adherent cells. J Supramol Struct. 1977;6(4):551–557. doi: 10.1002/jss.400060408. [DOI] [PubMed] [Google Scholar]
- Nowack H., Gay S., Wick G., Becker U., Timpl R. Preparation and use in immunohistology of antibodies specific for type I and type III collagen and procollagen. J Immunol Methods. 1976;12(1-2):117–124. doi: 10.1016/0022-1759(76)90101-0. [DOI] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell. 1977 Aug;11(4):957–969. doi: 10.1016/0092-8674(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Pontén J. The relationship between in vitro transformation and tumor formation in vivo. Biochim Biophys Acta. 1976 Dec 23;458(4):397–422. doi: 10.1016/0304-419x(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Stenman S., Wartiovaara J., Vaheri A. Changes in the distribution of a major fibroblast protein, fibronectin, during mitosis and interphase. J Cell Biol. 1977 Aug;74(2):453–467. doi: 10.1083/jcb.74.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. B., Goldberg B. The processing of procollagen in cultures of human and mouse fibroblasts. Arch Biochem Biophys. 1976 Apr;173(2):490–494. doi: 10.1016/0003-9861(76)90286-1. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wick G., Gay S. Antibodies to distinct types of collagens and procollagens and their application in immunohistology. J Immunol Methods. 1977;18(1-2):165–182. doi: 10.1016/0022-1759(77)90168-5. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Fibroblast surface antigen produced but not retained by virus-transformed human cells. J Exp Med. 1975 Aug 1;142(2):530–535. doi: 10.1084/jem.142.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Wartiovaara J., Linder E., Ruoslahti E., Vaheri A. Distribution of fibroblast surface antigen: association with fibrillar structures of normal cells and loss upon viral transformation. J Exp Med. 1974 Dec 1;140(6):1522–1533. doi: 10.1084/jem.140.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartiovaara J., Stenman S., Vaheri A. Changes in expression of fibroblast surface antigen (SFA) during cytodifferentiation and heterokaryon formation. Differentiation. 1976 Jun 4;5(2-3):85–89. doi: 10.1111/j.1432-0436.1976.tb00896.x. [DOI] [PubMed] [Google Scholar]