Abstract
The induction of drug-metabolizing enzymes by chemicals is one of the major reasons for drug-drug interactions. In the present study, the regulation of mRNA expression of one arylacetamide deacetylase (Aadac) and 11 carboxylesterases (Cess) by 15 microsomal enzyme inducers (MEIs) was examined in livers of male C57BL/6 mice. The data demonstrated that Aadac mRNA expression was suppressed by three aryl hydrocarbon receptor (AhR) ligands, two constitutive androstane receptor (CAR) activators, two pregnane X receptor (PXR) ligands, and one nuclear factor erythroid 2-related factor 2 (Nrf2) activator. Ces1 subfamily mRNA expression was not altered by most of the MEIs, whereas Ces2 subfamily mRNA was readily induced by the activators of CAR, PXR, and Nrf2 but not by peroxisome proliferator-activated receptor α activators. Studies using null mice demonstrated that 1) AhR was required for the 2,3,7,8-tetrachlorodibenzo-p-dioxin–mediated suppression of Aadac and Ces3a; 2) CAR was involved in the 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene–mediated induction of Aadac, Ces2c, Ces2a, and Ces3a; 3) PXR was required for the pregnenolone-16α-carbonitrile–mediated induction of Aadac, Ces2c, and Ces2a; 4) Nrf2 was required for the oltipraz-mediated induction of Ces1g and Ces2c; and 5) PXR was not required for the DEX-mediated suppression of Cess in livers of mice. In conclusion, the present study systematically investigated the regulation of Cess by MEIs in livers of mice and demonstrated that MEIs modulated mRNA expression of mouse hepatic Cess through the activation of AhR, CAR, PXR, and/or Nrf2 transcriptional pathways.
Introduction
Carboxylesterases (CESs) (for rodents, Cess) catalyze the hydrolysis of many ester- and amide-containing chemicals, as well as drugs and prodrugs, to their respective free acids (Satoh and Hosokawa, 1998). Satoh and Hosokawa (1998, 2006) classified the CES/Ces enzymes across species into five groups, on the basis of amino acid homology and substrate selectivity, and revealed that the majority of identified CES/Ces enzymes belong to either the CES1 or CES2 subfamily. Arylacetamide deacetylase (AADAC) (for rodents, Aadac) is an important enzyme in the metabolic activation of arylamine substrates to ultimate carcinogens and also functions as a microsomal lipase during lipoprotein secretion (Probst et al., 1994; Trickett et al., 2001). AADAC/Aadac was categorized into the CES5 family, with a structure different from those of the other four CES families (Satoh and Hosokawa, 2006; Hosokawa et al., 2008).
Because of the confusion in naming rodent Ces genes, a new nomenclature system has been recommended for the five mammalian carboxylesterase gene families for human, mouse, and rat genes (Holmes et al., 2010). In the new nomenclature system, six human CES genes on human chromosome 16, 15 rat Ces genes on rat chromosome 19, and 20 mouse Ces genes on mouse chromosome 8 have been recognized and described (Holmes et al., 2010). AADAC/Aadac genes were not included in this new system, however, probably because of their different chromosomal location, compared with other CES/Ces genes.
CESs/Cess are found in a number of tissues, including liver, kidney, small intestine, heart, lung, brain, testis, nasal, respiratory, and adipose tissues, leukocytes, and blood (Satoh and Hosokawa, 1998). Among various tissues, the highest level of hydrolase activity is present in the liver. Microsomal enzyme inducers (MEIs) exert their effects on target genes through direct or indirect activation of transcription factors, such as the aryl hydrocarbon receptor (AhR), pregnane X receptor (PXR), constitutive androstane receptor (CAR), peroxisome proliferator-activated receptor α (PPARα), and nuclear factor erythroid 2-related factor 2 (Nrf2) (Handschin and Meyer, 2003; Numazawa and Yoshida, 2004). CES/Ces activity was shown previously to be induced by phenobarbital (PB), Aroclor 1254, polycyclic aromatic hydrocarbons, synthetic glucocorticoids, pregnenolone-16α-carbonitrile (PCN), and clofibrate (CLOF) (Satoh and Hosokawa, 1998). CAR and PXR were shown to play important roles in the regulation of mouse Cess (Staudinger et al., 2010). Potential transcriptional factors that depict binding sites in CES/Ces promoters include specificity protein 1 and 3, CCAAT/enhancer-binding protein, upstream stimulatory factor 1, neurofibromin 1, nuclear factor κB, PPARα, glucocorticoid receptor (GR), sterol regulatory element-binding protein, and hepatocyte nuclear factor 1, 3, and 4 binding sites (Satoh and Hosokawa, 2006).
CES enzymes are considered to be one of the major determinants of the metabolism and disposition of ester-containing drugs, through their actions in the liver and intestine. CES substrates include numerous clinically prescribed anticancer prodrugs, carbamate and pyrethroid insecticides, and environmental toxicants and procarcinogens (Hosokawa et al., 2008). Moreover, ester linkage methods are frequently used in rational drug design to target a prodrug or to improve the water solubility of a novel compound. Therefore, it is of clinical significance to investigate the regulation of CESs/Cess. Previous studies focused mainly on the regulation of human and rat CESs/Cess. However, information on the regulation of mouse Cess is limited. The purpose of the present study was to investigate systematically the regulation of Cess in livers of mice by prototypical MEIs that activate five transcription factors. The present study focused on one mouse Aadac gene and 11 mouse Ces genes that were investigated previously, including Ces1c (previously termed Es1) (Genetta et al., 1988), Ces1d (previously Ces3) (Dolinsky et al., 2001), Ces1e (previously Es22 or egasyn) (Ovnic et al., 1991), Ces1f (previously Es4 or TGH-2) (Poole et al., 2001), Ces1g (previously Ces1 or Est1) (Ellinghaus et al., 1998), Ces2a (previously Ces6) (Gerhard et al., 2004), Ces2b (previously Ces2A7) (Satoh and Hosokawa, 2006), Ces2c (previously Ces2) (Furihata et al., 2003), Ces2e (previously Ces5) (Gerhard et al., 2004), Ces3a (previously esterase 31 or Est31) (Aida et al., 1993), and Ces5a (previously Ces7) (Miyazaki et al., 2003).
