Abstract
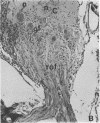
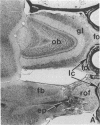
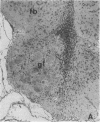
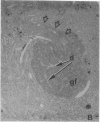
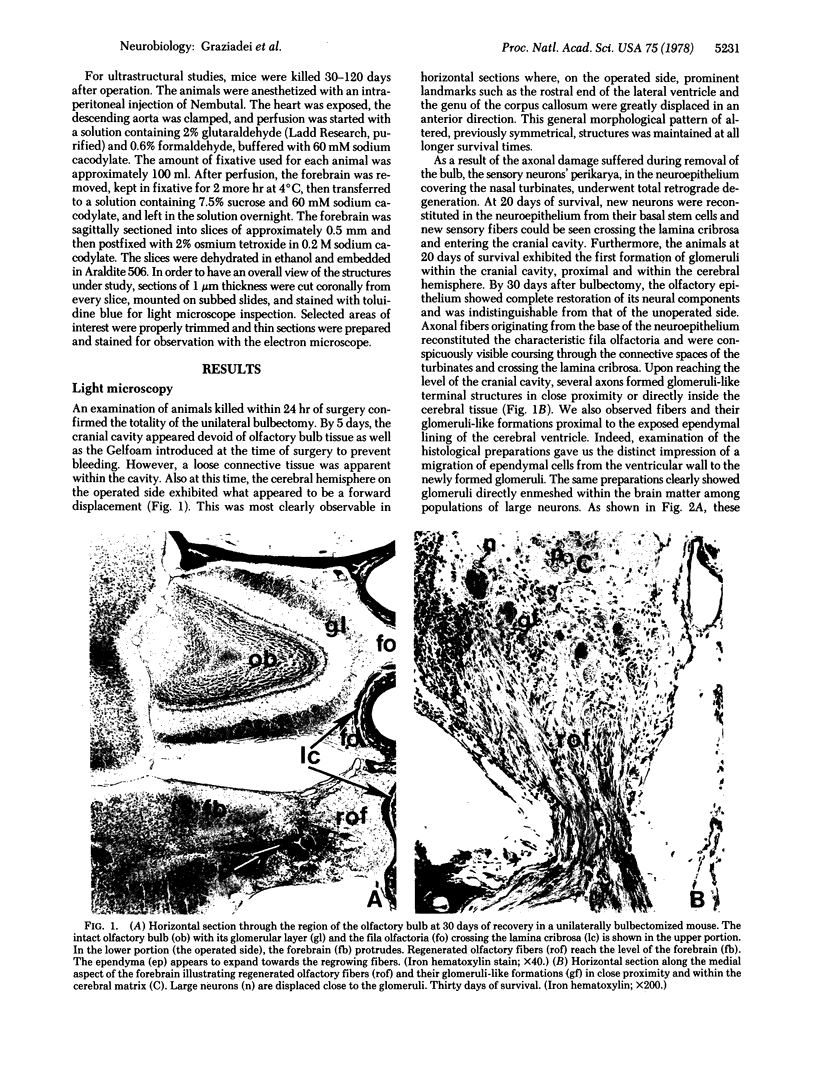
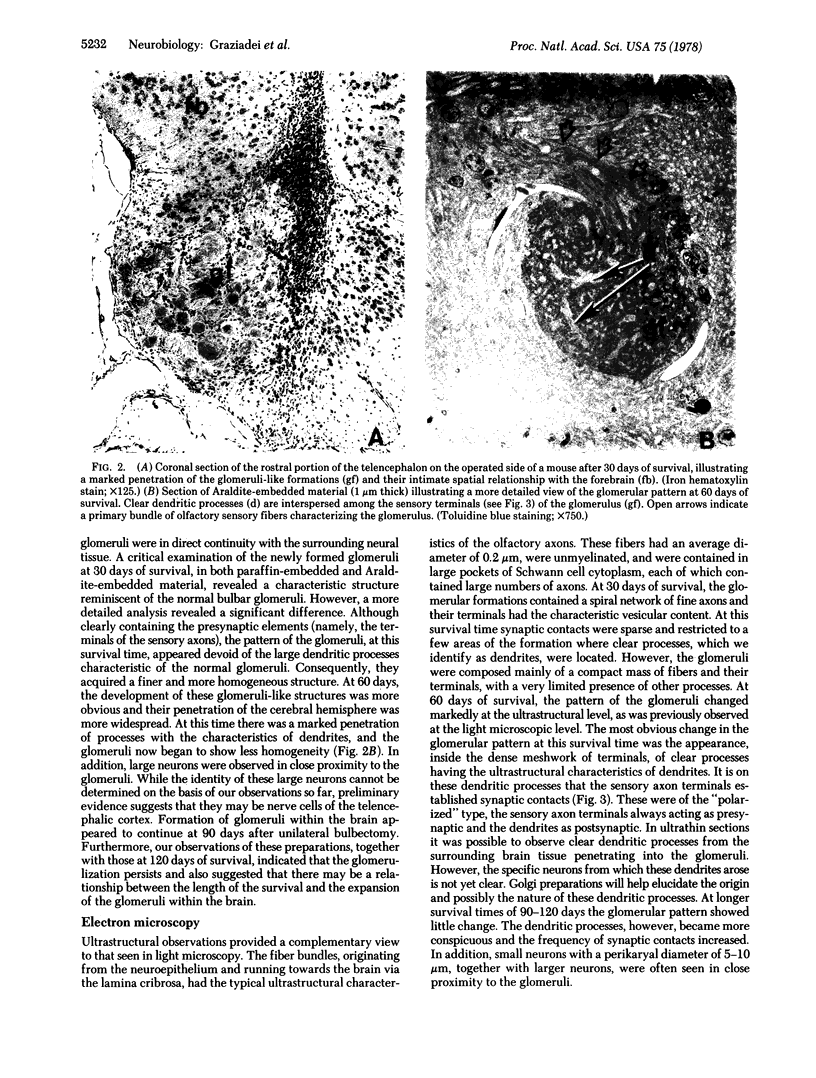
We removed the right olfactory bulb in neonatal mice, leaving the bulb on the left side intact as an internal control. At 5 days of survival time, we observed that the right cerebral hemisphere was displaced forward to occupy the region made vacant by removal of the bulb. The frontal cortex was, consequently, in close proximity to the lamina cribrosa. As a result of bulb ablation and severance of the fila olfactoria, the sensory perikarya underwent total retrograde degeneration, which peaked at 8 days. New neurons differentiated in the neuroepithelium from basal stem cells and, at 30 days of survival, mature sensory neurons were reconstituted. These new elements sent their axons through the lamina cribrosa to reach the protruding cerebral hemisphere, penetrating it and forming glomeruli-like structures directly in the host tissue. The "glomerulization" of the sensory fibers persisted and actually expanded between 60 and 120 days. The new glomeruli were organized intimately within the brain tissue, and large neurons of the cortex were observed to be in close proximity. Ultrastructural observations of the newly formed glomeruli demonstrated that typical sensory axon terminals profusely branched and synapsed with unidentified postsynaptic processes that penetrated the glomeruli from the surrounding cerebral tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Graziadei P. P. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5(1):113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., DeHan R. S. Neuronal regeneration in frog olfactory system. J Cell Biol. 1973 Nov;59(2 Pt 1):525–530. doi: 10.1083/jcb.59.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei P. P., Metcalf J. F. Autoradiographic and ultrastructural observations on the frog's olfactory mucosa. Z Zellforsch Mikrosk Anat. 1971;116(3):305–318. doi: 10.1007/BF00330630. [DOI] [PubMed] [Google Scholar]
- Guth L. History of central nervous system regeneration research. Exp Neurol. 1975 Sep;48(3 Pt 2):3–15. doi: 10.1016/0014-4886(75)90168-5. [DOI] [PubMed] [Google Scholar]
- Harding J. W., Getchell T. V., Margolis F. L. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978 Jan 27;140(2):271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Harding J., Graziadei P. P., Monti Graziadei G. A., Margolis F. L. Denervation in the primary olfactory pathway of mice. IV. Biochemical and morphological evidence for neuronal replacement following nerve section. Brain Res. 1977 Aug 19;132(1):11–28. doi: 10.1016/0006-8993(77)90703-x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977 Sep 9;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kerr F. W. Structural and functional evidence of plasticity in the central nervous system. Exp Neurol. 1975 Sep;48(3 Pt 2):16–31. doi: 10.1016/0014-4886(75)90169-7. [DOI] [PubMed] [Google Scholar]
- Moulton D. G. Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci. 1974 Sep 27;237(0):52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- Oley N., DeHan R. S., Tucker D., Smith J. C., Graziadei P. P. Recovery of structure and function following transection of the primary olfactory nerves in pigeons. J Comp Physiol Psychol. 1975 Feb;88(2):477–495. doi: 10.1037/h0076401. [DOI] [PubMed] [Google Scholar]