Abstract
Cytochromes P450 (P450s) are a superfamily of enzymes that have critical functions in liver to catalyze the biotransformation of numerous drugs. However, the functions of most P450s are not mature at birth, which can markedly affect the metabolism of drugs in newborns. Therefore, characterization of the developmental profiles and regulatory mechanisms of P450 expression is needed for more rational drug therapy of pediatric patients. An animal model is indispensable for studying the mechanisms of postnatal development of the P450s. Hence we used RNA sequencing (RNA-Seq) to provide a “true quantification” of mRNA expression of all P450s in mouse liver during development. Liver samples of male C57BL/6 mice at 12 different ages from prenatal to adulthood were used. Total mRNAs of the 103 mouse P450s displayed two rapid increasing stages after birth, reflecting critical functional transition of liver during development. Four ontogenic expression patterns were identified among the 71 significantly expressed P450s, which categorized genes into neonatal-, adolescent-, adolescent/adult-, and adult-enriched groups. The 10 most highly expressed subfamilies of mouse P450s in livers of adult mice were CYP2E, -2C, -2D, -3A, -4A, -2F, -2A, -1A, -4F, and -2B, which showed diverse expression profiles during development. The expression patterns of multiple members within a P450 subfamily were often classified to different groups. RNA-Seq also enabled the quantification of known transcript variants of CYP2C44, CYP2C50, CYP2D22, CYP3A25, and CYP26B1 and identification of novel transcripts for CYP2B10, CYP2D26, and CYP3A13. In conclusion, this study reveals the mRNA abundance of all the P450s in mouse liver during development and provides a foundation for mechanistic studies in the future.
Introduction
Cytochromes P450s (P450s) are a superfamily of enzymes that catalyze the oxidation of organic substances, including the biotransformation of numerous endobiotics (e.g., steroids, fatty acids, and eicosanoids) as well as the detoxification or bioactivation of a variety of xenobiotics (e.g., drugs, chemical carcinogens, and environmental contaminants) (Nebert and Gonzalez, 1987; Danielson, 2002). P450s are the major enzymes involved in the metabolism and bioactivation of drugs, accounting for approximately 75% of drug biotransformation (Guengerich, 2008). Liver expresses the largest number of individual P450 enzymes (Hrycay and Bandiera, 2009). However, most P450s in the liver are expressed at low levels at birth. The expression of P450s changes during liver development and has been categorized into several different developmental patterns, and considerable interindividual variability occurs in the immediate postnatal period (Hines and McCarver, 2002; Hines, 2007). Low P450 expression in liver during postnatal development is thought to be responsible for the substantial pharmacokinetic differences between newborns and adults and thus contributes to differences in therapeutic efficacy and adverse drug reactions in pediatric patients (Blake et al., 2005; Hines, 2008). One example is that the low CYP3A4 in neonatal livers results in a low capacity to oxidize cisapride, which can result in QT prolongation in pediatric patients (Pearce et al., 2001; Tréluyer et al., 2001). An in-depth understanding of the regulation of the ontogeny of human P450s is needed for safer and more effective drug therapy for pediatric patients.
The paucity of suitable tissue samples and limitations due to ethical and technical issues have made it difficult to study the mechanisms controlling the ontogenic expression of P450s in human liver (Rowell and Zlotkin, 1997). Animal models would be advantageous in overcoming these difficulties and minimizing the influence of genetic variations and the environment. In recent years, the mouse and rat have surpassed many other laboratory animals as the experimental models of choice for the study of physiology, metabolism, and disease (Muruganandan and Sinal, 2008; Hrycay and Bandiera, 2009). Advantages of these models include rapid growth, easy maintenance, and the development of genetic manipulation techniques for mechanistic studies with gain-of-function and loss-of-function strategies. Several researchers have examined the ontogenic gene expression profiles of a few P450s in mouse or rat liver (Choudhary et al., 2004; Alcorn et al., 2007; Cherala et al., 2007; Hart et al., 2009; Li et al., 2009a). Developmental expression patterns of some P450s in mice and rats are similar to those in humans.
Previous studies quantified P450 gene expression at the mRNA level by either microarrays or multiplex suspension arrays (Hart et al., 2009; Li et al., 2009a), which only provides relative quantification of a given P450. These technologies detect mRNA levels by probe hybridization and fluorescence signal intensity, which cannot compare expression levels among various P450s, because different probes may have different hybridization efficiency. With the development of next-generation sequencing technology, RNA sequencing (RNA-Seq) can define a transcriptome with low levels of background noise, no upper limit for quantification, and a high degree of reproducibility for both technical and biological replicates (Mortazavi et al., 2008; Nagalakshmi et al., 2008). More importantly, compared with microarrays, RNA-Seq quantifies the true abundance of mRNA molecules in biological samples and enables the comparison of expression levels of all genes (Malone and Oliver, 2011). Furthermore, RNA-Seq has the power to quantify expression levels of alternative transcripts of the same gene and to identify novel transcripts efficiently (Pan et al., 2008; Wang et al., 2008; Malone and Oliver, 2011). In the current study, RNA-Seq was used to systematically quantify the abundance of 103 mouse P450 mRNAs in liver during development to define the ontogenic profiles of these P450 mRNAs, with an additional focus on the identification and quantification of alternative transcripts. Therefore, the purpose of this study was to generate comprehensive information on the ontogeny of the mRNAs of the P450 family in the livers of mice, which will provide a foundation for determining the regulatory mechanisms controlling the various transcription patterns of P450s during liver development.
Materials and Methods
Animals.
Eight-week-old C57BL/6 breeding pairs of mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed according to the American Animal Association Laboratory animal care guidelines and were bred under standard conditions in the Laboratory Animal Resources Facility at the University of Kansas Medical Center. The use of these mice was approved by the Institute of Laboratory Animal Resources. Breeding pairs were established at 4:00 PM and separated at 8:00 AM the following day. The body weights of the females were recorded each day to determine pregnancy status. Livers (n = 3) from male offspring were collected at the following 12 ages: day −2 (gestational day 17), day 0 (right after birth and before the start of suckling), day 1 (exactly 24 h after birth), and day 3, 5, 10, 15, 20, 25, 30, 45, and 60 (collected at approximately 9:00 AM), which represent periods of prenatal (day −2), neonatal (day 0–10), juvenile (day 15–30), and young adult (day 45–60). Livers were immediately frozen in liquid nitrogen after removal and stored at −80°C.