Materials and Methods
Chemicals.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a kind gift from Dr. Karl Rozman (University of Kansas Medical Center, Kansas City, KS). Oltipraz (OPZ) was purchased from LKT Labs (St. Paul, MN). Polychlorinated biphenyl (PCB) 126 was obtained from AccuStandard (New Haven, CT). β-Naphthoflavone (BNF), diallyl sulfide (DAS), CLOF, di(2-ethylhexyl) phthalate, ethoxyquin (ETH), dexamethasone (DEX), PCN, ciprofibrate (CPFB), butylated hydroxyanisole (BHA), spironolactone (SPR), PB, and 1,4-bis[2-(3,5-dichloropuridyloxy)]benzene (TCPOBOP) were purchased from Sigma-Aldrich (St. Louis, MO). Sodium chloride, HEPES sodium salt, HEPES free acid, lithium lauryl sulfate, EDTA, and d-(+)-glucose were purchased from Sigma-Aldrich. Micro-O-protect was purchased from Roche Diagnostics (Indianapolis, IN). Chloroform, agarose, and ethidium bromide were purchased from AMRESCO (Solon, OH). RNA Bee was purchased from Tel-Test Inc. (Friendswood, TX). All other chemicals, unless indicated, were purchased from Sigma-Aldrich.
Animals.
Eight-week-old, male, C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Male AhR-null mice (>99% congenic for C57BL/6J background) from The Jackson Laboratory (stock no. 002831) were described previously (Schmidt et al., 1996). Breeding pairs of CAR-null mice in the C57BL/6 background were obtained as described previously (Ueda et al., 2002). Breeding pairs of PXR-null mice (Staudinger et al., 2001) in the C57BL/6 background were kindly provided by Dr. Frank J. Gonzalez (National Institutes of Health National Cancer Institute, Bethesda, MD). PPARα-null mice, which were engineered originally in the laboratory of Dr. Frank J. Gonzalez (Lee et al., 1995), were backcrossed to a C57BL/6 background (Akiyama et al., 2001). Breeding pairs of Nrf2-null mice on a mixed C57BL/6/AKR background were provided by Dr. Jefferson Chan (University of California, Irvine, CA) (Chan et al., 1996) and were backcrossed to a C57BL/6 background in our animal care facility. All mice were housed in an American Animal Associations Laboratory Animal Care–accredited facility, with a 12-h light/dark cycle, and were provided chow and water ad libitum.
Microsomal Enzyme Inducer Treatment.
Adult (∼8-week-old), male, C57BL/6 mice were treated with five groups of prototypical drug-metabolizing enzyme inducers, as described previously (Cheng et al., 2005). Each class contained three chemicals that are known to activate a specific signaling pathway. The doses and dosing regimens for these compounds were shown previously to induce the corresponding target genes in mice, namely, Cyp1A1 for AhR ligands, Cyp2B1 for CAR activators, Cyp3A11 for PXR ligands, Cyp4A14 for PPARα ligands, and NAD(P)H:quinine oxidoreductase 1 for Nrf2 activators (Cheng et al., 2005). Similar inductions were observed in the present study (Supplemental Fig. 1).
In another experiment, mice in five knockout mouse models and the corresponding wild-type mice (n = 5 per group) were treated with one of five prototypical MEIs once daily for 4 days, as follows: AhR-null mice, TCDD (40 μg/kg i.p. in corn oil); CAR-null mice, TCPOBOP (300 μg/kg i.p. in corn oil); PXR-null mice, PCN (200 mg/kg i.p. in corn oil); PPARα-null mice, CLOF (500 mg/kg i.p. in saline solution); Nrf2-null mice, OPZ (150 mg/kg p.o. in corn oil). Livers were collected on day 5, frozen in liquid nitrogen, and stored at −80°C.
Low-Dose and High-Dose Dexamethasone Treatment.
DEX at higher doses (20–50 mg/kg) was shown to activate PXR in vivo (Xie et al., 2000), whereas lower doses of DEX (2–6 mg/kg) had little effect on PXR activation (Heuman et al., 1982). To investigate the mechanism of DEX in the regulation of mouse Cess, both a low dose (2 mg/kg daily i.p. for 2 days) and a high dose (50 mg/kg daily i.p. for 1 day) of DEX were administered to wild-type (WT) and PXR-null mice.
Total RNA Isolation.
Total RNA was isolated by using RNA-Bee reagent (Tel-Test Inc.), according to the manufacturer's protocol. Total RNA concentrations were quantified spectrophotometrically at 260 nm, and purity was confirmed on the basis of 260/280-and 260/230-nm ratios. Total RNA was diluted to 1 μg of total RNA per microliter with diethyl pyrocarbonate-treated deionized water. The integrity of RNA samples was determined through formaldehyde-agarose gel electrophoresis, with visualization with ethidium bromide fluorescence monitoring under UV light.
Branched DNA Signal Amplification Assay.
Mouse Ces mRNA expression was quantified by using a branched DNA assay (QuantiGene branched DNA signal amplification kit; Panomics, Fremont, CA). The GenBank accession numbers of all genes, the sequences, and the functions of the probe sets for each mouse CES are shown in Supplemental Table 1. Reagents required for RNA analysis were supplied in the QuantiGene branched DNA signal amplification kit (Bayer Diagnostics, East Walpole, MA). The mRNAs were analyzed as described previously (Hartley and Klaassen, 2000). Data are presented as relative light units per 8 μg of total RNA.
Multiplex Suspension Array.
Mouse Ces mRNA expression from the chemical treatment experiment involving both WT and knockout mice was determined with a Mutiplex suspension array (Panomics). Individual gene accession numbers can be accessed at www.panomics.com (set no. 21086). Samples were analyzed by using a Bio-Plex 200 system array reader with Luminex 100 xMAP technology (Bio-Rad Laboratories, Hercules, CA), and the data were acquired by using Bio-Plex Data Manager 5.0 (Bio-Rad Laboratories). Assays were performed according to each manufacturer's protocol. RNA data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels and are presented as relative light units.
Statistical Analysis.
