Abstract
The survival, proliferation, self-renewal and differentiation of human pluripotent stem cells (hPSCs, including human embryonic stem cells and human induced pluripotent stem cells) involve a number of processes that require cell-cell and cell-matrix interactions. The cell adhesion molecules (CAMs), a group of cell surface proteins play a pivotal role in mediating such interactions. Recent studies have provided insights into the essential roles and mechanisms of CAMs in the regulation of hPSC fate decisions. Here, we review the latest research progress in this field and focus on how E-cadherin and several other important CAMs including classic cadherins, Ig-superfamily CAMs, integrins and heparin sulfate proteoglycans control survival and differentiation of hPSCs
Keywords: CAMs, E-cadherin, hESCs, hiPSCs, HSPGs, IgSF CAMs, Integrins, pluoripotency, self-renewal
Introduction
Our understanding of the molecular and cellular mechanisms that control self-renewal and pluripotency of human pluripotent stem cells and our progress toward harnessing the regenerative potential of these cells to treat human diseases are advancing at a rapid rate. Human pluripotent stem cells include human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). The first hESCs were generated in 1998 from the inner cell mass of blastocyst-stage embryos.1 Their unique capacity for indefinite self-renewal (unlimited proliferation) in vitro coupled with their ability to differentiate into almost any cell type present in the adult body (pluripotency) provide a potentially unlimited source of cells for cell replacement therapies. The demonstration, in 2007, that hiPSCs could be generated from human fibroblast cells has further expanded this therapeutic potential.2,3 Like hESCs, hiPSCs are capable of differentiating into almost any tissue type in the body.2-8 Production of hiPSCs, therefore, have been hailed as a transformative breakthrough in regenerative medicine with potential to lead to disease-based modeling and patient-tailed therapies. Clearly, understanding the mechanisms that control self-renewal, pluripotency and differentiation of hESCs and hiPSCs is key to realizing this potential and validating new therapies for many human degenerative diseases that currently have no cure.
Our understanding of the regulatory networks underlying the initiation and maintenance of hESC and hiPSC self-renewal and pluripotent states has expanded considerably during the past 14 years. Key transcription factors, including Oct4, Sox2 and Nanog, have been found to act in autoregulatory modules to specify the pluripotent state of both mouse and human pluripotent stem cells.9 Other regulatory cues, such as supporting cells,1,10,11 extracellular matrix,12,13 low oxygen culture,14,15 growth factors,16-18 small molecules19-23 and various signaling pathways24-29 have also been extensively studied and their relevance in self-renewal and differentiation of human pluripotent stem cells is now well recognized. However, the long-standing question as to why hESCs are so sensitive to the disruption of cell-cell contact, undergoing massive cell death when dissociated into single cell suspensions has only begun to be understood in recent years, which is key to propagating sufficient numbers of hESCs and hiPSCs for regenerative therapy.
A growing number of reports now reveal that, in addition to the support cells, extracellular matrix and growth factor cues, cell adhesion molecule (CAM)-mediated cohesive interaction among hESCs (and hiPSCs) and between the cells and their neighboring instructive/support cells and extracellular matrix contribute significantly to the self-renewal and the pluripotent state of human pluripotent stem cells. CAMs are the proteins on the surface of mammalian cells that contribute to juxtacrine cell-cell binding or cell-matrix binding. Two decades of structural biology studies have characterized domain topologies and binding mechanisms at the molecular/atomic level for key members of all the main classes of CAMs: the cadherins, integrins, selectins and immunoglobulin superfamily (IgSF).30 Cell biology approaches have shown that CAMs perform a wide range of functions at cell-cell and cell-matrix interfaces, such as mechanical support, target recognition, cell differentiation, initiation and regulation of signaling platforms, etc.30 Notably, numerous CAM family members have recently been identified on the surface of human pluripotent stem cells and found to regulate their self-renewal and pluripotency.12,31-35
This review will summarize recent progress in the study of CAM function in human pluripotent stem cells. We will focus on the roles of E-cadherin and several other molecules from following CAM superfamilies: the cadherins (N-cadherin and VE-cadherin), the integrins and the IgSF CAMs. In addition, we will also briefly discuss the significant role of heparin sulfate proteoglycans (HSPGs), a group of cell-matrix adhesion molecules critical in mediating the interaction between human pluripotent stem cells and extracellular matrix, in the regulation of hESC and hiPSC fate.
Cell-Cell Adhesion Molecules in Human Pluripotent Stem Cells: E-, N- and VE-Cadherins
The cadherin superfamily is comprised of more than 100 members in vertebrates and can be further divided into classic and non-classic subfamilies. Cadherins were originally identified as cell surface glycoproteins responsible for Ca2+-dependent homophilic cell-cell adhesion during morula compaction in the preimplantation mouse embryo and during chick development in the early 1980s.36-39 In the three decades since their discovery, it has become clear that the role of cadherins is not limited to mechanical adhesion between cells. While beyond the scope of this review, the history, general features, structure and model of homophilic binding of cadherin have already been reviewed extensively in a number of different contexts.30,40
Among the classic cadherin subfamily, the roles of E-cadherin, N-cadherin and VE-cadherin have been mostly studied in human pluripotent stem cells. These three classical cadherins were originally named for the tissue in which they were prominently expressed: epithelial cadherin (E-cadherin) in skin epithelia, neural cadherin (N-cadherin) in the central nervous system and vascular endothelial cadherin (VE-cadherin) in blood vessel endothelia. Our current understanding of how these three classical cadherins modulate hESC phenotype is described below.
The molecular structure and the binding sites of E-cadherin
E-cadherin is a transmembrane glycoprotein that mediates calcium-dependent, homophilic cell-cell adhesion in all epithelial tissues including embryonic stem cells.40 As shown in Figure 1, E-cadherin has long extracellular and cytoplasmic domains. The extracellular domain of E-cadherin establishes homophilic interactions between neighboring cells, while its cytoplasmic tail associates with an array of multifunctional adaptor proteins. These intracellular adaptor proteins link cell-cell adhesion to the actin-myosin network and other intracellular signaling pathways.
