Abstract
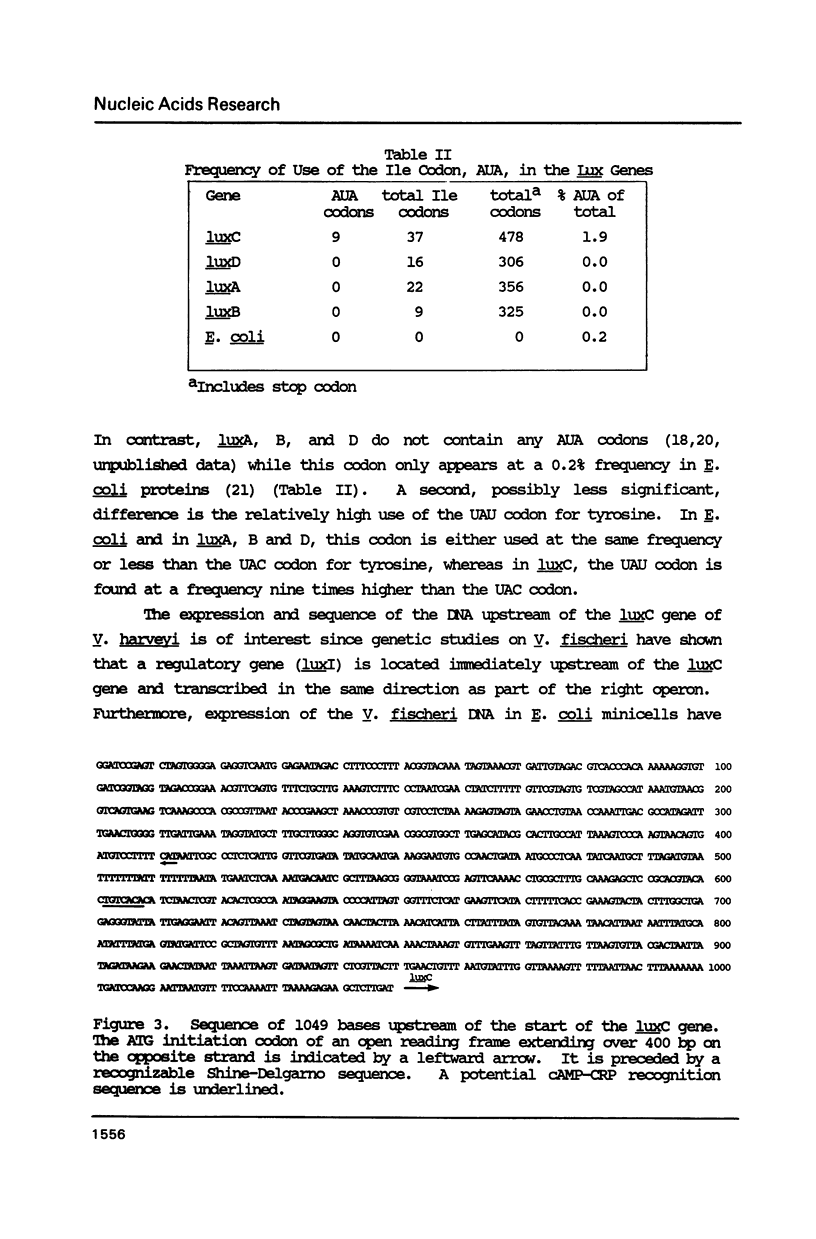
The nucleotide sequence of the luxC gene (1431 bp) and the upstream DNA (1049 bp) of the luminescent bacterium Vibrio harveyi has been determined. The luxC gene can be translated into a polypeptide of 55 kDa in excellent agreement with the molecular mass of the reductase polypeptide required for synthesis of the aldehyde substrate for the bioluminescent reaction. Analyses of codon usage showed a high frequency (1.9%) of the isoleucine codon, AUA, in the luxC gene compared to that found in Escherichia coli genes (0.2%) and its absence in the luxA, B and D genes. The low G/C content of the luxC gene and upstream DNA (38-39%) compared to that found in the other lux genes of V. harveyi (45%) was primarily due to a stretch of 500 nucleotides with only a 24% G/C content, extending from 200 bp inside lux C to 300 bp upstream. Moreover, an open reading frame did not extend for more than 48 codons between the luxC gene and 600 bp upstream at which point a gene transcribed in the opposite direction started. As the lux system in the luminescent bacterium, V. fischeri, contains a regulatory gene immediately upstream of luxC transcribed in the same direction, these results show that the organization and regulation of the lux genes have diverged in different luminescent bacteria.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers D., Meighen E. Purification and characterization of a bioluminescence-related fatty acyl esterase from Vibrio harveyi. J Biol Chem. 1985 Jun 10;260(11):6938–6944. [PubMed] [Google Scholar]
- Carey L. M., Rodriguez A., Meighen E. Generation of fatty acids by an acyl esterase in the bioluminescent system of Photobacterium phosphoreum. J Biol Chem. 1984 Aug 25;259(16):10216–10221. [PubMed] [Google Scholar]
- Cohn D. H., Mileham A. J., Simon M. I., Nealson K. H., Rausch S. K., Bonam D., Baldwin T. O. Nucleotide sequence of the luxA gene of Vibrio harveyi and the complete amino acid sequence of the alpha subunit of bacterial luciferase. J Biol Chem. 1985 May 25;260(10):6139–6146. [PubMed] [Google Scholar]
- Cohn D. H., Ogden R. C., Abelson J. N., Baldwin T. O., Nealson K. H., Simon M. I., Mileham A. J. Cloning of the Vibrio harveyi luciferase genes: use of a synthetic oligonucleotide probe. Proc Natl Acad Sci U S A. 1983 Jan;80(1):120–123. doi: 10.1073/pnas.80.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1985 Oct;164(1):45–50. doi: 10.1128/jb.164.1.45-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston T. C., Thompson R. B., Baldwin T. O. Nucleotide sequence of the luxB gene of Vibrio harveyi and the complete amino acid sequence of the beta subunit of bacterial luciferase. J Biol Chem. 1986 Apr 15;261(11):4805–4811. [PubMed] [Google Scholar]
- Maruyama T., Gojobori T., Aota S., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1986;14 (Suppl):r151–r197. doi: 10.1093/nar/14.suppl.r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. D., Boylan M., Evans J. F., Hasel K. W., Meighen E. A., Graham A. F. Polycistronic mRNAs code for polypeptides of the Vibrio harveyi luminescence system. J Bacteriol. 1985 Mar;161(3):995–1001. doi: 10.1128/jb.161.3.995-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C., Byers D., Graham A. F., Meighen E. A. Expression of bioluminescence by Escherichia coli containing recombinant Vibrio harveyi DNA. J Bacteriol. 1987 Jan;169(1):247–253. doi: 10.1128/jb.169.1.247-253.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M. C., Amass K., de Crombrugghe B. Molecuar model of the DNA interaction site for the cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2213–2217. doi: 10.1073/pnas.78.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Riendeau D., Meighen E. Purification of the acyl coenzyme A reductase component from a complex responsible for the reduction of fatty acids in bioluminescent bacteria. Properties and acyltransferase activity. J Biol Chem. 1983 Apr 25;258(8):5233–5237. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L., Rodriquez A., Meighen E. Differential acylation in vitro with tetradecanoyl coenzyme A and tetradecanoic acid (+ATP) of three polypeptides shown to have induced synthesis in Photobacterium phosphoreum. J Biol Chem. 1984 Feb 10;259(3):1409–1414. [PubMed] [Google Scholar]