Data were analyzed with one-way analysis of variance, followed by Duncan's post hoc test. Statistical significance was set at p < 0.05. Bars represent mean ± S.E.M. (n = 5).
Results
Regulation of Mouse Aadac, Ces1c, Ces1d, Ces1e, Ces1f, and Ces1g.
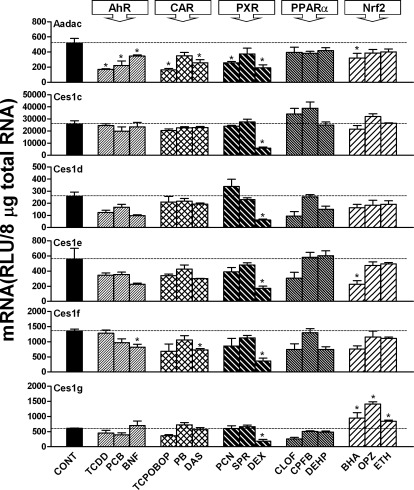
As shown in Fig. 1, Aadac mRNA was highly expressed in mouse livers and was suppressed by all three AhR ligands, i.e., TCDD (67%), PCB (57%), and BNF (33%); two CAR activators, i.e., TCPOBOP (68%) and DAS (50%); two PXR ligands, i.e., PCN (50%) and DEX (63%); and the Nrf2 activator BHA (38%). Ces1c expression was decreased by the PXR ligand DEX (75%). Ces1d expression was decreased 75% by the PXR ligand DEX. Ces1e expression was decreased ∼70% by the PXR ligand DEX and the Nrf2 activator BHA. Ces1f expression was decreased by the AhR ligand BNF (40%), the CAR activator DAS (47%), and the PXR ligand DEX (74%). Ces1g expression was decreased ∼70% by the PXR ligand DEX but was increased by all three Nrf2 activators, i.e., BHA (56%), OPZ (132%), and ethoxyquin (38%).
Fig. 1.
mRNA expression of Aadac, Ces1c, Ces1d, Ces1e, Ces1f, and Ces1g in mouse livers after treatment with prototypical drug-metabolizing enzyme inducers. Total RNA from livers of treated male C57BL/6 mice (n = 5 per treatment) was analyzed with the branched DNA assay. All data are expressed as mean ± S.E. for five mice in each group except for control groups, for which data were combined from the four individual control groups after it was determined that they were not statistically different. *, statistically significant differences between control and treated mice (p < 0.05). CONT, control.
Regulation of Mouse Ces2a, Ces2b, Ces2c, Ces2e, Ces3a, and Ces5a.
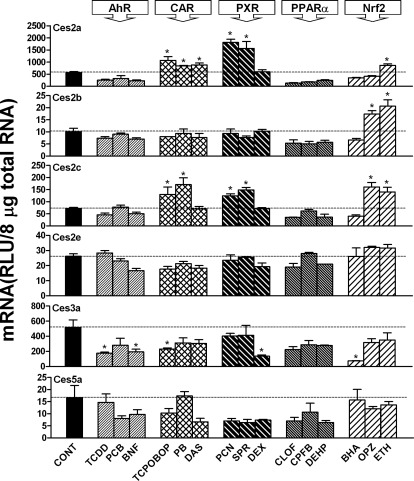
As shown in Fig. 2, Ces2a expression was increased by all three CAR activators, i.e., TCPOBOP (90%), PB (49%), and DAS (57%); two PXR ligands, i.e., PCN (222%) and SPR (177%); and the Nrf2 activator ETH (53%). Ces2b was minimally expressed in livers of mice, and expression was induced by two Nrf2 activators, i.e., OPZ (70%) and ETH (100%). Ces2c expression was increased markedly by two CAR activators, i.e., TCPOBOP (85%) and PB (150%); two PXR ligands, i.e., PCN (70%) and SPR (90%); and two Nrf2 activators, i.e., OPZ (140%) and ETH (110%). Ces2e was minimally expressed in livers of mice, and expression was not altered by any MEI. Ces3a expression was decreased by two AhR ligands, i.e., TCDD (75%) and BNF (63%); the CAR activator TCPOBOP (56%); the PXR ligand DEX (74%); and the Nrf2 activator BHA (86%). Ces5a was minimally expressed in mouse livers, and expression was not altered by any of the MEIs. Because of the minimal expression in liver, the regulation of Ces2b, Ces2e, and Ces5a was not investigated further in the present study.
Fig. 2.
mRNA expression of Ces2a, Ces2b, Ces2c, Ces2e, Ces3a, and Ces5a in mouse livers after treatment with prototypical drug-metabolizing enzyme inducers. Total RNA from livers of treated male C57BL/6 mice (n = 5 per treatment) was analyzed with the branched DNA assay. All data are expressed as mean ± S.E. for five mice in each group except for control groups, for which data were combined from the four individual control groups after it was determined that they were not statistically different. *, statistically significant differences between control and treated mice (p < 0.05). CONT, control.
Effects of TCDD on Aadac and Ces3a mRNA Expression in Livers of WT and AhR-Null Mice.
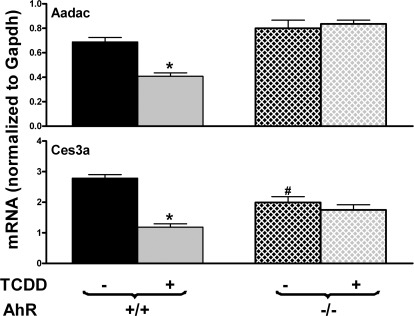
To determine whether the inhibition of Aadac and Ces3a by TCDD was dependent on AhR, both WT and AhR-null mice were treated with TCDD. As shown in Fig. 3, Aadac was similarly expressed in control WT and AhR-null mice. TCDD decreased Aadac expression ∼40% in WT mice but not in AhR-null mice. Ces3a exhibited lower expression in AhR-null mice than in WT mice. TCDD suppressed Ces3a expression approximately 57% in WT mice but not in AhR-null mice. Therefore, TCDD-mediated inhibition of Aadac and Ces3a expression is AhR-dependent.
Fig. 3.