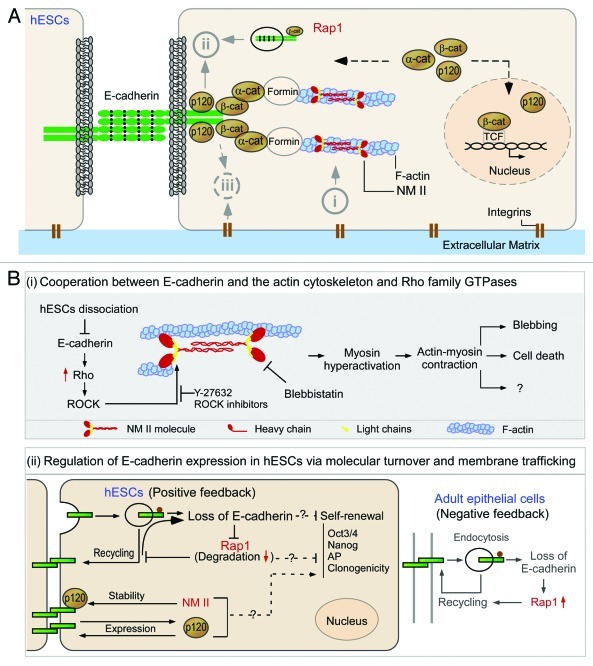
Figure 1. E-cadherin and its associated molecules in the survival and differentiation of human embryonic stem cells. (A) The molecular structure and the binding sites of E-cadherin and its connection with actin cytoskeleton. The extracellular region of E-cadherin consists of five cadherin-type repeats (extracellular cadherin domains) that are bound together by Ca2+ ions. The cytoplasmic tails of E-cadherin bind to p120-catenin (p120) and β-catenin (β-cat), while β-catenin binds to monomeric α-catenin (α-cat) which indirectly anchors the cadherin-catenin complexes to the actin filaments (F-actin). NM II molecules assemble into bipolar filaments by interactions between their rod domains. These filaments bind to actin through their head domains where the ATPase activity enables a conformational change that moves actin filaments in an anti-parallel manner. (B) Schematic view of the pathways associated with E-cadherin in the survival of human embryonic stem cells. (i) Cooperation between E-cadherin and the actin cytoskeleton and Rho family GTPases in the regulation of survival of dissociated human embryonic stem cells (hESCs). The subunit of non-muscle myosin II (NM II) forms a dimer through interactions between the α-helical coiled-coil rod domains. The globular head domain contains the actin-binding regions and the enzymatic Mg2+-ATPase motor domains. The light chains bind to the heavy chains at the lever arms that link the globular head and rod domains. Dissociation of hESCs results in disruption of E-cadherin mediated cell-cell adhesions and subsequently leads to Rho/ROCK activation and phosphorylation of myosin light chain. Y-27632 inhibits ROCK activation and therefore suppresses the phosphorylation of myosin light chain and promotes the survival and cloning efficiency of hESCs. Blebbistatin inhibit heavy chains and facilitate survival of dissociated hESCs. (ii) Regulation of E-cadherin expression in hESCs via molecular turnover and membrane trafficking. Unlike epithelial cells, hESCs lack a negative feedback-like mechanism between Rap1 and E-cadherin. After disassembly of adherens junctions, Rap1 is delivered to lysosomes and degraded, which further hampers hESC clonogenicity. There is another unique positive feedback loop between p120-catenin (p120) and E-cadherin in hESCs. Unlike epithelial cells, E-cadherin increases p120 production, while p120 boosts E-cadherin expression. p120 seems to facilitate E-cadherin accumulation at cell-cell junctions, thereby enhancing the self-renewal of hESCs.
The cytoplasmic part of E-cadherins contains binding sites for two catenins, p120-catenin and β-catenin. While p120-catenin is involved in the delivery and stabilization of adhesion complexes at the plasma membrane,41-43 β-catenin provides the connection to α-catenin. The α-catenin complex in turn interacts with formin to nucleate actin filaments at the adherens junctions and uses an unknown mechanism to link cadherin-catenin complexes at the membrane with the actin cytoskeleton.44-46 Strengthening of E-cadherin-mediated cell-cell adhesion is dependent on intact actin cytoskeleton, which includes motor protein non-muscle myosin (NM) II. The NM II motor protein is a hexamer of three subunits (two heavy chains with a globular N-terminal motor domain for each, two regulatory light chains and two essential light chains) that converts the energy from ATP hydrolysis into mechanical work and is post-translationally regulated by the phosphorylation of its light and heavy chains.47 In addition to linking cadherin and α-catenin with the actin-myosin cytoskeleton, β-catenin is also a central part of the canonical Wnt signaling pathway.48 The molecular structure and the binding sites of E-cadherin, and its connection with the actin-myosin network, are illustrated in Figure 1A.
The role of E-cadherin in hESCs
As one of the archetypal cadherin families, E-cadherin is essential for epithelialization of the early mouse embryo, cell rearrangement, tissue morphogenesis, establishment of cell polarity and maintenance of tissue architecture.40 Given the inherent origin of undifferentiated hESCs (derived from the inner cell mass of the blastocyst1 wherein E-cadherin was found on human oocytes and early embryos49), one may speculate that E-cadherin expression reflects the tissue of origin from which the hESCs are initially derived and, as such, that E-cadherin mediated cell-cell cohesion plays an important role in the survival and self-renewal of hESCs. Indeed, E-cadherin has been used as an undifferentiated marker to identify hESCs. E-cadherin is co-expressed with other typical undifferentiated markers, such as SSEA4, Tra-1-60, Tra-1-81, alkaline phosphatase, as well as pluripotency factors Oct4 (also known as Pou5f1), Nanog and Sox2 in undifferentiated hESCs.21,50-52 The use of E-cadherin to demarcate differentiated and undifferentiated hESCs is based on definitive expression kinetics. Expression in undifferentiated hESCs is decreased immediately after induction of differentiation.50,51 The positive expression of E-cadherin in characteristic colonies is usually considered as one of the undifferentiated properties during the hESC maintenance.21,53,54 Accordingly, in the recent derivation of iPSCs from somatic cells, development of compact colonies with tight cellular association and E-cadherin expression has been used and is now widely accepted as a simple and reliable readout for conversion of non-ESCs to an ESC-like state.2,3,55,56
Beyond use as a supplementary marker for undifferentiated hESCs,21,50-52 the active role of E-cadherin in hESC self-renewal was not further pursued until recently. One potential explanation for this might be a clear demonstration in 1996 that E-cadherin was dispensable for mouse ESC maintenance.57 More recent studies, however, report that hESCs may resemble mouse epiblast stem cells (EpiSCs)58,59 to a greater extent rather than mouse ESCs in their molecular profile and growth factors requirements.60,61 Intriguingly, a current report described a mouse EpiSC-like cell type, termed FAB-SCs (i.e., fibroblast growth factor, Activin and BIO-derived stem cells),62 that share expression markers with EpiSCs but are unable to differentiate. Remarkably, these differentiation-resistant FAB-SCs can be steered to convert into their naïve pluripotent state (like ESCs) by ectopic expression of E-cadherin, suggesting an unexpected role for E-cadherin-mediated cell-cell adhesions in the maintenance of pluripotent state.62 Taken together, the molecular and biological similarities between hESCs and mouse EpiSCs indicate that E-cadherin corresponds, at least partially, to the survival, self-renewal and pluripotency state of hESCs.