Total RNA Extraction.
Total RNA was isolated by using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. Whole livers without gallbladders at various ages were collected for RNA isolation. RNA concentrations were quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE) at a wavelength of 260 nm. The integrity of the total RNA was evaluated on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and the samples with RNA integrity values larger than 7.0 were accepted for sequencing library construction.
cDNA Library Construction.
The cDNA libraries from all samples were prepared by using an Illumina TruSeq RNA Sample Prep Kit (Illumina, San Diego, CA). Three micrograms of total RNA were used as the RNA input on the basis of the manufacturer's recommendation. mRNAs were isolated from the total RNAs by poly(A) selection using poly(T) primers. RNA fragmentation, first- and second-strand cDNA syntheses, end repair, adapter ligation, and PCR amplification were performed according to the manufacturer's protocol. Fragments of the cDNA library ranged from 220 to 500 bp with a peak size at at 280 bp (including 120-bp adapter sequences). Quality of the cDNA libraries was evaluated using an Agilent 2100 Bioanalyzer before sequencing.
RNA-Seq.
RNA-Seq was performed on an Illumina HiSeq 2000 instrument by the Genome Sequencing Facility at Kansas University Medical Center. Clusters of the cDNA libraries were generated on a TruSeq flow cell and sequenced for 100-bp paired end reads (2 × 100) with a TruSeq 200 cycle SBS kit (Illumina). A PhiX bacteriophage genome and a universal human reference RNA were used as controls and sequenced in parallel with other samples to ensure that the data generated for each run were accurately calibrated during the image analysis and data analysis. In addition, the PhiX was spiked into each cDNA sample at approximately 1% as an internal quality control.
RNA-Seq Data Analysis.
After the sequencing images were generated by the sequencing platform, the pixel-level raw data collection, image analysis, and base calling were performed using Illumina Real Time Analysis software. The output bcl files were converted to qseq files by Illumina BCL Converter 1.7 software and subsequently converted to FASTQ files for downstream analysis. The RNA-Seq reads from the FASTQ files were mapped to the mouse reference genome (NCBI37/mm9), and the splice junctions were identified by TopHat 1.2. The output files in BAM (binary sequence alignment) format were analyzed by Cufflinks 1.0.3 to estimate the transcript abundance (Trapnell et al., 2010). The mRNA abundance was expressed as the number of fragments per kilobase of exon per million reads mapped (FPKM).
Data Visualization and Statistics.
The P450 genes (103 in total) were retrieved from Cufflinks output for further analysis. Significant gene expression was determined by the drop-in-deviance F test of the fitted FPKM data to a generalized linear model with a Poisson link function, a statistic designed to measure the significance of a gene's measured FPKM value relative to a zero FPKM value. The p values were adjusted for extra Poisson variation and corrected for false discovery by the Benjamini-Hochberg method (FDR-BH) with a threshold set at 0.05 for significant expression (Benjamini and Hochberg, 1995). A two-way hierarchical clustering dendrogram was generated by JMP (version 9.0; SAS Institute, Inc., Cary, NC) to determine the expression patterns of the P450 mRNAs during liver development.
Validation of Alternative mRNA Transcripts.
Alternative splicing events in CYP2B10 and CYP2D26 were validated by reverse transcription and endpoint PCR. Total RNA (5 μg) was reverse-transcribed with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and random primers in a final volume of 50 μl. Endpoint PCRs with reaction volumes of 20 μl were performed. Primer sequences are listed in Table 1. The PCR products were separated on a 2% agarose gel. Bands of the expected sizes were excised and purified with a QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA). Subsequently, DNA was sequenced with sequencing primers (Table 1) by ACGT Inc. (Wheeling, IL). The alternative polyadenylation of CYP3A13 mRNA was validated by rapid amplification of cDNA 3′-end (3′-RACE) using RNA ligase-mediated rapid amplification of cDNA ends (FirstChoice RLM-RACE Kit; Ambion, Austin, TX) as described previously (Li et al., 2012). Primer sequences are shown in Table 1.
TABLE 1.
Primer sequences for validation of novel transcripts identified by RNA-Seq
| Gene | Method | Amplification | Direction | Sequence (5′ to 3′) | PCR Product |
|---|---|---|---|---|---|
| bp | |||||
| Cyp2b10 | Endpoint PCR | From exon 6 to exon 9 (including cassette exon) | Forward | ATGGCTTCCTGCTCATGCTCAAGT | 411 |
| Reverse | GACAAATGCGCTTTCCCACAGACT | ||||
| Sequencing | From exon 7 to exon 9 | AGTGCCACACAGAGTGACCAAAGA | |||
| Cyp2d26 | Endpoint PCR | From exon 5 to cassette exon | Forward | ACTACACATCCCTGGTTTGCCTGA | 520 |
| Reverse | AGCCTCTGAGCACCTTCTCTTGTA | ||||
| Sequencing | From exon 6 to cassette exon | TGATTGACCTGTTCATGGCAGGGA | |||
| Endpoint PCR | From cassette exon to exon 9 | Forward | TACAAGAGAAGGTGCTCAGAGGCT | 327 | |
| Reverse | TAGGGCTCTGGAGTAACTGGCATT | ||||
| Sequencing | From exon 8 to cassette exon | CATGAAGGCCTCGTGCTTCACAAA | |||
| Cyp3a13 | 3′-RACE | cDNA synthesis | GCGAGCACAGAATTAATACGACTCACTATAGGT12VN | ||
| Outer PCR | Forward | AAGTTGCTCTTGTCAGAGTCCTGC | |||
| Reverse | GCGAGCACAGAATTAATACGACT | ||||
| Inner PCR | Forward | CACTGTCCAGCCTTGTAAGGAAAC | |||
| Reverse | CGCGGATCCGAATTAATACGACTCACTATAGG | ||||
| Sequencing | AAGTTGCTCTTGTCAGAGTCCTGC |
Results
Total Expression and Proportions of Individual P450s during Liver Development.