Effects of TCDD on mRNA expression of Aadac and Ces3a in livers of WT and AhR-null mice. Total RNA from livers of control and TCDD-treated, male, C57BL/6 and AhR-null mice (n = 5 per group) was analyzed with the QuantiGene Plex assay. Data are reported as the carboxylesterase mRNA/GAPDH mRNA expression ratio in 500 ng of total RNA. All data are expressed as mean ± S.E. *, statistically significant differences between control and treated mice of the same genotype (p < 0.05); #, statistically significant differences between control WT and control null mice (p < 0.05).
Effects of TCPOBOP on Aadac, Ces2a, Ces2c, and Ces3a mRNA Expression in Livers of WT and CAR-Null Mice.
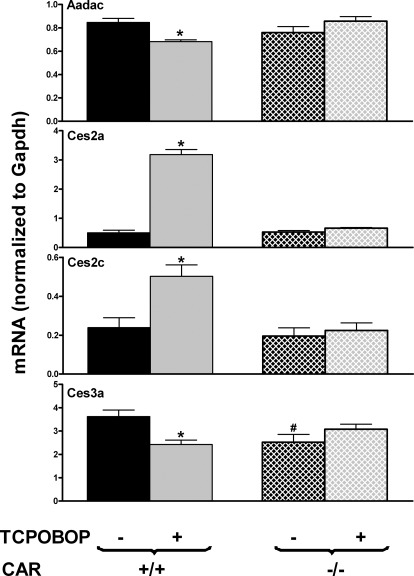
To determine whether TCPOBOP regulation of Aadac, Ces2a, Ces2c, and Ces3a was dependent on CAR, both WT and CAR-null mice were treated with TCPOBOP. As shown in Fig. 4, Aadac, Ces2a, and Ces2c exhibited similar expression in WT and CAR-null mice, whereas Ces3a expression was lower in CAR-null mice. Aadac and Ces3a expression was suppressed by TCPOBOP in WT mice but not in CAR-null mice. TCPOBOP strongly induced Ces2a and Ces2c expression in WT mice but not in CAR-null mice. Taken together, these findings indicate that CAR is required for the TCPOBOP-mediated suppression of Aadac and Ces3a expression and the induction of Ces2a and Ces2c expression in livers of mice.
Fig. 4.
Effects of TCPOBOP on mRNA expression of Aadac, Ces2a, Ces2c, and Ces3a in livers of WT and CAR-null mice. Total RNA from livers of control and TCPOBOP-treated, male, C57BL/6 and CAR-null mice (n = 5 per group) was analyzed with the QuantiGene Plex assay. Data are reported as the carboxylesterase mRNA/GAPDH mRNA expression ratio in 500 ng of total RNA. All data are expressed as mean ± S.E. *, statistically significant differences between control and treated mice of the same genotype (p < 0.05); #, statistically significant differences between control WT and control null mice (p < 0.05).
Effects of PCN on Aadac, Ces2a, and Ces2c mRNA Expression in Livers of WT and PXR-Null Mice.
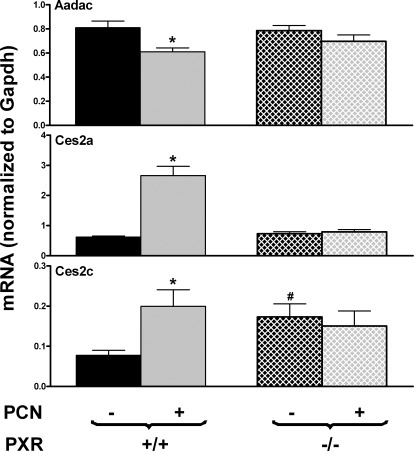
To determine the role of PXR in PCN-mediated regulation of Aadac, Ces2a, and Ces2c, both WT and PXR-null mice were treated with PCN. As shown in Fig. 5, Aadac and Ces2a were similarly expressed in livers of WT and PXR-null mice, whereas Ces2c expression was higher in livers of PXR-null mice than WT mice. Aadac expression was decreased by PCN in WT mice but not in PXR-null mice. PCN increased Ces2a and Ces2c expression in WT mice but not in PXR-null mice. Therefore, PXR is required for the PCN-mediated suppression of Aadac expression and the induction of Ces2a and Ces2c expression in livers of mice.
Fig. 5.
Effects of PCN on mRNA expression of Aadac, Ces2a, and Ces2c in livers of WT and PXR-null mice. Total RNA from livers of control and PCN-treated, male, C57BL/6 and PXR-null mice (n = 5 per group) was analyzed with the QuantiGene Plex assay. Data are reported as the carboxylesterase mRNA/GAPDH mRNA expression ratio in 500 ng of total RNA. All data are expressed as mean ± S.E. *, statistically significant differences between control and treated mice of the same genotype (p < 0.05); #, statistically significant differences between control WT and control null mice (p < 0.05).
Effects of OPZ on Ces1g and Ces2c mRNA Expression in Livers of WT and Nrf2-Null Mice.
To determine whether the induction of Ces1g and Ces2c expression by OPZ was Nrf2-dependent, both WT and Nrf2-null mice were treated with OPZ. Ces1g and Ces2c mRNA levels were lower in Nfr2-null mice than in WT mice (Fig. 6). OPZ increased Ces1g and Ces2c expression in WT mice but not in Nrf2-null mice. Therefore, OPZ-mediated induction of Ces1g and Ces2c is Nrf2-dependent.
Fig. 6.
Effects of OPZ on mRNA expression of Ces1g and Ces2c in livers of WT and Nrf2-null mice. Total RNA from livers of control and OPZ-treated, male, C57BL/6 and Nrf2-null mice (n = 5 per group) was analyzed with the QuantiGene Plex assay. Data are reported as the carboxylesterase mRNA/GAPDH mRNA expression ratio in 500 ng of total RNA. All data are expressed as mean ± S.E. *, statistically significant differences between control and treated mice (p < 0.05); #, statistically significant differences between control WT and control null mice (p < 0.05).
Effects of Low-Dose and High-Dose DEX on Aadac, Ces1c, Ces1d, Ces1e, Ces1f, Ces1g, and Ces3a mRNA Expression in Livers of WT and PXR-Null Mice.