Recent reports from several groups, including our own, have indeed demonstrated that E-cadherin directly contributes to hESC survival and self-renewal.22,32,63-66 Unlike mouse ESCs, dissociated hESCs are highly susceptible to apoptosis and undergo massive cell death upon seeding as single cells at low cell density21,67 or in suspension culture.63 Several lines of evidence recently suggest that the primary cause of hESC death following cell dissociation comes from an irreparable disruption of E-cadherin signaling.22,32,63-66,68 Even attenuation of E-cadherin interactions will cause hESC blebbing and apoptosis, such as treatment with a Ca2+ chelator, incubation with an E-cadherin blocking antibody, or knockdown E-cadherin with siRNA or shRNA.64-66 Conversely, timely reestablishment of E-cadherin-mediated cell-cell contact (within temporal windows of intervention) facilitates survival and proliferation of dissociated single hESCs.32,63,69 At a high plating-density, single hESCs can reaggregate together via cell motility and reestablish cell-cell contacts and form typical undifferentiated polyclonal colonies.32,63 Such motility-induced reaggregation occurred very often (~70%) if the distance between two single hESCs was < 6.4 μm and very rarely if the distance was > 150 µm. As a result, if single hESC could not efficiently reaggregate together at a low plating density, they would lose E-cadherin and alkaline phosphatase expression, eventually undergoing cell death or differentiation.63 Consistently, 2,4-disubstituted thiazole, a small molecule capable of stabilizing E-cadherin after cell dissociation, was shown to reduce hESC death.22 Together, these results suggest that E-cadherin-mediated cellular adhesion plays a determinative role in the control of hESC death or survival after dissociation.
In addition to modulating survival and proliferation, E-cadherin also contributes to the self-renewal and pluripotency of hESCs. Upregulation of E-cadherin expression markedly enhances the cloning efficiency and self-renewal capacity of hESCs.63 Further, small molecules capable of upregulating E-cadherin expression facilitate hESC self-renewal.22 Conversely, inhibition of E-cadherin expression with specific blocking antibody suppresses hESC self-renewal32,66 and results in not only an altered response to growth factor stimulation but also an overall decrease in hESC proliferation.51 Moreover, suppression of E-cadherin expression by siRNA or by blocking antibodies lead to a rapid loss in the expression of pluripotency markers Oct4, Nanog and Sox2 in hESCs.32,66 Oct4, Nanog and Sox2 constitute a core set of transcription factors crucial for the maintenance of pluripotent state of ESCs.70,71 It has yet to be established how (or indeed if) signaling events downstream of E-cadherin connect with these core set of transcriptional factors.
Taken together, the aforementioned findings yield a conclusion that E-cadherin is not only a marker for undifferentiated hESCs but also actively contributes to the death/survival, self-renewal and pluripotent state of hESCs.22,32,63-66
Molecular mechanisms underlying the role of E-cadherin in hESCs
Recent studies have identified multiple regulatory mechanisms involved in the interplay between E-cadherin and cell signaling in the regulation of cell fate decisions of hESCs. As illustrated in Figure 1B, several key mechanistic elements participate in a range of regulatory events in hESCs, such as cooperation between E-cadherin and the actin cytoskeleton and Rho family GTPases,63-66,72 regulation of E-cadherin expression at the hESC surface by turnover and membrane trafficking32,66 and interplay between E-cadherin and cell signaling.22,32,51,69
Cooperation between E-cadherin and the actin cytoskeleton and Rho family GTPases
Increasing evidence has shown that the activity of NM II motor protein, a major component of actin cytoskeleton, is involved in (or regulates) the survival and self-renewal of hESCs, likely through E-cadherin.64-66,72 These studies have demonstrated that NM II exhibits dual functions in hESCs.64,65,72 For hESC colonies where E-cadherin-mediated cell-cell contacts have been established, NM II may help stabilize Oct4-Nanog-Sox2 circuitry possibly by regulating E-cadherin-mediated intercellular adhesion and by stabilizing p120-catenin protein.66 For dissociated single hESCs, NM II may accelerate the apoptosis of single hESCs through the Rho-Rock (Rho-associated kinase)-Myosin signaling axis.64,65,72,73
For hESC colonies, NM II forms a network with E-cadherin and p120-catenin. This regulatory network helps hESCs remain in close contact and thus maintain their undifferentiated conditions.66 NM II, the two-headed conventional myosin, consists of three isoforms, IIA, IIB and IIC.74 The isoform IIA and IIB (not IIC) are predominantly localized to plasma membranes, and NM IIA has been observed to colocalize with E-cadherin in undifferentiated hESCs.66,72 Depletion of NM IIA (but not NM IIB), or treatment with blebbistatin (a small molecule specifically inhibiting the myosin heavy chain ATPase), reduces E-cadherin accumulation at the junctional sites and impairs the formation of characteristic hESC colonies. As a result, a great number of hESCs within the colonies can be dissociated from one another and spread out as single cells. Moreover, those hESC cells also show lower levels of alkaline phosphatase activity and lower level expressions of Sox2, Nanog and Oct4 proteins, indicating an impaired status of self-renewal. This study suggests that NM IIA is necessary for the formation of E-cadherin-mediated cell-cell contacts and that E-cadherin is essential for the formation of characteristic hESC colonies, for the stability of the Oct4-Nang-Sox2 circuitry and for the long-term survival of hESCs.
