Abstract
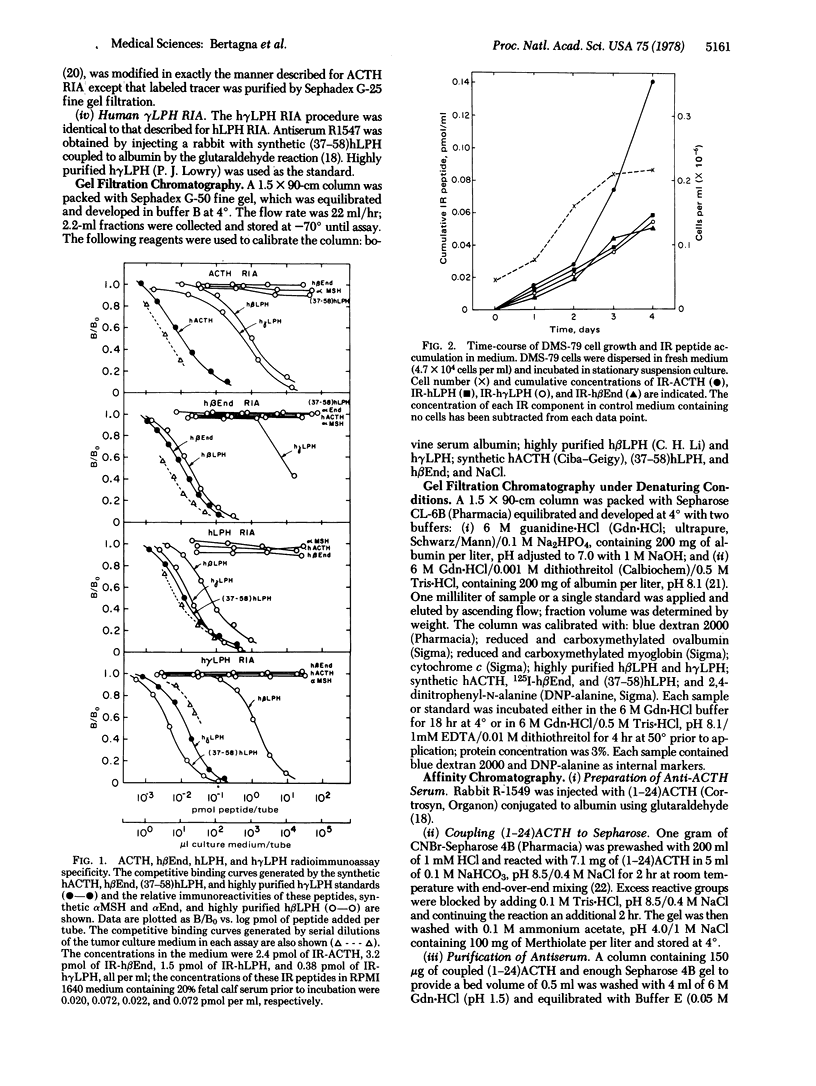
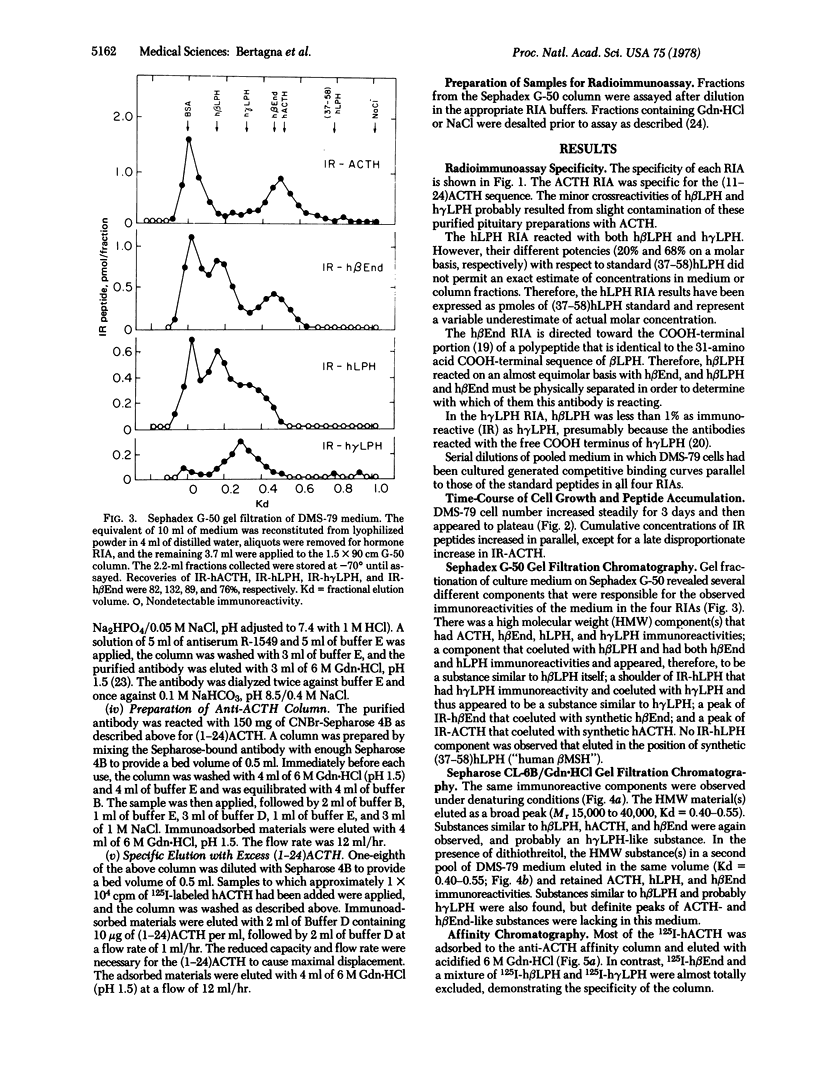
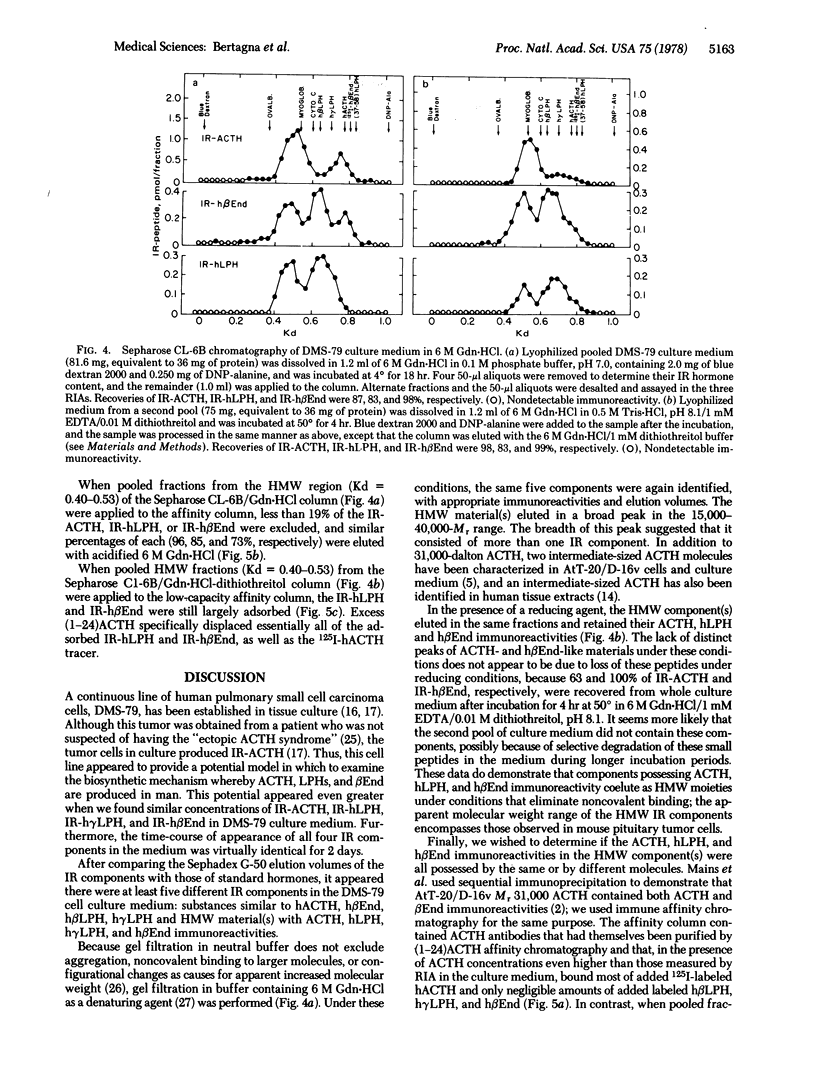
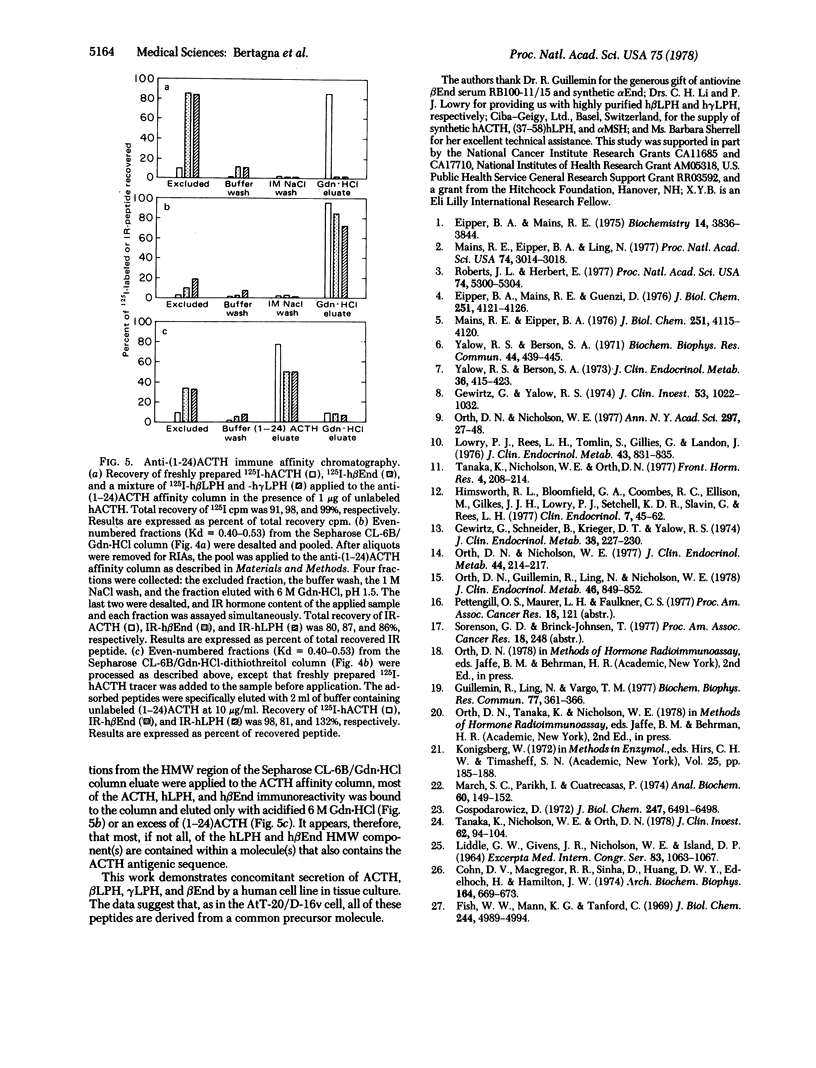
A continuous line (DMS-79) of human pulmonary small cell carcinoma cells was shown to secrete immunoreactive adrenocorticotropin (ACTH), lipotropin, and beta-endorphin concomitantly into the culture medium. Gel filtration of the culture medium demonstrated at least five components: high molecular weight material(s) that had ACTH, lipotropin, and beta-endorphin immunoreactivities and materials similar to ACTH, beta-lipotropin, gamma-lipotropin, and beta-endorphin in their immunoreactivities and apparent molecular weights. The same components were observed when gel filtration was carried out in 6 M guanidine-HCl, and the high molecular weight material(s) appeared to consist of more than one component, with molecular weights in the range of 15,000-40,000. Immune affinity chromatography of the high molecular weight component(s) from gel filtration with a specific anti-(1-24)ACTH serum demonstrated that the ACTH, lipotropin, and beta-endorphin immunoreactivities were possessed by the same molecule(s), suggesting that ACTH, lipotropins, and beta-endorphin were derived from a common, high molecular weight precursor.