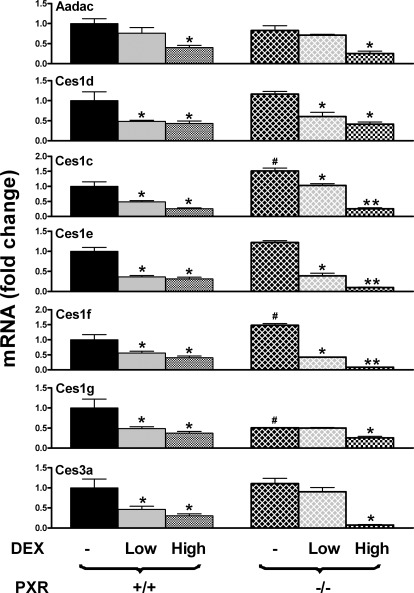
Both PXR and GR were shown to be activated by DEX (Shi et al., 2008). Activation of PXR requires micromolar concentrations of DEX, whereas activation of GR requires nanomolar concentrations. In the present study, WT and PXR-null mice were treated with DEX at both low and high doses. Aadac, Ces1d, Ces1e, and Ces3a were similarly expressed in control WT and control PXR-null mice (Fig. 7). In contrast, PXR-null mice had lower Ces1g levels but higher Ces1c and Ces1f levels than did WT mice. Aadac expression was suppressed by high-dose but not low-dose DEX in WT mice. In contrast, Ces1c, Ces1d, Ces1e, Ces1f, Ces1g, and Ces3a expression was suppressed by both low-dose and high-dose DEX in WT mice. Aadac, Ces1g, and Ces3c expression was suppressed by high-dose but not low-dose DEX in PXR-null mice. In contrast, Ces1c, Ces1d, Ces1e, and Ces1f expression was suppressed by both low- and high-dose DEX in PXR-null mice. It is noteworthy that Ces1c, Ces1d, Ces1e, and Ces1f expression was suppressed more by high-dose DEX than by low-dose DEX in PXR-null mice. Taken together, PXR does not seem to be involved in the DEX-mediated regulation of Cess in livers of mice.
Fig. 7.
Effects of low-dose (2 mg/kg) and high-dose (50 mg/kg) DEX on mRNA expression of Aadac, Ces1c, Ces1d, Ces1e, Ces1f, Ces1g, and Ces3a in livers of WT and PXR-null mice. Wild-type and PXR-null mice were treated with both a low dose (2 mg/kg daily i.p. for 2 days) and a high dose (50 mg/kg daily i.p. for 1 day) of DEX. Total RNA from livers of control and DEX-treated, male, C57BL/6 and PXR-null mice (n = 5 per group) was analyzed with the branched DNA assay. The data are reported as the mRNA fold change with respect to the carboxylesterase mRNA of control WT mice. All data are expressed as mean ± S.E. *, statistically significant differences between treated and control mice of the same genotype (p < 0.05); **, statistically significant differences between low-dose and high-dose DEX-treated mice (p < 0.05); #, statistically significant differences between control WT and control null mice (p < 0.05).
Discussion
The majority of identified CES/Ces enzymes belong to either the CES1 or CES2 subfamily (Satoh and Hosokawa, 2006). The CES1 subfamily mainly hydrolyzes substrates with small alcohol and large acyl groups, whereas the CES2 subfamily prefers to hydrolyze substrates with large alcohol or small acyl groups. The present study demonstrates that the mRNA expression of the Ces1 subfamily is generally not altered by most MEIs, whereas the mRNA expression of the Ces2 subfamily is readily induced by CAR, PXR, and Nrf2 ligands in the livers of mice. This suggests that MEIs increase the liver hydrolysis of ester-containing drugs, particularly those with large alcohol and small acyl groups, such as irinotecan, which is a carbamate prodrug used in the treatment of colorectal cancers (Humerickhouse et al., 2000). Of the 11 Cess investigated, Ces2b, Ces2e, and Ces5a were minimally expressed in livers of mice and their expression was not altered by most of the MEIs, except that Ces2b expression was induced by two Nrf2 ligands, OPZ and ETH. Because of the minimal expression of these Cess in liver, we did not investigate their regulatory mechanisms further.
AADAC was purified from human liver in studies of the metabolism of carcinogens and was reported to be involved in the hydrolysis of various clinically therapeutic drugs, such as flutamide, phenacetin, and rifamycins (Probst et al., 1994; Watanabe et al., 2009, 2010; Nakajima et al., 2011). In the present study, mouse liver Aadac mRNA was suppressed by three AhR ligands, two CAR ligands, two PXR ligands, and one Nrf2 activator. The suppression of Aadac by TCDD, TCPOBOP, and PCN was dependent on AhR, CAR, and PXR, respectively. Trickett et al. (2001) reported that two PPARα ligands, i.e., fibrate and Wy-14,643, increased Aadac mRNA levels in the intestines but not livers of mice. Consistently, the present findings demonstrate that Aadac mRNA levels in livers of mice are not altered by any of three PPARα ligands.
In general, AhR ligands have little effect on most Cess in livers of mice. It was reported that the AhR ligand PCB (Aroclor 1254) tended to decrease rat Ces activities in both whole liver and liver microsomes (Carr et al., 2002). In contrast, the present data indicated that PCB had no effect on mouse liver Cess. The AhR ligands BNF and TCDD were reported to decrease Ces1f mRNA levels in livers of rats (Morgan et al., 1994; Yang et al., 2001). In the present study, mouse liver Ces1f mRNA expression was suppressed by BNF but was not altered by TCDD. It is noteworthy that both BNF and TCDD suppressed mouse liver Ces3a expression, and the TCDD-mediated suppression of Ces3a expression was AhR-dependent. Ces3a (or Es31) is expressed predominantly in male mouse livers (Aida et al., 1993). There is little information on the substrate specificity of CES3/Ces3a. However, the pig homolog of Ces3a was shown to be involved in the hydrolysis of indomethacin, a drug commonly used to reduce fever, pain, and swelling (Terashima et al., 1996). Therefore, AhR ligands in livers of mice may decrease the hydrolytic activity toward substrates such as indomethacin.