On the other hand, for dissociated single hESCs, blebbistatin treatment or selective knockdown of myosin heavy and light chains (or knockdown of ROCK1 or ROCK2) reduces apoptosis, promotes the expression of Oct4 and Nanog and enhances the survival and self-renewal of the dissociated hESCs.64-66,72,73 Three independent groups have recently elucidated a basic molecular mechanism regarding ROCK-dependent cell death in hESCs.64,65,72 Using multidisciplinary approaches (e.g., Ca2+ chelator EGTA to disrupt the cadherin-mediated cell attachment, E-cadherin-blocking antibodies, siRNA knockdown, FRET analysis, etc.), Ohgushi and colleagues65 found that the continuous loss of E-cadherin-dependent intercellular adhesion was responsible for blebbing and apoptosis of hESCs. Apoptosis was induced by actomyosin hyperactivation and triggered by the loss of E-cadherin-dependent intercellular contacts following ROCK activation. Further support has been provided by Chen and colleagues64 who observed that ROCK inhibition suppresses the phosphorylation of myosin light chain and promotes the cloning efficiency of hESCs, possibly by hampering actin-myosin contraction. It has been suggested that Rho activation coupled with Rac inhibition is a driver for dissociation-induced hESC apoptosis via ROCK-mediated myosin light chain phosphorylation. The Abr (actin binding region)-dependent “Rho-high/Rac-low” state may play a decisive role in initiating the dissociation-induced actomyosin hyperactivation and apoptosis in hESCs.65 Therefore, the actomyosin machinery downstream of Rho activation plays an important role in cell blebbing and apoptosis after hESC dissociation. This may explain why a selective ROCK inhibitor (Y-27632) exhibits a significant anti-apoptotic effect on dissociated single hESCs that was first reported in 2007.21
Regulation of E-cadherin expression in hESCs via molecular turnover and membrane trafficking
The dynamic properties of E-cadherin molecule and its complexes are observed both in vivo in Drosophila embryos75,76 and in vitro in epithelial cell culture.77-79 A pivotal role for various membrane trafficking pathways in regulating cellular transitions between quiescent adhesive states and more dynamic phenotypes have been reported.80-83 Regulation of E-cadherins expression by membrane trafficking is emerging as a key player in hESC self-renewal, and studies are also beginning to reveal how this process occurs.
It is apparent that endocytic recycling pathway involves the formation and maintenance of E-cadherin-mediated cell-cell cohesion in hESCs. A temporal interplay between the small G protein Rap1 and E-cadherin serves as a timely and efficient mechanism to regulate hESC self-renewal.32 Rap1 is a member of the Ras family of small G proteins.84 Rap1 is co-localized with E-cadherin on hESC membrane and also localized to the perinuclear region, which is required for the proper delivery of E-cadherin to the nascent cell-cell contact sites in hESCs.32 Rap1 impacts hESC clonogenicity by affecting the initiation and formation of E-cadherin mediated cell-cell contacts. In turn, the inadequate formation of E-cadherin-mediated cell-cell adhesion in hESCs exacerbates the diminution of E-cadherin by accelerating Rap1 degradation, which further hampers the clonogenic capacity and self-renewal of hESCs.32 Unlike epithelial cells,85-87 hESCs lack a negative feedback-like mechanism between Rap1 and E-cadherin. After disassembly of adherens junctions, Rap1 is delivered to lysosomes and degraded, which further hampers hESC clonogenicity. Thus, hESC dissociation to reseeding should be completed within 30 min to limit Rap1 delivery to the lysosome.32,63 It can be concluded that a functional crosstalk between Rap1 and E-cadherin along the endocytic recycling pathway contributes to the self-renewal of hESCs.
In addition, p120-catenin has recently received a great attention in cadherin trafficking.30 A role of p120-catenin in stabilizing cadherin junctions has been observed previously in epithelial tumor cell lines41,42 and in endothelial cells.43 Loss of p120 results in an increase in turnover rate of E-cadherin has been observed in epithelial cells.88 In hESCs, however, E-cadherin and p120-catenin uniquely work in a positive feedback loop: E-cadherin increases p120-catenin production, while p120-catenin boosts E-cadherin expression. p120-catenin seems to facilitate E-cadherin accumulation at cell-cell junctions, thereby enhancing the self-renewal of hESCs.66 It should be noted that E-cadherin dynamics and trafficking is a relatively new topic in hESCs. The ability for individual E-cadherin molecule or complexes to be continually formed and disassembled is vital for the preservation of hESC property and colony integrity during cell proliferation and colony reorganization and requires further investigation.
The interplay between E-cadherin and cell signaling
In addition to Rho family small GTPases,64-66,72 small G protein Rap132 and the p120-catenin,66 the interplay between E-cadherin and other signaling molecules is also important for the regulation of hESC fate. Xu and colleagues22 discovered that the primary cause of hESC death following enzymatic dissociation comes from an irreparable disruption of E-cadherin signaling, which leads to a fatal perturbation of integrin signaling. Furthermore, they found that cultivating hESCs under conditions that favor mouse ESC growth (e.g., in the presence of leukemia inhibitor factor instead of basic fibroblast growth factor, etc.) significantly increases E-cadherin levels and inhibits integrin signaling. Subsequently, self-renewal and survival of hESCs under mouse ESC culture conditions becomes less dependent on integrin signaling but more dependent on E-cadherin signaling.22 This study suggests that different growth factors/cytokines modify hESC utilization of different cell adhesion systems to maintain cell identity. hESCs may have a broad dynamic range of pluripotent states, likely regulated by different inputs from the E-cadherin and integrin and influenced by growth factors.
In addition, controllable overexpression of E-cadherin in hESCs using a doxycycline-inducible lentiviral system enhances the expression of the apoptotic inhibitory gene Bcl-xL, while suppressing the expression of the pro-apoptotic gene Caspase-3.63 Consistently, two recent studies showed a significant increase of survival and colonies in the single-cell suspension cultures after overexpression of Bcl-xL89 and Bcl290 genes in hESCs. Moreover, upregulation of E-cadherin expression leads to high clonogenicity in hESCs while knockdown results in the opposite outcome.63 Interestingly, E-cadherin-expressing feeder cells promote neural lineage restriction of hESCs,11 suggesting that direct E-cadherin engagement between hESCs and other type of cells may favor neural specification. It is apparent that the roles of E-cadherin signaling in hESC self-renewal and lineage specification is far from simple and in need of further mechanistic investigation.
E-cadherin expression enhances the derivation of induced pluripotent stem cells
The active role and function of E-cadherin in the regulation of pluripotent state of ESCs is further demonstrated in the derivation of iPSCs. Using Yamanaka’s factors (Oct4, Sox2, Klf4 and c-Myc), Aasen and colleagues91 have shown that reprogramming efficiency is 100-fold greater and 2-fold faster for the conversion of human epithelial cells (keratinocytes that already express E-cadherin) to hiPSCs than for human fibroblasts. Bypassing a requirement for mesenchymal-to-epithelial transition (MET) has been speculated for the high reprogramming efficiency of epithelial cells, although this hypothesis has yet to be assessed directly.