RNA-Seq generated an average of 175 million 100-bp paired end reads per sample for the 36 samples from 12 different ages (n = 3). More than 80% of the reads from each sample were mapped to the mouse reference genome (NCBI37/mm9) by TopHat 1.2 (data not shown). Transcript abundances of the 103 mouse P450 genes (including nine pseudogenes) were calculated by Cufflinks and are presented as FPKM values (Supplemental Table 1). The FPKM values were highly reproducible in the triplicate samples at each age as indicated by the small S.E.M. If the Benjamini-Hochberg adjusted drop-in-deviance F test p value (FDR-BH) in at least one of the 12 ages were less than 0.05 for a P450 gene, then that gene was considered to be expressed in liver during development. Of the 103 mouse P450 genes, 71 genes were expressed during liver development.
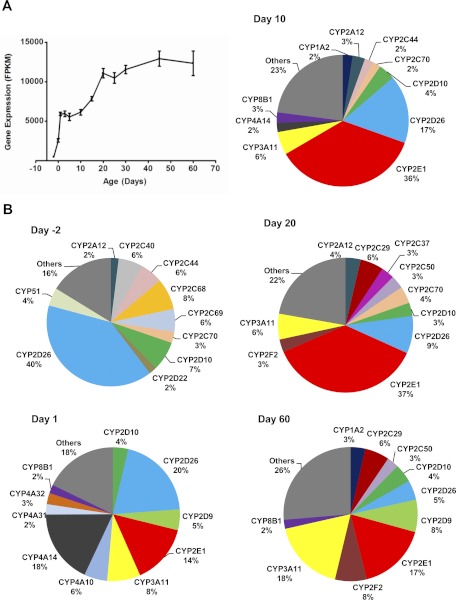
Total FPKM values of all P450 mRNAs increased approximately 25-fold during postnatal liver development from day −2 (479) to day 60 (12,345), with two surges followed by a plateau stage after each surge (Fig. 1A). Figure 1B shows the composition of the P450s, represented as percentages of the total P450 mRNA FPKM, at the beginning and end ages of these surge and plateau stages, i.e., day −2, 1, 10, 20, and 60. The first surge occurred from day −2 (FPKM = 479) to day 1 (5926) with more than a 10-fold increase of total P450 mRNA FPKM values. During the first surge, the top 10 highly expressed P450s changed dramatically, with only CYP2D22 and CYP2D10 in the top 10 P450s at both day −2 and day 1. During the first plateau stage, although the total P450 mRNA FPKM values were relatively constant between day 1 and 10, the individual P450 mRNAs were altered, with CYP2E1 the most abundant at day 10. The second surge occurred between day 10 and 20, with nearly a 2-fold increase of total P450 FPKM values from 6139 to 11,087. The individual P450 mRNAs, which increased during the second surge from day 10 to 20, were similar, because 6 P450s appeared at both ages in the top 10 highly expressed P450s, and the top 3 are the same in an order of CYP2E1, CYP3A11, followed by CYP2D26. During the second plateau stage of day 20 to 60, the individual P450 mRNAs continued to change and became more diverse. At day 60, the top 10 highly expressed P450s consisted of only 74% of the total P450 mRNAs, and many additional P450s were expressed.
Fig. 1.
A, total mRNAs of the 103 mouse P450 genes in liver during development. RNA-Seq was done for liver mRNAs of male C57BL/6 mice at 12 ages from 2 days before birth to 60 days after birth. The FPKM values of all 103 mouse P450 genes at each age were added and plotted to show the developmental pattern of total P450 mRNAs. Bars represented the mean ± S.E.M. of three individual animals. B, individual P450 mRNAs (shown as percentages of total P450 mRNAs) at 2 days before birth and 1, 10, 20, and 60 days after birth. Each gene is presented in a unique color for all ages. Only the top 10 P450 mRNAs at each age are listed in alphabetical order, and the rest were grouped as “Others.”
Expression Patterns of P450s during Liver Development.
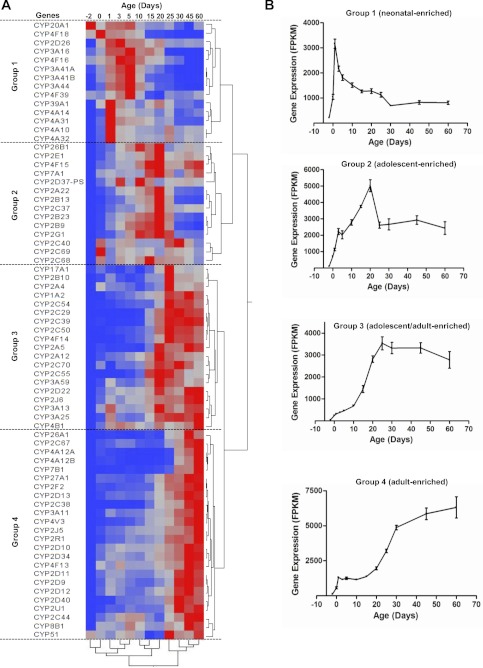
P450 genes with a maximum over minimum expression ratio of <2 among the 12 ages studied were to be excluded for this part of the study. The data indicate that all 71 significantly expressed P450 genes had >2-fold differences in expression during development. Two-way hierarchical clustering analysis of the 71 P450 genes revealed four distinct patterns of gene expression with respect to age, which are defined as neonatal-enriched (group 1), adolescent-enriched (group 2), adolescent/adult-enriched (group 3), and adult-enriched (group 4) (Fig. 2A). The sum of the FPKM values in each group was plotted against age in Fig. 2B. Group 1 (neonatal-enriched) contained CYP2D26, CYP3A16, CYP3A41a/b, CYP3A44, CYP4A10, CYP4A14, CYP4A31, CYP4A32, CYP4F16, CYP4F18, CYP4F39, CYP20A1, and CYP39A1. The P450s in this group increased markedly after birth and reached a peak at approximately day 1 of age and then decreased and remained relatively low after day 30. Group 2 P450s (adolescent-enriched) consisted of CYP2A22, CYP2B9, CYP2B13, CYP2B23, CYP2C37, CYP2C40, CYP2C68, CYP2C69, CYP2D37-ps, CYP2E1, CYP2G1, CYP4F15, CYP7A1, and CYP26B1. The P450s in this group increased rapidly after birth, reached maximal expression at approximately day 20, and then decreased to lower levels in adult mice. Group 3 P450s (adolescent/adult-enriched) included CYP1A2, CYP2A4, CYP2A5, CYP2A12, CYP2B10, CYP2C29, CYP2C39, CYP2C50, CYP2C54, CYP2C55, CYP2C70, CYP2D22, CYP2J6, CYP3A13, CYP3A25, CYP3A59, CYP4B1, CYP4F14, and CYP17A1. The mRNAs of the P450s in this group were expressed at very low levels at birth, but increased substantially between day 10 and 25, and reached a plateau in adult mice. Group 4 P450s (adult-enriched) consisted of CYP2C38, CYP2C44; CYP2C67, CYP2D9, CYP2D10, CYP2D11, CYP2D12, CYP2D13, CYP2D34, CYP2D40, CYP2F2, CYP2J5, CYP2R1, CYP2U1, CYP3A11, CYP4A12A/B, CYP4F13, CYP4V3, CYP7B1, CYP8B1, CYP26A1, CYP27A1, and CYP51. The P450s in this group were expressed at low levels 2 days before birth, gradually increased after birth with a rapid increase at approximately 25 days of age, and reached their highest expression in adults (45 and 60 days of age). The largest correlation distance with respect to neighboring ages (as shown by the clade separation on the dendrogram scale) was observed between 20 and 25 days of age.