CAR and PXR are two closely related, xenobiotic-sensing, nuclear receptors that are expressed mainly in hepatic tissue. The CAR activator PB has been shown to induce hepatic microsomal CES activity in mice and the activity of two Ces isozymes in rat liver microsomes (Ketterman et al., 1987; Hosokawa et al., 1988). Compared with CAR, a critical role of PXR in the regulation of CES gene expression in both humans and rodents has been suggested in many studies. PXR is implicated in the induction of human CES2 by 8-methoxypsoralen (Yang and Yan, 2007). PCN markedly induces the expression of rat Ces1e (also known as ES-3, Egasin, or Ces RL2) (Hosokawa et al., 1993). Overexpression of constitutively active human PXR has a positive effect on Ces gene expression in livers of mice (Rosenfeld et al., 2003). The present study demonstrated that both Ces2a and Ces2c in livers of mice were induced by CAR and PXR ligands, and the induction by TCPOBOP and PCN was dependent on CAR and PXR, respectively. Xu et al. (2009) also reported that Ces2a is a target gene of both CAR and PXR in livers of mice. Together with numerous other drug-metabolizing enzymes and drug transporters, CAR and PXR regulate the expression of key CES enzymes that coordinately determine the pharmacokinetic and pharmacodynamic properties of drugs and other xenobiotics.
PPARα is one of three subtypes of PPARs and is expressed predominantly in tissues with high oxidative capacity, such as liver and heart. Feeding of clofibrate, a PPARα ligand, has little effect on the expression of Ces1d (also known as TGH) in livers of wild-type or PPARα-null mice (Dolinsky et al., 2003). This is consistent with the present study. Poole et al. (2001) showed that Ces1f (also known as Es-4) was down-regulated by the peroxisome proliferator Wy-14,643. In the present study, Ces1f mRNA levels in livers of male mice tended to be decreased by two PPARα ligands, i.e., CLOF and DEHP, but not significantly. Ces1f exhibited higher levels of expression in livers of female mice, compared with male mice (Supplemental Fig. 2a). It is noteworthy that the PPARα ligand ciprofibrate decreased Ces1f mRNA expression in a PPARα-dependent manner in livers of female but not male mice (Supplemental Fig. 2b). Feeding of 2% DEHP in the diet for 7 days significantly increased the Ces protein and hydrolytic activity levels in mouse liver microsomes (Hosokawa et al., 1994). Furihata et al. (2004) reported that Ces2c (also known as mCES2) is induced by DEHP. In contrast, in the present study, DEHP had little effect on Cess expression in livers of mice. This might be attributable to the shorter-term (4-day) treatment in the present study. Taken together, these findings indicated that PPARα ligands had little effect on the levels of most Cess, which was confirmed with inducer treatment in PPARα-null mice (Supplemental Fig. 3).
Nrf2 is the most important regulator of antioxidant proteins and phase II enzymes that protect against oxidative stress and electrophiles in cells. The Nrf2 ligand BHA induces CES1 expression in human hepatocytes (Takakusa et al., 2008). Maruichi et al. (2010) reported that human CES1 (also known as CES1A1) is induced by tert-butylhydroquinone and sulforaphane in a Nrf2-dependent manner. Sulforaphane has been shown to induce CES activity moderately in livers of WT but not Nrf2-null mice (Thimmulappa et al., 2002). The Nrf2 activator BHA increases microsomal Ces activity in mice (Ketterman et al., 1987). The present study demonstrated that the Nrf2 ligand BHA increased Ces1g expression but decreased Ces1e and Ces3a expression in mouse livers. Our previous study demonstrated that Ces1g and Ces2c levels were lower in Nrf2-null mice and higher in Keap1-knockdown mice (Nrf2 activated) than in wild-type mice (Reisman et al., 2009). Consistently, the Nrf2 ligand OPZ induced Ces1g and Ces2c expression in mouse livers in a Nrf2-dependent manner. It is noteworthy that whereas OPZ had no effect on Ces2a or Ces3a expression in livers of male mice, OPZ increased Ces2a expression and decreased Ces3a expression in livers of female WT mice but not female Nrf2-null mice (Supplemental Fig. 4).
Species differences have been observed in DEX regulation of CESs. DEX treatment decreased expression of Ces1d (also known as Es-10) and Ces1f (also known as Es-4 or hydrolase B) in rat livers, whereas it slightly increased expression of human CES1 and CES2 in cultured human hepatocytes (Zhu et al., 2000; Furihata et al., 2005). In the present study, DEX suppressed almost all of the Ces1 isozymes, as well as Ces3a, in livers of mice. Both PXR and GR are known to mediate DEX regulation of rat Cess, and the concentration of DEX determines the involvement of PXR and/or GR in the suppression of rat Ces1d and Ces1f (Shi et al., 2008). To determine the role of PXR in DEX suppression of mouse Cess, both a low dose (2 mg/kg) and a high dose (50 mg/kg) of DEX were administered to WT and PXR-null mice. Low-dose DEX is proposed to activate the GR, whereas high-dose DEX is able to activate both the GR and PXR. Low-dose DEX tended to increase Cyp3a11 expression (but not significantly), whereas high-dose DEX significantly increased Cyp3a11 expression in livers of WT mice (Supplemental Fig. 5). Neither low-dose nor high-dose DEX had effects on Cyp3a11 in livers of PXR-null mice (Supplemental Fig. 5). This suggests that high-dose DEX is able to induce Cy3a11 in a PXR-dependent manner. High-dose DEX suppressed Aadac, Ces1c, Ces1d, Ces1e, Ces1f, Ces1g, and Ces3a expression in both WT and PXR-null mice, which suggests that PXR may not be required for the DEX-mediated suppression of Cess in livers of mice.
The current study provides important insights into the chemical regulation of mouse liver Cess. Cess in livers of mice are shown to be regulated by AhR, CAR, PXR, Nrf2, and PPARα. Future studies with primary human hepatocytes should be performed to determine whether these five signaling pathways are conserved in humans. To conclude, the present study on the inductive abilities and regulatory mechanisms of MEIs for CESs/Cess should aid in rational drug design and the development of novel prodrugs and should be clinically beneficial for predicting drug-drug interactions and clarifying the cause of individual responses to various drugs.
