Abstract
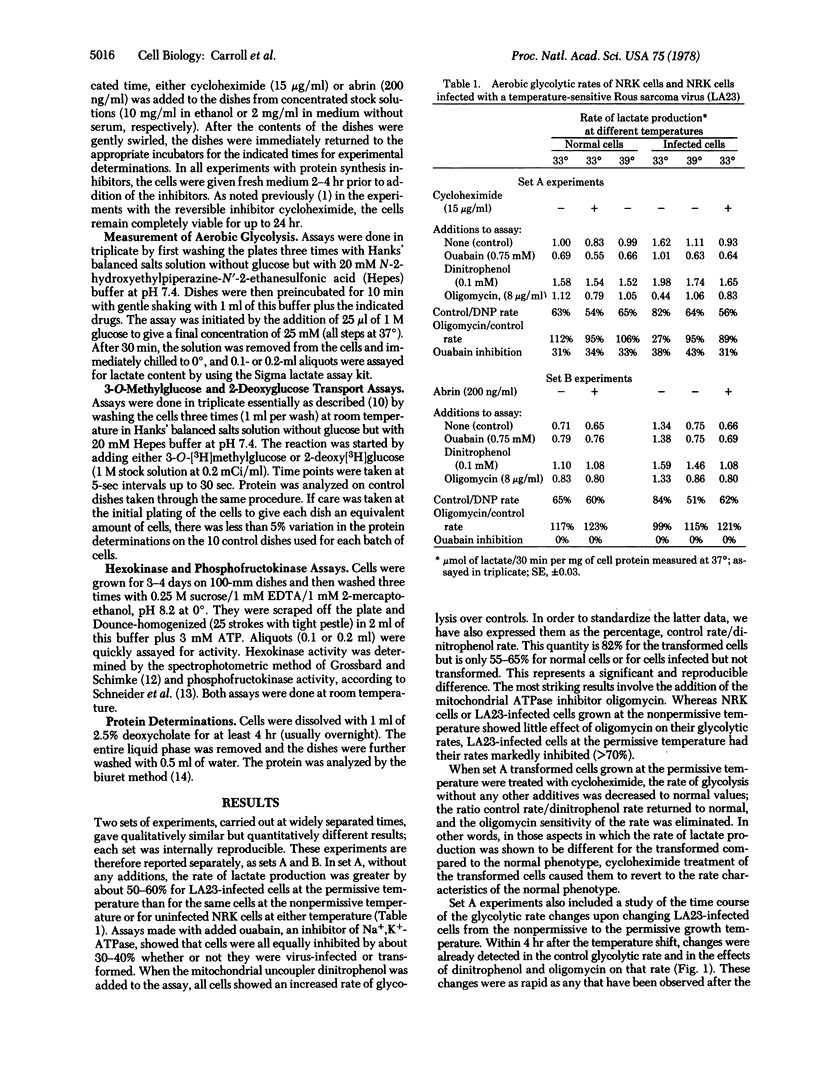
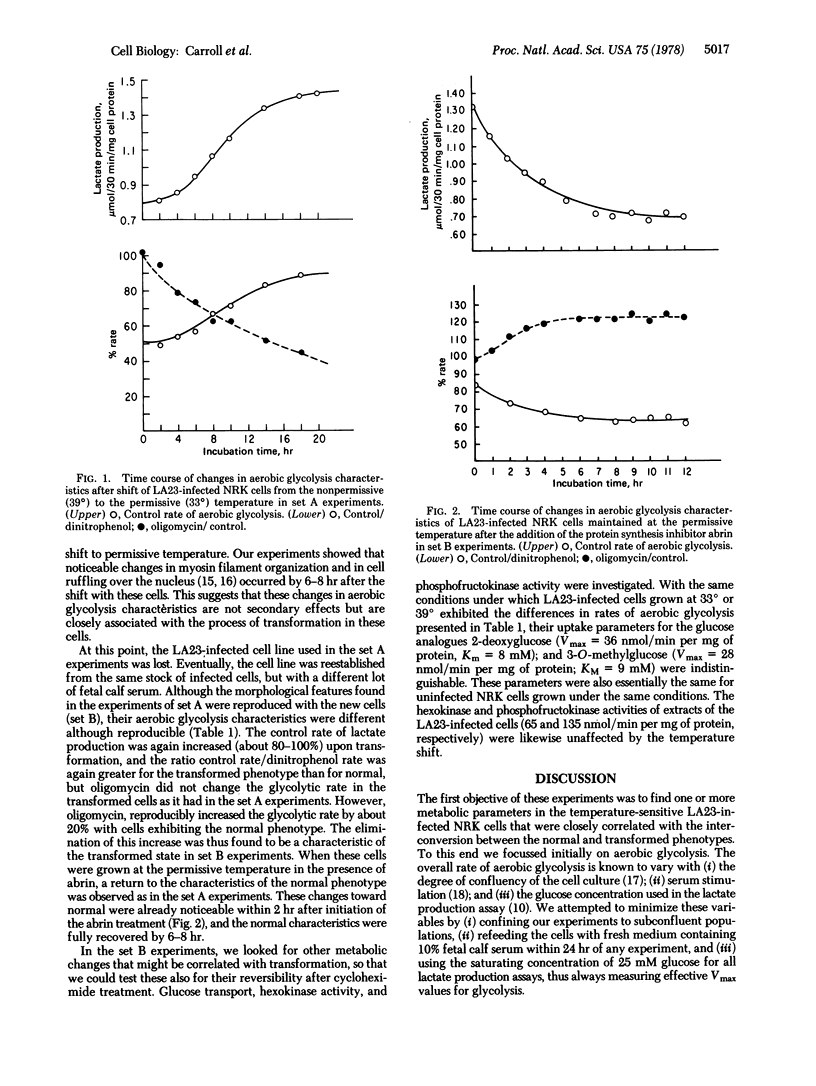
Normal rat kidney cells infected with a temperature-sensitive mutant (LA23) of Rous sarcoma virus exhibit the transformed phenotype when grown at 33 degrees and the normal phenotype at 39 degrees. We have previously shown [Ash, J.F., Vogt, P.K. & Singer, S.J. (1976) Proc. Natl. Acad. Sci. USA 73, 3603-3607] that the addition of protein synthesis inhibitors to LA23-infected cells grown at 33 degrees causes them to revert, over a period of 12 hr, to the normal phenotype with respect to morphological and cytoskeletal characteristics. We now show that reversion of the metabolic characteristics of the transformed phenotype to those of the normal also occurs under these conditions. LA23-infected cells show an increased rate of aerobic glycolysis at 33 degrees compared to that at 39 degrees. They also show a different sensitivity of that rate to dinitrophenol and oligomycin at 33 degrees compared to 39 degrees. Such cells grown at 33 degrees in the presence of cycloheximide or abrin rapidly recover the aerobic glycolysis characteristics of the normal phenotype. These results support the thesis that transformation by the src gene of the Rous sarcoma virus is a pleiotypic and reversible process, such as is involved in a pleiotypic enzymic modification reaction and its reversal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V. R., Chen L. B., Buchanan J. M. Surface ruffles as markers for studies of cell transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3144–3148. doi: 10.1073/pnas.72.8.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Bissell M. J., Hatié C., Rubin H. Patterns of glucose metabolism in normal and virus-transformed chick cells in tissue culture. J Natl Cancer Inst. 1972 Aug;49(2):555–565. [PubMed] [Google Scholar]
- Bissell M. J. Transport as a rate limiting step in glucose metabolism in virus-transformed cells: studies with cytochalasin B. J Cell Physiol. 1976 Dec;89(4):701–709. doi: 10.1002/jcp.1040890430. [DOI] [PubMed] [Google Scholar]
- Bustamante E., Pedersen P. L. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Hayman M. J., Vogt P. K. Properties of mammalian cells transformed by temperature-sensitive mutants of avian sarcoma virus. Cell. 1977 Jul;11(3):513–521. doi: 10.1016/0092-8674(77)90069-1. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M., Scheffler I. E. Energy metabolism in respiration-deficient and wild type Chinese hamster fibroblasts in culture. J Cell Physiol. 1976 Sep;89(1):39–51. doi: 10.1002/jcp.1040890105. [DOI] [PubMed] [Google Scholar]