Fig. 2.
A, hierarchical clustering of expression profiles for 71 significantly expressed P450 mRNAs in livers of male C57BL/6 mice. Significant expression was determined by the FDR-BH with a threshold of <0.05. The two trees describe the relationship between different gene expression profiles (right tree) and various ages (bottom tree). The dendrogram scale represents the correlation distances. Average FPKM values of three replicates per age are given by colored squares: red, relatively high expression; blue, relatively low expression. The dashed lines categorize the expression profiles into four groups. B, changes in total FPKM values of the P450 mRNAs in each group on the basis of the hierarchical clustering in A through different ages during development. Bars represent the mean ± S.E.M. of three individual samples.
Comparison of Transcript Abundance of Some P450 Subfamilies during Liver Development.
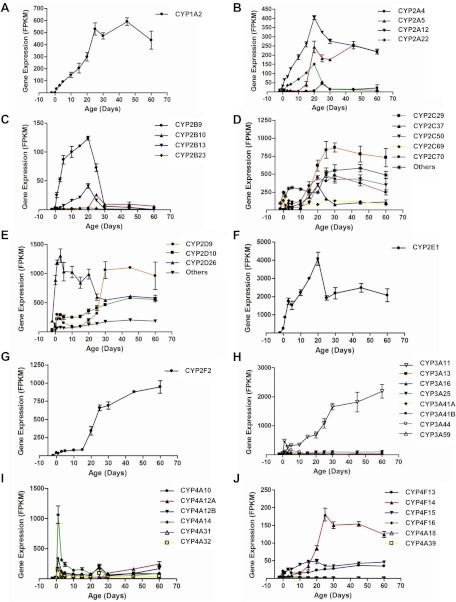
The 103 mouse P450 genes belong to 16 families and 39 subfamilies. Ontogenic patterns of the major P450 subfamilies in liver are summarized in Fig. 3.
Fig. 3.
Expression patterns of the selected P450 subfamilies during liver development: A, CYP1A; B, CYP2A; C, CYP2B; D, CYP2C; E, CYP2D; F, CYP2E; G, CYP2F; H, CYP3A; I, CYP4A; and J, CYP4F. Data are presented as mean FPKM ± S.E.M. of three individual samples.
CYP1A subfamily.
The CYP1A subfamily has two members, CYP1A1 and CYP1A2. CYP1A1 mRNA was expressed at very low levels in liver at all ages. CYP1A2 had a developmental pattern belonging to group 3 (adolescent/adult-enriched) (Fig. 3A).
CYP2A subfamily.
The CYP2A subfamily consists of five members, CYP2A4, CYP5, CYP12, CYP22, and CYP2AB1. Except for CYP2AB1, the other four genes in liver were expressed with the adolescent- or adolescent/adult-enriched patterns (Fig. 3B).
CYP2B subfamily.
The CYP2B subfamily contains five members, CYP2B9, -10, -13, -19, and -23. CYP2B9 and CYP2B13 contributed the most to the expressed levels of this subfamily with an adolescent-enriched pattern (Fig. 3C).
CYP2C subfamily.
The CYP2C subfamily includes 16 members, CYP2C29, -37, -38, -39, -40, -44, -50, -53-ps, -54, -55, -65, -66, -67, -68, -69, and -70. CYP2C29, -37, -50, -69, and -70 were the most highly expressed members of this P450 subfamily in livers of mice. CYP2C69 was neonatal-enriched, CYP2C37 was adolescent-enriched, and CYP2C29, -50, and -70 were adolescent/adult-enriched (Fig. 3D).
CYP2D subfamily.
The CYP2D subfamily consists of 10 members, CYP2D9, -10, -11, -12, -13, -22, -26, -34, -37-ps, and -40. Three genes, CYP2D9, -10, and -26, were expressed to a higher extend than the other CYP2D genes. CYP2D26 increased remarkably after birth and remained at a consistent level until day 20 and then decreased after 30 days of age (neonatal-enriched). CYP2D9 was expressed at a low level until day 20 and increased markedly to reach a plateau at 30 days of age (adolescent/adult-enriched). CYP2D10 increased gradually throughout development (Fig. 3E).
CYP2E subfamily.
The CYP2E subfamily has only one member, CYP2E1, which was one of the most abundant P450s expressed in liver. CYP2E1 increased sharply from day −2 to day 5 and then continually increased to a peak at day 20, followed by a decrease and then increase to a consistently high level thereafter (Fig. 3F).
CYP2F subfamily.
The CYP2F subfamily also contains only one member, CYP2F2, which was expressed at a low level until 15 days of age and thereafter gradually increased (Fig. 3G).
CYP3A subfamily.