Supplementary Material
Acknowledgments
We thank the members of Dr. Klaassen's laboratory group for their assistance in tissue collection and manuscript review.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES009649, ES019487, ES081461].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- CES
- carboxylesterase
- AADAC
- arylacetamide deacetylase
- AhR
- aryl hydrocarbon receptor
- BHA
- butylated hydroxyanisole
- BNF
- β-naphthoflavone
- CAR
- constitutive androstane receptor
- CLOF
- clofibric acid
- DAS
- diallyl sulfide
- DEHP
- diethylhexylphthalate
- DEX
- dexamethasone
- ETH
- ethoxyquin
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GR
- glucocorticoid receptor
- MEI
- microsomal enzyme inducer
- Nrf2
- nuclear factor erythroid 2-related factor 2
- OPZ
- oltipraz
- PB
- phenobarbital
- PCB
- polychlorinated biphenyl
- PCN
- pregnenolone-16α-carbonitrile
- PXR
- pregnane X receptor
- PPARα
- peroxisome proliferator-activated receptor α
- SPR
- spironolactone
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- TCPOBOP
- 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
- WT
- wild type.
Authorship Contributions
Participated in research design: Zhang, Cheng, and Klaassen.
Conducted experiments: Zhang, Cheng, and Aleksunes.
Contributed new reagents or analytic tools: Cheng and Aleksunes.
Performed data analysis: Zhang, Cheng, and Aleksunes.
Wrote or contributed to the writing of the manuscript: Zhang, Cheng, and Klaassen.
References
- Aida K, Moore R, Negishi M. (1993) Cloning and nucleotide sequence of a novel, male-predominant carboxylesterase in mouse liver. Biochim Biophys Acta 1174:72–74 [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, Lee SS, Gonzalez FJ, Peters JM. (2001) Peroxisome proliferator-activated receptor-α regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. J Biol Chem 276:39088–39093 [DOI] [PubMed] [Google Scholar]
- Carr RL, Richardson JR, Guarisco JA, Kachroo A, Chambers JE, Couch TA, Durunna GC, Meek EC. (2002) Effects of PCB exposure on the toxic impact of organophosphorus insecticides. Toxicol Sci 67:311–321 [DOI] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. (1996) NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA 93:13943–13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. (2005) Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33:1276–1282 [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Gilham D, Hatch GM, Agellon LB, Lehner R, Vance DE. (2003) Regulation of triacylglycerol hydrolase expression by dietary fatty acids and peroxisomal proliferator-activated receptors. Biochim Biophys Acta 1635:20–28 [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Sipione S, Lehner R, Vance DE. (2001) The cloning and expression of a murine triacylglycerol hydrolase cDNA and the structure of its corresponding gene. Biochim Biophys Acta 1532:162–172 [DOI] [PubMed] [Google Scholar]
- Ellinghaus P, Seedorf U, Assmann G. (1998) Cloning and sequencing of a novel murine liver carboxylesterase cDNA. Biochim Biophys Acta 1397:175–179 [DOI] [PubMed] [Google Scholar]
- Furihata T, Hosokawa M, Fujii A, Derbel M, Satoh T, Chiba K. (2005) Dexamethasone-induced methylprednisolone hemisuccinate hydrolase: its identification as a member of the rat carboxylesterase 2 family and its unique existence in plasma. Biochem Pharmacol 69:1287–1297 [DOI] [PubMed] [Google Scholar]
- Furihata T, Hosokawa M, Koyano N, Nakamura T, Satoh T, Chiba K. (2004) Identification of di-(2-ethylhexyl) phthalate-induced carboxylesterase 1 in C57BL/6 mouse liver microsomes: purification, cDNA cloning, and baculovirus-mediated expression. Drug Metab Dispos 32:1170–1177 [DOI] [PubMed] [Google Scholar]
- Furihata T, Hosokawa M, Nakata F, Satoh T, Chiba K. (2003) Purification, molecular cloning, and functional expression of inducible liver acylcarnitine hydrolase in C57BL/6 mouse, belonging to the carboxylesterase multigene family. Arch Biochem Biophys 416:101–109 [DOI] [PubMed] [Google Scholar]
- Genetta TL, D'Eustachio P, Kadner SS, Finlay TH. (1988) cDNA cloning of esterase 1, the major esterase activity in mouse plasma. Biochem Biophys Res Commun 151:1364–1370 [DOI] [PubMed] [Google Scholar]
- Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G, Klein SL, Old S, Rasooly R, Good P, et al. (2004) The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC). Genome Res 14:2121–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Meyer UA. (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 55:649–673 [DOI] [PubMed] [Google Scholar]
- Hartley DP, Klaassen CD. (2000) Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab Dispos 28:608–616 [PubMed] [Google Scholar]
- Heuman DM, Gallagher EJ, Barwick JL, Elshourbagy NA, Guzelian PS. (1982) Immunochemical evidence for induction of a common form of hepatic cytochrome P-450 in rats treated with pregnenolone-16α-carbonitrile or other steroidal or non-steroidal agents. Mol Pharmacol 21:753–760 [PubMed] [Google Scholar]
- Holmes RS, Wright MW, Laulederkind SJ, Cox LA, Hosokawa M, Imai T, Ishibashi S, Lehner R, Miyazaki M, Perkins EJ, et al. (2010) Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome 21:427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M, Furihata T, Yaginuma Y, Yamamoto N, Watanabe N, Tsukada E, Ohhata Y, Kobayashi K, Satoh T, Chiba K. (2008) Structural organization and characterization of the regulatory element of the human carboxylesterase (CES1A1 and CES1A2) genes. Drug Metab Pharmacokinet 23:73–84 [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Hattori K, Satoh T. (1993) Differential responses of rat hepatic microsomal carboxylesterase isozymes to glucocorticoids and pregnenolone 16α-carbonitrile. Biochem Pharmacol 45:2317–2322 [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Hirata K, Nakata F, Suga T, Satoh T. (1994) Species differences in the induction of hepatic microsomal carboxylesterases caused by dietary exposure to di(2-ethylhexyl)phthalate, a peroxisome proliferator. Drug Metab Dispos 22:889–894 [PubMed] [Google Scholar]
- Hosokawa M, Maki T, Satoh T. (1988) Differences in the induction of carboxylesterase isozymes in rat liver microsomes by xenobiotics. Biochem Pharmacol 37:2708–2711 [DOI] [PubMed] [Google Scholar]