In 2010, several research groups independently demonstrated that undergoing MET is an essential early step of reprogramming92,93 and E-cadherin is required in the initial stages of this process.34,66,92,93 Reprogramming of fibroblasts into hiPSCs is a slow (2–3 weeks) and inefficient (< 1%) progress in which somatic cells gradually lose their differentiated identity and assume embryonic gene expression patterns and growth behaviors. Several groups have chronologically traced the events that occur during the first 2–3 weeks upon induction of the reprogramming factors in mouse embryonic fibroblasts. The first change in gene expression is the downregulation of somatic markers including key mesenchymal genes such as Snail and N-cadherin.92-94 Concomitantly, E-cadherin is upregulated when cells undergo a MET and start proliferating.92-94 Similar observations have been reported in reprogramming of human somatic cells.66 In addition, ectopic expression of human E-cadherin in mouse embryonic fibroblasts results in a 4-fold increase in iPSC colonies.34 In contrast, E-cadherin knockdown or inhibition reduces the reprogramming efficiency of mouse fibroblasts,34,92,93 human primary fibroblasts and keratinocytes.66 Further mechanistic study has demonstrated that the adhesive binding activity of E-cadherin is essential for the reprogramming process.34 Collectively, these data as well as previous work62 suggest that E-cadherin is crucial for the self-renewal of human pluripotent stem cells and reprogramming of adult cells to iPSCs.
Most recently, Redmer and colleagues have further demonstrated the importance of E-cadherin in reprogramming.95 They found that the forced expression of E-cadherin together with other three Yamanaka factors (Sox2, Klf4 and c-Myc) was sufficient to reprogram murine fibroblasts to iPSCs, without the need of Oct4.95 Although further testing needs to be done on human cells, their data suggest the spatial and mechanical input provided by E-cadherin alters cell fate. E-cadherin may directly or indirectly tie into the pluripotent transcription factor circuit thereby influencing the master transcription factor Oct4. However, considering their different biological activities, it is unlikely that E-cadherin can functionally replace Oct4. Clearly, molecular mechanism underlying the cross-talk between cell surface molecules and nuclear machinery is key to furthering our understanding of this crucial interaction.
Recent findings have, however, provided some new insights into this issue. It seems that Oct4 and Sox2 suppress the Snail (an E-cadherin repressor), Klf4 induces E-cadherin expression, and c-Myc downregulates the expression of TGF-β1 and TGF-β receptor 2.92 It is well known that TGF-β signals induce the epithelial-to-mesenchymal transition, at least in part, through the activation of the E-cadherin repressor Snail and thereby negatively regulate the MET. Furthermore, the crucial role and possible mechanism of E-cadherin in reprogramming34,66,92,93 has been further highlighted by failed rescue experiments with β-catenin.34 β-catenin is an adaptor molecule that links to the cytoplasmic tail of E-cadherin mediating cell-cell adhesion and is also a pivotal effector of the canonical Wnt signaling pathway. It is known that β-catenin enhances Oct4 activity and plays an important role in the regulation of self-renewal of ESCs.96 Unexpectedly, β-catenin is incapable of rescuing the poor reprogramming efficiency induced by E-cadherin inhibition,34 suggesting an indispensable role of E-cadherin in reprogramming. Finally, microRNAs (miRNAs) are emerging as critical regulators of cell function within this network. Using a strategy that avoids the caveats associated with transient transfection of chemically synthesized miRNA mimics, Liao and colleagues97 have shown that miRNA clusters 302 and 367 target TGFβ receptor 2, promote E-cadherin expression and accelerate MET necessary for colony formation.97 Together, these observations have provided important biological insights into how E-cadherin plays an overarching role in reprogramming, self-renewal and pluripotency.
The role of N-cadherin and VE-cadherin in human pluripotent stem cells
Another two members of classic cadherin subfamily studied in hESCs are N-cadherin and VE-cadherin. Similar to E-cadherin, both family members are single-pass transmembrane glycoproteins that form Ca2+-dependent homophilic cis- and trans-interactions with their extracellular regions and also link to catenins through their cytoplasmic tails.98 Unlike E-cadherin, however, they are not expressed by undifferentiated hESCs.50,51 The appearance of N-cadherin and VE-cadherin often represent a specific cell lineage transition differentiated from hESCs.
N-cadherin is expressed by a variety of cell types, including neuroepithelial cells, neurons, mesenchymal cells,40 as well as fetal and adult hepatocytes,99 but is not expressed by undifferentiated hESCs.50,51 N-cadherin has therefore been used as a neuroepithelial marker or a mesenchymal marker in the studies of hESC differentiation, depending on the status of other co-markers. During neural induction, hESCs and hiPSCs change their morphology into compactly assembled cells and then into tubular rosette-like structures expressing neural precursor cell specific markers such as Pax6, nestin and Sox2.100 Expression of N-cadherin is asymmetrically localized on the luminal side of the rosettes, a characteristic feature of primitive neuroepithelial rosette structures.100-103 An early switch from E-cadherin expression in undifferentiated hESCs to N-cadherin expression is retained in rosette-stage neural stem cells.100,101 This scenario recapitulates embryonic development in vivo. For instance, during the formation of the neural tube, E-cadherin is switched off in a subset of cells, whereas N-cadherin expression is turned on in those cells.30 Additionally, N-cadherin is also expressed in mesodermal tissues.40 A switch from E-cadherin to N-cadherin expression, indicating epithelial-to-mesenchymal transition, is observed in hESC differentiation.50 Recently, N-cadherin has also been reported as a surface marker for the enrichment of hepatic endoderm cells from differentiated hESCs.99
VE-cadherin, an endothelial-specific cell-cell adhesion protein of the adherens junction complex, plays a key role in endothelial barrier function and angiogenesis.98,104 VE-cadherin is absent in undifferentiated hESCs but is upregulated prior to hematopoietic emergence between days 3 and 10 of human embryoid body (hEB) development.105 Several studies have identified a population of intermediate-stage precursors defined, in part, by their expression VE-cadherin and other specific surface markers that possess primitive endothelial properties during hESC differentiation. These precursors are capable of giving rise to endothelial and hematopoietic cells.28,105-107 Additionally, screens using green fluorescent protein driven by VE-cadherin promoter to identify factors that promote vascular commitment have revealed that the expansion and maintenance of hESC-derived endothelial cells by TGFβ inhibition is dependent on Id1 (an inhibitor of a group of basic helix-loop-helix transcription factors), providing a further correlative link between VE-cadherin and hESC fate determination.108
Cell-Cell Adhesion Molecules in Human Pluripotent Stem Cells: L1-CAM, NCAM and PECAM-1
The immunoglobulin superfamily (IgSF) is another class of CAMs. IgSF CAMs are either homophilic or heterophilic and bind integrins or different IgSF CAMs. IgSF CAMs contain one or more of the extracellular Ig-like domains characteristic of antibody molecules.109 Analysis of the human genome reveals that this Ig-like domain has the widest representation of any protein domain, being encoded by 765 genes.109 Expression and function of IgSF CAMs in undifferentiated hESCs and hiPSCs have not been extensively studied. Our knowledge of the expression patterns or levels and the roles or functions of IgSF CAMs on hESCs and hiPSCs remain limited. For example, a molecule called L1-CAM (CD171) that belongs to IgSF CAM family has been shown to be displayed by undifferentiated hESCs but little is known about its function.35 Other IgSF CAM molecules, if detected, most often appear first during hESC differentiation into a specific lineage and are thus used as surface markers to fractionate hESC-derived stage-specific subpopulations. These molecules include NCAM (Neural Cell Adhesion Molecule/CD56) and PECAM-1 (Platelet-Endothelial Cell Adhesion Molecule-1/CD31).