The CYP3A subfamily includes nine members, CYP3A11, -13, -16, 25, -41A, -41B, -44, -57, and -59. CYP3A11 was the most abundantly expressed gene in this subfamily, which gradually increased throughout postnatal development (Fig. 3H). Several CYP3A members, including CYP3A16, -41A, -41B, and -44, were neonatal-enriched with the highest expressed levels at approximately 5 days of age (Fig. 3H).
CYP4A subfamily.
The CYP4A subfamily has eight members, CYP4A10, -12A, -12B, -14, -29-ps, -30B-ps, -31, and -32. Four members in this subfamily, CYP4A10, -14, -31, and -32, had a neonatal-enriched expression pattern with the highest expression at 1 day of age. The other two members, CYP4A12A and CYP4A12B, were not expressed until day 30 and then increased rapidly in adult mice (Fig. 3I).
CYP4F subfamily.
The CYP4F subfamily contains nine genes, CYP4F13, -14, -15, -16, -17, -18, -39, -40, and -41-ps. CYP4F13, -14, and -15 were expressed in liver, and they appeared to have an adolescent or adolescent/adult enriched pattern (Fig. 3J). CYP4F16, -18, and -39 were also expressed, but at very low levels. Ontogenic patterns of the other P450 subfamilies are also presented (Supplemental Table 1).
Ontogeny of Known Transcript Variants of P450s during Liver Development.
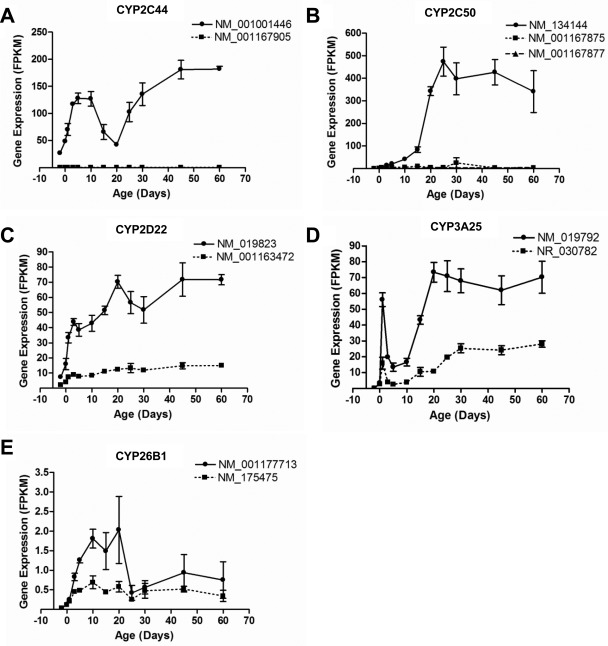
Among the 103 mouse P450 genes, six have known alternative transcripts annotated in the NCBI database, including Cyp1a1 (two isoforms), Cyp2c44 (two isoforms), Cyp2c50 (three isoforms), Cyp3a25 (two isoforms), and Cyp26b1 (two isoforms). CYP1A1 was expressed at a very low level in liver. RNA-Seq was able to provide the ontogenic patterns of the annotated alternative transcripts of the five P450 genes that were significantly expressed in liver (Fig. 4, A–E; Supplemental Table 2).
Fig. 4.
The mRNAs of known alternative transcripts of five P450s that were significantly expressed during liver development: A, CYP2C44; B, CYP2C50; C, CYP2D22; D, CYP3A25; and E, CYP26B1. The transcripts are labeled with their NCBI transcript identification number. Data are presented as mean FPKM ± S.E.M. of three individual samples.
For the two CYP2C44 transcripts, NM_001167905 lacks the last exon of NM_001001446 and has an alternative polyadenylation site. NM_001001446 was the major isoform in mouse liver throughout development. Its mRNA was high at birth and reached a peak at approximately 5 and 10 days of age, decreased between 10 and 20 days of age, and increased again and reached a high expression plateau after 45 days of age (Fig. 4A). NM_001167905 was not expressed throughout liver development.
Cyp2c50 has three transcripts. NM_001167875 lacks the fifth exon of NM_134144. NM_001167877 is a truncated isoform with the coding region containing only the first 4 exons of NM_134144. NM_134144 was the major isoform in mouse liver throughout development. Its expression profile was a typical group 3 pattern (adolescent/adult-enriched) (Fig. 4B). NM_001167875 and NM_001167877 were expressed at very low levels throughout liver development.
The two CYP2D22 transcripts encode the same protein, but NM_019823 has six nucleotides less at the end of the first exon than NM_001163472, which results in a shorter 5′-UTR. NM_019823 was the major transcript of CYP2D22 expressed throughout liver development. mRNA of NM_019823 increased rapidly from 2 days before birth to 3 days after birth and then remained relatively consistent with a small peak at 20 days of age. mRNA of NM_001163472 remained consistently low at all ages (Fig. 4C).
Cyp3a25 has two transcripts. NR_030782 is a noncoding RNA with a skipped sixth exon of NM_019792. The expression profiles of these two transcripts were similar during development, but NM_019792 had higher expression levels at all postnatal ages. Both transcripts had a “day 1 surge,” which decreased markedly by 5 days of age and then rapidly increased between 10 and 20 days of age and reached a plateau at 20 days of age (Fig. 4D).
The two CYP26B1 transcripts encode the same protein but have different transcription start sites. NM_001177713 is shorter than NM_175475 in the 5′-UTR. Both transcripts were expressed at very low levels during mouse liver development (Fig. 4E).
Identification of Novel Transcripts of P450s in Liver by RNA-Seq.
Potential novel transcripts of P450s, which are not annotated in the NCBI database, were identified by examining sequencing results in the UCSC genome browser (http://genome.ucsc.edu/cgi-bin/hgGateway). Alignment results from TopHat were converted to bigWig files and uploaded to the browser to display the distribution of reads in the genome. Junction files were also used to determine alternative splicing events. In the browser, each junction consists of two connected blocks, where each block is as long as the maximal overhang of any read spanning the junction. The score is the number of alignments spanning the junction.