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn D. V., Macgregor R. R., Sinha D., Huang D. W., Edelhoch H., Hamilton J. W. The migration behavior of proparathyroid hormone, parathyroid hormone, and their peptide fragments during gel filtration. Arch Biochem Biophys. 1974 Oct;164(2):669–673. doi: 10.1016/0003-9861(74)90079-4. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Guenzi D. High molecular weight forms of adrenocorticotropic hormone are glycoproteins. J Biol Chem. 1976 Jul 10;251(13):4121–4126. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. High molecular weight forms of adrenocorticotropic hormone in the mouse pituitary and in a mouse pituitary tumor cell line. Biochemistry. 1975 Aug 26;14(17):3836–3844. doi: 10.1021/bi00688a016. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Gewirtz G., Schneider B., Krieger D. T., Yalow R. S. Big ACTH: conversion to biologically active ACTH by trypsin. J Clin Endocrinol Metab. 1974 Feb;38(2):227–230. doi: 10.1210/jcem-38-2-227. [DOI] [PubMed] [Google Scholar]
- Gewirtz G., Yalow R. S. Ectopic ACTH production in carcinoma of the lung. J Clin Invest. 1974 Apr;53(4):1022–1032. doi: 10.1172/JCI107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Single step purification of ovine luteinizing hormone by affinity chromatography. J Biol Chem. 1972 Oct 25;247(20):6491–6498. [PubMed] [Google Scholar]
- Guillemin R., Ling N., Vargo T. Radioimmunoassays for alpha-endorphin and beta-endorphin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):361–366. doi: 10.1016/s0006-291x(77)80205-2. [DOI] [PubMed] [Google Scholar]
- Himsworth R. L., Bloomfield G. A., Coombes R. C., Ellison M., Gilkes J. J., Lowry P. J., Setchell K. D., Slavin G., Rees L. H. 'Big ACTH' and calcitonin in an ectopic hormone secreting tumour of the liver. Clin Endocrinol (Oxf) 1977 Jul;7(1):45–62. doi: 10.1111/j.1365-2265.1977.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Lowry P. J., Rees L. H., Tomlin S., Gillies G., Landon J. Chemical characterization of ectopic ACTH purified from a malignant thymic carcinoid tumor. J Clin Endocrinol Metab. 1976 Oct;43(4):831–835. doi: 10.1210/jcem-43-4-831. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Guillemin R., Ling N., Nicholson W. E. Immunoreactive endorphins, lipotropins and corticotropins in a human nonpituitary tumor: evidence for a common precursor. J Clin Endocrinol Metab. 1978 May;46(5):849–852. doi: 10.1210/jcem-46-5-849. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E. High molecular weight forms of human ACTH are glycoproteins. J Clin Endocrinol Metab. 1977 Jan;44(1):214–217. doi: 10.1210/jcem-44-1-214. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. Molecular size and plasma levels of immunoreactive beta-MSH under physiological and pathological conditions in man. Front Horm Res. 1977;4:208–214. doi: 10.1159/000400368. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. The nature of the immunoreactive lipotropins in human plasma and tissue extracts. J Clin Invest. 1978 Jul;62(1):94–104. doi: 10.1172/JCI109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Characteristics of "big ACTH" in human plasma and pituitary extracts. J Clin Endocrinol Metab. 1973 Mar;36(3):415–423. doi: 10.1210/jcem-36-3-415. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]