- Humerickhouse R, Lohrbach K, Li L, Bosron WF, Dolan ME. (2000) Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res 60:1189–1192 [PubMed] [Google Scholar]
- Ketterman AJ, Pond SM, Becker CE. (1987) The effects of differential induction of cytochrome P-450, carboxylesterase and glutathione S-transferase activities on malathion toxicity in mice. Toxicol Appl Pharmacol 87:389–392 [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. (1995) Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15:3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruichi T, Fukami T, Nakajima M, Yokoi T. (2010) Transcriptional regulation of human carboxylesterase 1A1 by nuclear factor-erythroid 2 related factor 2 (Nrf2). Biochem Pharmacol 79:288–295 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Kamiie K, Soeta S, Taira H, Yamashita T. (2003) Molecular cloning and characterization of a novel carboxylesterase-like protein that is physiologically present at high concentrations in the urine of domestic cats (Felis catus). Biochem J 370:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EW, Yan B, Greenway D, Parkinson A. (1994) Regulation of two rat liver microsomal carboxylesterase isozymes: species differences, tissue distribution, and the effects of age, sex, and xenobiotic treatment of rats. Arch Biochem Biophys 315:513–526 [DOI] [PubMed] [Google Scholar]
- Nakajima A, Fukami T, Kobayashi Y, Watanabe A, Nakajima M, Yokoi T. (2011) Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine. Biochem Pharmacol 82:1747–1756 [DOI] [PubMed] [Google Scholar]
- Numazawa S, Yoshida T. (2004) Nrf2-dependent gene expressions: a molecular toxicological aspect. J Toxicol Sci 29:81–89 [DOI] [PubMed] [Google Scholar]
- Ovnic M, Swank RT, Fletcher C, Zhen L, Novak EK, Baumann H, Heintz N, Ganschow RE. (1991) Characterization and functional expression of a cDNA encoding egasyn (esterase-22): the endoplasmic reticulum-targeting protein of β-glucuronidase. Genomics 11:956–967 [DOI] [PubMed] [Google Scholar]
- Poole M, Bridgers K, Alexson SE, Corton JC. (2001) Altered expression of the carboxylesterases ES-4 and ES-10 by peroxisome proliferator chemicals. Toxicology 165:109–119 [DOI] [PubMed] [Google Scholar]
- Probst MR, Beer M, Beer D, Jenö P, Meyer UA, Gasser R. (1994) Human liver arylacetamide deacetylase: molecular cloning of a novel esterase involved in the metabolic activation of arylamine carcinogens with high sequence similarity to hormone-sensitive lipase. J Biol Chem 269:21650–21656 [PubMed] [Google Scholar]
- Reisman SA, Yeager RL, Yamamoto M, Klaassen CD. (2009) Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci 108:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JM, Vargas R, Jr, Xie W, Evans RM. (2003) Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol 17:1268–1282 [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. (1998) The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol 38:257–288 [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. (2006) Structure, function and regulation of carboxylesterases. Chem Biol Interact 162:195–211 [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. (1996) Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA 93:6731–6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Yang J, Yang D, Yan B. (2008) Dexamethasone suppresses the expression of multiple rat carboxylesterases through transcriptional repression: evidence for an involvement of the glucocorticoid receptor. Toxicology 254:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Xu C, Cui YJ, Klaassen CD. (2010) Nuclear receptor-mediated regulation of carboxylesterase expression and activity. Expert Opin Drug Metab Toxicol 6:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusa H, Masumoto H, Mitsuru A, Okazaki O, Sudo K. (2008) Markers of electrophilic stress caused by chemically reactive metabolites in human hepatocytes. Drug Metab Dispos 36:816–823 [DOI] [PubMed] [Google Scholar]
- Terashima K, Takai S, Usami Y, Adachi T, Sugiyama T, Katagiri Y, Hirano K. (1996) Purification and partial characterization of an indomethacin hydrolyzing enzyme from pig liver. Pharm Res 13:1327–1335 [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62:5196–5203 [PubMed] [Google Scholar]
- Trickett JI, Patel DD, Knight BL, Saggerson ED, Gibbons GF, Pease RJ. (2001) Characterization of the rodent genes for arylacetamide deacetylase, a putative microsomal lipase, and evidence for transcriptional regulation. J Biol Chem 276:39522–39532 [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61:1–6 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Fukami T, Nakajima M, Takamiya M, Aoki Y, Yokoi T. (2009) Human arylacetamide deacetylase is a principal enzyme in flutamide hydrolysis. Drug Metab Dispos 37:1513–1520 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Fukami T, Takahashi S, Kobayashi Y, Nakagawa N, Nakajima M, Yokoi T. (2010) Arylacetamide deacetylase is a determinant enzyme for the difference in hydrolase activities of phenacetin and acetaminophen. Drug Metab Dispos 38:1532–1537 [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. (2000) Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406:435–439 [DOI] [PubMed] [Google Scholar]
- Xu C, Wang X, Staudinger JL. (2009) Regulation of tissue-specific carboxylesterase expression by pregnane X receptor and constitutive androstane receptor. Drug Metab Dispos 37:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li Y, Yuan X, Matoney L, Yan B. (2001) Regulation of rat carboxylesterase expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): a dose-dependent decrease in mRNA levels but a biphasic change in protein levels and activity. Toxicol Sci 64:20–27 [DOI] [PubMed] [Google Scholar]
- Yang J, Yan B. (2007) Photochemotherapeutic agent 8-methoxypsoralen induces cytochrome P450 3A4 and carboxylesterase HCE2: evidence on an involvement of the pregnane X receptor. Toxicol Sci 95:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Song L, Zhang H, Matoney L, LeCluyse E, Yan B. (2000) Dexamethasone differentially regulates expression of carboxylesterase genes in humans and rats. Drug Metab Dispos 28:186–191 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.