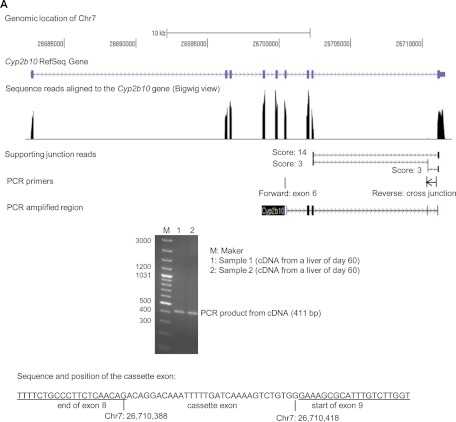
The Cyp2b10 RefSeq gene in assembly of NCBI37/mm9 was located on chromosome 7 between 26,682,683 and 26,711,643 with nine exons. A tiny peak of read alignment between the peaks of exon 8 and 9 was observed around chromosome position 26,710,000 in the bigWig view (Fig. 5A). Two junctions with a score of 3 indicated alternative splicing events that could generate a cassette exon in this region. The cassette exon was validated by endpoint PCR using cDNAs from two liver samples of 60-day-old mice. The forward PCR primer mapped to exon 6 and the reverse PCR primer mapped across the junction between the cassette exon and exon 9 (Table 1), which produced an amplicon containing the cassette exon with a size of 411 bp in all samples examined (Fig. 5A). Sequencing of the PCR product revealed the presence of a 31-bp cassette exon, which could alter the protein translation reading frame when retained in the transcript.
Fig. 5.
A, identification of a cassette exon in CYP2B10 mRNA.
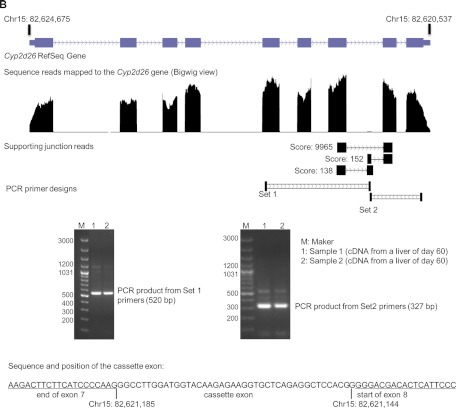
A similar cassette exon peak and alternative splicing junctions were also found in the browser view of the Cyp2d26 gene, which is located on the minus strand of chromosome 15 between 82,620,537 and 82,624,675 (Fig. 5B). The cassette exon was located between exon 7 and 8 and validated by endpoint PCR with two sets of primers. Primer set 1 bound to exon 5 and the cassette exon, which amplified a fragment with an expected size of 520 bp. Primer set 2 bound to the cassette exon and exon 9, which amplified a fragment with an expected size of 327 bp. The cassette exon sequence contained 42 nucleotides, which would change the protein product if retained in the transcript.
Fig. 5.
B, identification of a cassette exon in CYP2D26 mRNA.
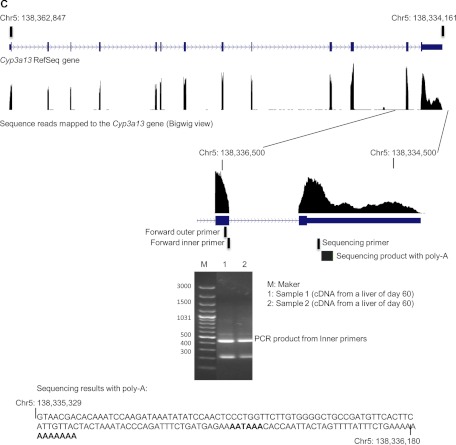
Thirteen peaks of sequencing reads were observed along the RefSeq Cyp3a13 gene. The first 12 exons were covered by a single peak. However, in exon 13, a region with high-level reads followed by a region with low-level reads was found. A sharp decrease in the sequencing reads occurred around chromosome position 138,335,200 in the 3′-UTR of Cyp3a13 (Fig. 5C). The RNA-Seq reads ended at coordinate 138,334,161 of chromosome 5, which matched with the termination of this gene in the reference sequence. This suggested an alternative polyadenylated mRNA transcript in the 3′-UTR region of CYP3A13. To validate the shorter 3′-UTR transcript, we performed 3′-RACE-PCR with two RNA samples from livers of 60-day-old mice. The forward PCR primers shown in Fig. 5C, together with reverse primers provided by the RLM-RACE Kit, generated a band that was sequenced using the Cyp3a13-specific sequencing primer (Table 1). Sequencing results revealed a poly(A)-containing sequence that uniquely mapped to the 3′-UTR of CYP3A13. Termination of the shorter 3′-UTR transcript occurred at chromosome location 138,336,180, which was more than 2 kilobases upstream of the end of the reference gene sequence. In addition, an AATAAA sequence was noted at 23 bases upstream of the termination site, which might serve as a recognition signal for the poly(A) polymerase complex.
Fig. 5.
C, identification of an alternative polyadenylation site in CYP3A13 mRNA. RNA-Seq reads of a day 60 sample were aligned to the reference genome mm9 by TopHat and viewed as distribution peaks by the UCSC genome browser. Output junction reads from TopHat are also shown. The cassette exons or alternative polyadenylation was validated by endpoint PCR or 3′-RACE. The PCR products are shown by gel electrophoresis and further sequenced by single-pass DNA sequencing with a designed primer. The sequence was aligned back to the P450 gene regions.
Discussion
In the current study, RNA-Seq was used to truly quantify mRNA abundance of the 103 P450 genes in mouse liver during postnatal development. Compared with other commonly used methods for RNA quantification, such as microarray, branched DNA, or real-time PCR, RNA-Seq directly counts sequence reads of the nucleotide molecules in biological samples, which is the only method for true quantification of mRNA expression. Expression of a transcript is represented by FPKM, which normalizes sequencing depths between different samples and sizes between various genes, allowing direct comparison of mRNAs among various transcripts on a genome-wide scale. As shown in Fig. 1, the quantitative abundance of mRNAs encoded by the 103 P450 genes was determined in mice at 12 ages, which has not been reported previously.