- Fagan J. B., Racker E. Determinants of glycolytic rate in normal and transformed chick embryo fibroblasts. Cancer Res. 1978 Mar;38(3):749–758. [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Activation of phosphofructokinase by stimulants of cell multiplication. Nat New Biol. 1973 Dec 12;246(154):181–183. doi: 10.1038/newbio246181a0. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Regulation of sugar transport in chick embryo fibroblasts and in fibroblasts transformed by a temperature-sensitive mutant of the Rous sarcoma virus. J Cell Physiol. 1976 Dec;89(4):723–728. doi: 10.1002/jcp.1040890432. [DOI] [PubMed] [Google Scholar]
- Padgett T. G., Stubbledield E., Varmus H. E. Chicken macrochromosomes contain an endogenous provirus and microchromosomes contain sequences related to the transforming gene of ASV. Cell. 1977 Apr;10(4):649–657. doi: 10.1016/0092-8674(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Racker E. Why do tumor cells have a high aerobic glycolysis? J Cell Physiol. 1976 Dec;89(4):697–700. doi: 10.1002/jcp.1040890429. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Diamond I., Rozengurt E. Glycolysis of quiescent cultures of 3T3 cells. Addition of serum, epidermal growth factor, and insulin increases the activity of phosphofructokinase in a protein synthesis-independent manner. J Biol Chem. 1978 Feb 10;253(3):872–877. [PubMed] [Google Scholar]
- Scholnick P., Lang D., Racker E. Regulatory mechanisms in carbohydrate metabolism. IX. Stimulation of aerobic glycolysis by energy-linked ion transport and inhibition by dextran sulfate. J Biol Chem. 1973 Jul 25;248(14):5175–5175. [PubMed] [Google Scholar]
- Singh V. N., Singh M., August J. T., Horecker B. L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Suolinna E. M., Lang D. R., Racker E. Quercetin, an artificial regulator of the high aerobic glycolysis of tumor cells. J Natl Cancer Inst. 1974 Nov;53(5):1515–1519. doi: 10.1093/jnci/53.5.1515. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Hale A. H., Yau T. M., Buckman T., Johnson M., Brady T. M., LaRossa D. D. Transport changes associated with growth control and malignant transformation. J Cell Physiol. 1976 Dec;89(4):711–721. doi: 10.1002/jcp.1040890431. [DOI] [PubMed] [Google Scholar]
- Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972 Oct;32(10):2007–2016. [PubMed] [Google Scholar]