NCAM/CD56 is a homophilic binding glycoprotein. It is the first member of IgSF CAM family described in the central nervous system although its expression is also found in other cell types and not restricted to neural cells. NCAM/CD56 has been used to isolate hESC-derived neurons by fluorescence activated cell sorter (FACS) at late stage of neural differentiation since NCAM does not present on undifferentiated hESCs, neural stem cells, or neural precursor cells.110,111 FACS-sorted hESC-derived neurons survive in vivo after transplantation into the rodent brain.110 Furthermore, there is evidence that NCAM/CD56-positive and CD326 (epithelial cell adhesion/activating molecule, EpCAM)-negative populations may represent more lineage-restricted mesodermal progenitors differentiated from hESCs.112
PECAM-1/CD31 has been considered to be a marker associated with cells with early hematopoietic potential in the human embryo.113 PECAM-1/CD31 is undetectable in undifferentiated hESCs but upregulated prior to hematopoietic emergence between days 3 and 10 of hESC differentiation.105 A subset of embryonic endothelia lacking the common leukocyte marker CD45 but expressing surface markers PECAM-1/CD31, CD34, VEGFR2 (vascular endothelial growth factor receptor 2) and VE-cadherin are first identified within 3–10 d of hEB development. These cells have the ability to give rise to both endothelial and hematopoietic cells.17,105 Subsequent in vivo studies have shown that these hESC-derived hematopoietic cells possess hematopoietic stem/progenitor properties after transplantation into immunodeficient mice.28 A recent study further reveals PECAM/CD31-positive cells purified from differentiating EBs express high levels of ICAM-1 (intercellular adhesion molecule 1) and VCAM-1 (vascular cell adhesion molecule-1).107 They also express multiple endothelial genes and form lumenized vessels when seeded onto porous poly(2-hydroxyethyl methacrylate) scaffolds and subcutaneously implanted in athymic rats.107 Thus, it is likely that, during early hESC differentiation, the PECAM/CD31-positive cells may represent primitive endothelium capable of further development into committed hematopoietic and endothelial cells.
Cell-Matrix Adhesion Molecules in Human Pluripotent Stem Cells: Integrins
In addition to cadherin mediated cell-cell adhesion, the maintenance of hESCs and hiPSCs also requires contact with extracellular matrix components. The requirement for basement membrane formation in human pluripotent stem cells is consistent with their cellular origin.1 During embryonic development, cells not only interact with each other but also with the extracellular matrix composed of proteins such as collagen, fibronectin and laminin that interlace with proteoglycans.114 hESCs interact with extracellular matrix via cell-surface receptors including integrins, which allow for mechanically anchoring to the extracellular substrates as well as intracellular signal transduction.
Integrins are heterodimeric surface glycoproteins and constitute a large and widespread family of cell-matrix adhesion molecules. There are 24 known integrin heterodimers comprised of combinations of one of 18 α subunits and one of 8 β subunits.115 The integrin dimers bind to an array of different extracellular matrix molecules with overlapping binding affinities.116
Genomic data (microarray, expressed sequence tags analysis and massively parallel signature sequencing27,117,118) have provided the expression profiles of different integrin subunits in hESCs. Integrin expression and function in hESCs have also been examined by studying cell interactions with various extracellular matrix components,12,119-122 including vitronectin.33,123,124 Of 18 α subunits and 8 β subunits, α1, α2, α3, α5, α6, α7, α11, αV, αE and β1, β2, β3, β5 have been detected in undifferentiated hESCs in the aforementioned reports, albeit with some controversy. These controversial results have been attributed to inter-line variation, heterogeneity and/or highly plastic phenotype of hESCs. Additionally, culture methodologies (e.g., feeder layer vs. feeder free) and analysis methods vary significantly among different research groups.
A recent study shows that hiPSC lines reprogrammed by Oct4, Sox2, Nanog and Lin28 and cultured on Matrigel and vitronectin express a repertoire of integrins similar to that of hESCs, although hiPSCs prominently express subunits α5, α6, αV, β1 and β5.33 It appears that β1 integrin is required for hiPSC adhesion and proliferation on Matrigel, while integrin αVβ5 is required for the initial attachment to vitronectin.33 Furthermore, integrins αVβ3 and αVβ5 (also mediating hiPSC adhesion to vitronectin) have been found to contribute to self-renewal and pluripotency123 and to the clonal growth121 of hESCs.
Integrin-related pathways, such as PI3K-Akt22,24,65 and Mek/Erk24,125 also play important role in hESC survival. The PI3K-Akt pathway has been shown to inhibit apoptosis and attenuate cell death after hESC dissociation, and constitutive overexpression of Akt partially improves hESC survival.65
Cell-Matrix Adhesion Molecules in Human Pluripotent Stem Cells: Heparan Sulfate Proteoglycans
Heparan sulfate proteoglycans (HSPGs) reside on the plasma membrane of all animal cells studied to date and are a major component of extracellular matrices.126 HSPGs are composed of a core protein and one or more heparan sulfate glycosaminoglycan (GAG)-chains. There are three subfamilies of HSPGs: the membrane-spanning proteoglycans (namely syndecans, betaglycan and CD44v3), the glycophosphatidylinositol (GPI)-linked proteoglycans (namely glypicans) and the secreted extracellular matrix proteoglycans (namely agrin, collagen XVIII and perlecan). HSPGs contain long polysaccharide side chains that bind signal proteins and immobilize them, by which HSPGs help localize the action of secreted signal proteins.127 HSPGs may also control the stability of signal proteins, transport them through the extracellular space, or make them interact with cell-surface receptors. For example, the stability, movement and reception of diffusible growth factors such as FGF,128 BMP,129 TGF-β,130 Indian hedgehog131,132 and Wnt133,134 have been reported to be controlled by HSPGs.