From perinatal through neonatal to adult life, the total FPKM values of all P450 transcripts increased approximately 25-fold with two rapidly increasing stages (Fig. 1A). The first surge occurred from 2 days before birth to 1 day after birth. Not only the total abundance but also the composition of expressed P450s was markedly altered during this period, when the pup was born and lost the direct physiological connection with the mother. Multiple hormones are known to influence the expression of drug-metabolizing enzymes. During pregnancy, maternal hormones cross the placenta into the baby. After birth, the loss of maternal hormones may be a contributing factor to the changes in the expression of some P450s. The newborn mice also began to be exposed to the environment, which contains all kinds of xenobiotics. A critical function of P450s is xenobiotic metabolism; thus, the first surge of P450 expression may provide protections to the newborns against environmental toxic compounds. The second surge occurred from day 10 to day 20, with a rapid increase in abundance of the P450 mRNAs. However, the expression of each P450 was similar from 10 to 20 days of age, as the expression of most P450s increased simultaneously. This period is also the most rapidly growing stage of postnatal liver development, as studies have shown that cell proliferation in mouse liver is high until 20 days of age and then the hepatic architecture begins to resemble that of adult liver (Apte et al., 2007).
Hierarchical clustering of significantly expressed P450s revealed four hepatic ontogenic expression patterns (Fig. 2). The expression profiles of these patterns were similar to those in a previous study of P450 ontogeny in mice (Hart et al., 2009), with some genes enriched at younger ages and some enriched at older ages. However, the previous study examined only a few P450s, whereas the present study included all the expressed P450s in the liver of mice and therefore generated a more comprehensive summary of ontogenic patterns. Whereas the earlier study concluded that there were three patterns of P450 ontogeny in livers of mice (Hart et al., 2009), the present study concludes that there are four patterns. Clustering of the P450s from 12 developmental ages indicates that the largest correlation distance with respect to neighboring ages is between 20 and 25 days of age, which spanned the period when the diet changes from milk to chow. Because P450s have important functions in metabolizing chemicals absorbed from the gastrointestinal tract, it is reasonable to anticipate this change in P450 expression with a change in food intake.
The most highly expressed P450 mRNAs in livers of mice were from the CYP1–4 families, which belong to the xenobiotic-metabolizing P450s. They displayed changes in expression during development. Some P450 subfamilies were primarily expressed during the neonatal stage, whereas others were mainly expressed during the adolescent or adult stage of development. Even within each subfamily, individual members were expressed with various developmental patterns. In the CYP2D subfamily, CYP2D26 mRNA reached its maximum expression during the neonatal period, whereas CYP2D9 reached its maximum expression during the adolescent/adult period (Fig. 3E). Likewise, in the CYP3A subfamily, CYP3A16, CYP3A41A, CYP3A41B, and CYP3A44 were maximally expressed during the neonatal period, whereas CYP3A11 was maximally expressed in the adult mice (Fig. 3H). A similar developmental switch also exists in human CYP3A enzymes (Schuetz et al., 1994; Stevens et al., 2003; Leeder et al., 2005). Thus, mice might serve as a good model to study the mechanisms controlling the developmental switches of P450 expression in liver. Of note, it is difficult to directly compare our data in mice with other previously published data in humans. Our study determined levels of mRNA transcripts of P450s, whereas most other published studies determined protein concentrations and enzyme activities of human P450s.
Because RNA-Seq has the ability to examine the transcriptome at the resolution of a single base (Wang et al., 2009), it can quantify the mRNAs of genes as well as their individual transcript variants (Fig. 4). The known transcript variants of the P450s might be translated into different proteins, as in CYP2C44 and CYP2C50, or those that differ only in the UTR regions of the transcripts, as in CYP2D22 and CYP26B1. Because transcript variants can alter protein functions, mRNA stability, subcellular localization, and translation efficiency of the transcripts (Pesole et al., 2000), identification of the predominant transcripts of P450s and their developmental expression patterns might help to predict variations in protein activity.
Research has shown that 92 to 94% of human genes undergo alternative splicing, promoter usage, and polyadenylation (Wang et al., 2008). Only 6 of the 103 mouse P450 genes in the NCBI database have annotated transcript variants, so alternative splicing of P450 transcripts in mice is probably an underexplored area. With the advantage of RNA-Seq, the present study identified several novel transcript variants in CYP2B10 and CYP2D26, even though they are expressed at very low levels (very small peaks in bigWig view of Fig. 5, A and B). When the cassette exons are translated, the resultant P450 enzymes will have different protein sequences, which might lead to a difference in enzyme activities. The CYP3A13 transcripts, with the shorter 3′-UTR, appeared to be the predominant transcript of this gene. A similar shorter 3′-UTR transcript has been reported for the human CYP3A4 gene (Li et al., 2012). Although the CYP3A4 transcript with shorter 3′-UTR does not change amino acid sequences, it is more stable and can translate to protein more efficiently than the CYP3A4 transcript with the longer 3′-UTR (Li et al., 2012). The mouse Cyp3a13 gene might serve as a model to study how alternative polyadenylation events are regulated. Further studies are needed to investigate the functional significance of these novel transcripts.
P450 proteins and enzyme activities were not determined in this study because of technique limitations. Specific antibodies against most of the mouse P450 proteins, specific substrates, and inhibitors of many individual mouse P450 enzymes are not available. Technological breakthroughs in proteomics and metabolomics are essential to study the ontogeny of P450 proteins.
In summary, the present study describes the ontogeny of the mRNAs of all P450s in the mouse liver during development. These data provide fundamental knowledge for studying mechanisms of regulation of the transcription of P450 genes in liver during development. A previous study from our laboratories indicates that the developmental regulation of Cyp3a gene expression in mouse liver may be due to dynamic changes of histone modifications during postnatal liver maturation (Li et al., 2009b). No doubt, epigenetics and various hepatic transcription factors play an important role in regulating ontogenic expression of P450 enzymes (Kyrmizi et al., 2006). Studies on the networking of transcription factors and epigenetic signatures in liver may shed light on the regulatory mechanisms of the observed P450 developmental patterns. These mechanisms may be of considerable importance in understanding the kinetics of xenobiotic metabolism during the neonatal period.
Supplementary Material
Acknowledgments
We thank Drs. Julia Yue Cui, Helen Renaud, and Dan Li for help on sample collection and data analysis and Clark Bloomer (Kansas University Medical Center, Sequencing Core Facilities) for technical assistance on RNA-Seq.
This study was supported by the National Institutes of Health National Institute for Environmental Health Sciences [Grant ES-019487] (to X.Z., C.D.K., and H.L.); the National Institutes of Health National Institute of General Medical Sciences [Grant GM-087376] (to X.Z.); the National Institutes of Health National Institute for Environmental Health Sciences [Grant ES-009649] (to C.D.K.); and the National Institutes of Health National Center for Research Resources [Grant RR-021940] (to C.D.K. and X.Z.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- P450
- cytochrome P450
- RNA-Seq
- RNA sequencing
- bp
- base pairs
- NCBI
- National Center for Biotechnology Information
- FPKM
- fragments per kilobase of exon per million reads mapped
- FDR-BH
- Benjamini-Hochberg-adjusted false discovery rate
- PCR
- polymerase chain reaction
- 3′RACE
- rapid amplification of cDNA 3′ end
- UTR
- untranslated region.
Authorship Contributions
Participated in research design: Peng, Yoo, Gunewardena, Lu, Klaassen, and Zhong.
Conducted experiments: Peng.
Performed data analysis: Peng, Gunewardena, Yoo, and Zhong.
Wrote or contributed to the writing of the manuscript: Peng, Yoo, Gunewardena, Lu, Klaassen, and Zhong.
References
- Alcorn J, Elbarbry FA, Allouh MZ, McNamara PJ. (2007) Evaluation of the assumptions of an ontogeny model of rat hepatic cytochrome P450 activity. Drug Metab Dispos 35:2225–2231 [DOI] [PubMed] [Google Scholar]
- Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. (2007) β-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol 292:G1578–G1585 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300 [Google Scholar]
- Blake MJ, Castro L, Leeder JS, Kearns GL. (2005) Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med 10:123–138 [DOI] [PubMed] [Google Scholar]
- Cherala G, Shapiro BH, D'mello AP. (2007) Effect of perinatal low protein diets on the ontogeny of select hepatic cytochrome P450 enzymes and cytochrome P450 reductase in the rat. Drug Metab Dispos 35:1057–1063 [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Sarfarazi M, Schenkman JB. (2004) Xenobiotic-metabolizing cytochromes P450 in ontogeny: evolving perspective. Drug Metab Rev 36:549–568 [DOI] [PubMed] [Google Scholar]
- Danielson PB. (2002) The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab 3:561–597 [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (2008) Cytochrome p450 and chemical toxicology. Chem Res Toxicol 21:70–83 [DOI] [PubMed] [Google Scholar]
- Hart SN, Cui Y, Klaassen CD, Zhong XB. (2009) Three patterns of cytochrome P450 gene expression during liver maturation in mice. Drug Metab Dispos 37:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RN. (2007) Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol 21:169–175 [DOI] [PubMed] [Google Scholar]
- Hines RN. (2008) The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 118:250–267 [DOI] [PubMed] [Google Scholar]
- Hines RN, McCarver DG. (2002) The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther 300:355–360 [DOI] [PubMed] [Google Scholar]
- Hrycay EG, Bandiera SM. (2009) Expression, function and regulation of mouse cytochrome P450 enzymes: comparison with human P450 enzymes. Curr Drug Metab 10:1151–1183 [DOI] [PubMed] [Google Scholar]
- Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. (2006) Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev 20:2293–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeder JS, Gaedigk R, Marcucci KA, Gaedigk A, Vyhlidal CA, Schindel BP, Pearce RE. (2005) Variability of CYP3A7 expression in human fetal liver. J Pharmacol Exp Ther 314:626–635 [DOI] [PubMed] [Google Scholar]
- Li D, Gaedigk R, Hart SN, Leeder JS, Zhong XB. (2012) The role of CYP3A4 mRNA transcript with shortened 3′-untranslated region in hepatocyte differentiation, liver development, and response to drug induction. Mol Pharmacol 81:86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Huang J, Jiang Y, Zeng Y, He F, Zhang MQ, Han Z, Zhang X. (2009a) Multi-stage analysis of gene expression and transcription regulation in C57/B6 mouse liver development. Genomics 93:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cui Y, Hart SN, Klaassen CD, Zhong XB. (2009b) Dynamic patterns of histone methylation are associated with ontogenic expression of the Cyp3a genes during mouse liver maturation. Mol Pharmacol 75:1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JH, Oliver B. (2011) Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628 [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Sinal CJ. (2008) Mice as clinically relevant models for the study of cytochrome P450-dependent metabolism. Clin Pharmacol Ther 83:818–828 [DOI] [PubMed] [Google Scholar]
- Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. (2008) The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320:1344–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Gonzalez FJ. (1987) P450 genes: structure, evolution, and regulation. Annu Rev Biochem 56:945–993 [DOI] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 40:1413–1415 [DOI] [PubMed] [Google Scholar]
- Pearce RE, Gotschall RR, Kearns GL, Leeder JS. (2001) Cytochrome P450 Involvement in the biotransformation of cisapride and racemic norcisapride in vitro: differential activity of individual human CYP3A isoforms. Drug Metab Dispos 29:1548–1554 [PubMed] [Google Scholar]
- Pesole G, Grillo G, Larizza A, Liuni S. (2000) The untranslated regions of eukaryotic mRNAs: structure, function, evolution and bioinformatic tools for their analysis. Brief Bioinform 1:236–249 [DOI] [PubMed] [Google Scholar]
- Rowell M, Zlotkin S. (1997) The ethical boundaries of drug research in pediatrics. Pediatr Clin North Am 44:27–40 [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Beach DL, Guzelian PS. (1994) Selective expression of cytochrome P450 CYP3A mRNAs in embryonic and adult human liver. Pharmacogenetics 4:11–20 [DOI] [PubMed] [Google Scholar]
- Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ. (2003) Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307:573–582 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tréluyer JM, Rey E, Sonnier M, Pons G, Cresteil T. (2001) Evidence of impaired cisapride metabolism in neonates. Br J Clin Pharmacol 52:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. (2008) Alternative isoform regulation in human tissue transcriptomes. Nature 456:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.