HSPGs are present throughout embryonic development126,127 and potentially modulate signals essential for the maintenance of self-renewal or for the initiation of differentiation. Most recently, Shimokawa et al. revealed that cell surface-tethered heparan sulfate chains (key components of HSPGs) play pivotal roles in the local retention of FGF ligands and moreover, can “spread” FGF signaling to adjacent cells within a short-distance in heparan sulfate-deficient mouse embryos.135 However, the functional role of cell surface-HSPGs in hESCs and hiPSCs has not been elucidated until recently.
New data showed that secreted HSPGs produced by mouse embryonic fibroblast feeder cells coordinated hESC proliferation.136 It is well known that hESCs can be maintained in an undifferentiated state if the culture medium is first conditioned on a layer of mouse embryonic fibroblast feeder. Using column chromatography, immunoblotting and mass spectrometry-based proteomic analysis, multiple secreted HSPG species have been identified in mouse embryonic fibroblast-conditioned medium.136 These HSPGs and other heparinoids can stabilize basic fibroblast growth factor (bFGF) and also directly mediate binding of bFGF to the hESC surface, promoting hESC maintenance. In contrast, removal of HSPGs from conditioned medium impairs hESC proliferation,136 suggesting that HSPGs might be key signaling cofactors in hESC maintenance. One potential mechanism, infered from studies of mouse ESCs, could be that heparan sulfate chains mediate autocrine/paracrine Wnt/β-catenin signaling to regulate Nanog expression,137 although further validation will be required in human pluripotent stem cells.
Concluding Remarks
CAMs provide two-way communication links from hESCs (or hiPSCs) to their surrounding environment and from one cell to another. CAMs also mediate/regulate the transduction initiated by many chemical signals across cell membrane and enable connections with important intracellular signaling pathways. The molecular and cellular biology of CAMs and their functions in hESCs and hiPSCs represents a very exciting area of discovery with emerging links to self-renewal, pluripotency, reprogramming and differentiation. Further understanding the underlying mechanisms will greatly facilitate the maintenance and direct differentiation of hESCs and hiPSCs into a given cell type for potential applications necessary to validate their regenerative potential. There is little doubt that research into CAMs and human pluripotent stem cells will continue to produce interesting and surprising results key to the future cell replacement strategies.
Acknowledgments
We apologize to all those colleagues whose important work could not be discussed owing to space limitations. This work is supported by an operating grant from the Canadian Institutes of Health Research (CIHR) MOP-111224, a CIHR New Investigator Award MSH-166732 and an Early Research Award from the Ontario Government to L.W.; CIHR operating support (MOP 62826) to S.A.L.B.
Glossary
Abbreviations:
- bFGF
basic fibroblast growth factor
- CAMs
cell adhesion molecules
- EB
embryoid body
- EpiSCs
epiblast stem cells
- FACS
fluorescence-activated cell sorting
- hESCs
human embryonic stem cells
- hiPSCs
human induced pluripotent stem cells
- hPSCs
human pluripotent stem cells
- HSPGs
heparan sulfate proteoglycans
- IgSF CAMs
immunoglobulin superfamily CAMs
- Mek/Erk
mitogen-activated protein/extracellular signal-regulated kinase
- MET
mesenchymal-to-epithelial transition
- miRNA
microRNA
- NCAM
neural cell adhesion molecule (CD56)
- Oct4
octamer-binding transcription factor 3/4
- PECAM-1
platelet-endothelial cell adhesion molecule-1 (CD31)
- PI3K
phosphoinositide 3-kinase
- ROCK
Rho-associated coiled coil-forming protein kinase
- TGFβ
transforming growth factor β
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/19583
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Lee G, Ganat Y, Papapetrou EP, Lipchina I, Socci ND, et al. miR-371-3 expression predicts neural differentiation propensity in human pluripotent stem cells. Cell Stem Cell. 2011;8:695–706. doi: 10.1016/j.stem.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 7.Grigoriadis AE, Kennedy M, Bozec A, Brunton F, Stenbeck G, Park IH, et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010;115:2769–76. doi: 10.1182/blood-2009-07-234690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–6. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 9.Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–54. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–80. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- 11.Moore RN, Cherry JF, Mathur V, Cohen R, Grumet M, Moghe PV. E-cadherin-expressing feeder cells promote neural lineage restriction of human embryonic stem cells. Stem Cells Dev. 2012;21:30–41. doi: 10.1089/scd.2010.0434. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 14.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–8. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–7. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 16.Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–74. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005;105:4598–603. doi: 10.1182/blood-2004-10-4065. [DOI] [PubMed] [Google Scholar]
- 18.Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–21. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Tian R, Li L, Figeys D, Wang L. An enhanced chemically defined SILAC medium for quantitative proteomics study of human embryonic stem cells. Proteomics. 2011;11:4040–6. doi: 10.1002/pmic.201100052. [DOI] [PubMed] [Google Scholar]
- 20.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–12. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–34. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 2011;108:8299–304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, et al. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 25.Vijayaragavan K, Szabo E, Bosse´ M, Ramos-Mejia V, Moon RT, Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell. 2009;4:248–62. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian R, Wang S, Elisma F, Li L, Zhou H, Wang L, et al. Rare cell proteomic reactor applied to stable isotope labeling by amino acids in cell culture (SILAC)-based quantitative proteomics study of human embryonic stem cell differentiation. Mol Cell Proteomics. 2011;10:M110.000679. doi: 10.1074/mcp.M110.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, et al. Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation. Nat Biotechnol. 2004;22:707–16. doi: 10.1038/nbt971. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–14. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 30.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91:691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prokhorova TA, Rigbolt KT, Johansen PT, Henningsen J, Kratchmarova I, Kassem M, et al. Stable isotope labeling by amino acids in cell culture (SILAC) and quantitative comparison of the membrane proteomes of self-renewing and differentiating human embryonic stem cells. Mol Cell Proteomics. 2009;8:959–70. doi: 10.1074/mcp.M800287-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Wang S, Jezierski A, Moalim-Nour L, Mohib K, Parks RJ, et al. A unique interplay between Rap1 and E-cadherin in the endocytic pathway regulates self-renewal of human embryonic stem cells. Stem Cells. 2010;28:247–57. doi: 10.1002/stem.289. [DOI] [PubMed] [Google Scholar]
- 33.Rowland TJ, Miller LM, Blaschke AJ, Doss EL, Bonham AJ, Hikita ST, et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010;19:1231–40. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 34.Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, et al. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010;28:1315–25. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- 35.Son YS, Seong RH, Ryu CJ, Cho YS, Bae KH, Chung SJ, et al. Brief report: L1 cell adhesion molecule, a novel surface molecule of human embryonic stem cells, is essential for self-renewal and pluripotency. Stem Cells. 2011;29:2094–9. doi: 10.1002/stem.754. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida C, Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982;28:217–24. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]
- 37.Gallin WJ, Edelman GM, Cunningham BA. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983;80:1038–42. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyrie´ras N, Hyafil F, Louvard D, Ploegh HL, Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983;80:6274–7. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vestweber D, Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res. 1984;152:169–78. doi: 10.1016/0014-4827(84)90241-6. [DOI] [PubMed] [Google Scholar]
- 40.Oda H, Takeichi M. Evolution: structural and functional diversity of cadherin at the adherens junction. J Cell Biol. 2011;193:1137–46. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–76. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–34. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–45. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-J. [DOI] [PubMed] [Google Scholar]
- 45.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–77. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 49.Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD. Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995;10:1571–8. doi: 10.1093/humrep/10.6.1571. [DOI] [PubMed] [Google Scholar]
- 50.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–41. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 51.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–62. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 52.Costa M, Dottori M, Ng E, Hawes SM, Sourris K, Jamshidi P, et al. The hESC line Envy expresses high levels of GFP in all differentiated progeny. Nat Methods. 2005;2:259–60. doi: 10.1038/nmeth748. [DOI] [PubMed] [Google Scholar]
- 53.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–73. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 54.Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–55. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 57.Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, et al. A role for cadherins in tissue formation. Development. 1996;122:3185–94. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- 58.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 59.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 60.Rossant J. The impact of developmental biology on pluripotent stem cell research: successes and challenges. Dev Cell. 2011;21:20–3. doi: 10.1016/j.devcel.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 61.Nichols J, Smith A. The origin and identity of embryonic stem cells. Development. 2011;138:3–8. doi: 10.1242/dev.050831. [DOI] [PubMed] [Google Scholar]
- 62.Chou YF, Chen HH, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–61. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Wang BH, Wang S, Moalim-Nour L, Mohib K, Lohnes D, et al. Individual cell movement, asymmetric colony expansion, rho-associated kinase, and E-cadherin impact the clonogenicity of human embryonic stem cells. Biophys J. 2010;98:2442–51. doi: 10.1016/j.bpj.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–8. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–39. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Li D, Zhou J, Wang L, Shin ME, Su P, Lei X, et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol. 2010;191:631–44. doi: 10.1083/jcb.201006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–50. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, et al. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2010;123:853–60. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh H, Mok P, Balakrishnan T, Rahmat SN, Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4:165–79. doi: 10.1016/j.scr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 72.Walker A, Su H, Conti MA, Harb N, Adelstein RS, Sato N. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71. doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasza KE, Zallen JA. Dynamics and regulation of contractile actin-myosin networks in morphogenesis. Curr Opin Cell Biol. 2011;23:30–8. doi: 10.1016/j.ceb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature. 2006;442:580–4. doi: 10.1038/nature04935. [DOI] [PubMed] [Google Scholar]
- 76.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–6. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 77.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–87. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–31. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 79.de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A. 2009;106:7010–5. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palacios F, Tushir JS, Fujita Y, D’Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–32. [PMC free article] [PubMed] [Google Scholar]
- 82.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem. 2003;278:21050–7. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- 83.Ogata S, Morokuma J, Hayata T, Kolle G, Niehrs C, Ueno N, et al. TGF-beta signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis. Genes Dev. 2007;21:1817–31. doi: 10.1101/gad.1541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pizon V, Chardin P, Lerosey I, Olofsson B, Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the ‘effector’ region. Oncogene. 1988;3:201–4. [PubMed] [Google Scholar]
- 85.Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–32. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- 86.Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, et al. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–83. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- 88.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, et al. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bai H, Chen K, Gao YX, Arzigian M, Xie YL, Malcosky C, et al. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ardehali R, Inlay MA, Ali SR, Tang C, Drukker M, Weissman IL. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc Natl Acad Sci U S A. 2011;108:3282–7. doi: 10.1073/pnas.1019047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 92.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 93.Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 94.Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–6. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–27. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liao B, Bao X, Liu L, Feng S, Zovoilis A, Liu W, et al. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–64. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brasch J, Harrison OJ, Ahlsen G, Carnally SM, Henderson RM, Honig B, et al. Structure and binding mechanism of vascular endothelial cadherin: a divergent classical cadherin. J Mol Biol. 2011;408:57–73. doi: 10.1016/j.jmb.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao D, Chen S, Cai J, Guo Y, Song Z, Che J, et al. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One. 2009;4:e6468. doi: 10.1371/journal.pone.0006468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhee YH, Ko JY, Chang MY, Yi SH, Kim D, Kim CH, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121:2326–35. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LaVaute TM, Yoo YD, Pankratz MT, Weick JP, Gerstner JR, Zhang SC. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–9. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–20. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010;22:651–8. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–8. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 107.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2010;30:80–9. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–6. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aricescu AR, Jones EY. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Curr Opin Cell Biol. 2007;19:543–50. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 110.Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells. 2007;25:2257–68. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sundberg M, Jansson L, Ketolainen J, Pihlajamäki H, Suuronen R, Skottman H, et al. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009;2:113–24. doi: 10.1016/j.scr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, et al. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2010;107:13742–7. doi: 10.1073/pnas.1002077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oberlin E, Tavian M, Blazsek I, Pe´ault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–57. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 114.Kraehenbuehl TP, Langer R, Ferreira LS. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods. 2011;8:731–6. doi: 10.1038/nmeth.1671. [DOI] [PubMed] [Google Scholar]
- 115.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–51. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 116.Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, et al. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–53. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 117.Miura T, Luo Y, Khrebtukova I, Brandenberger R, Zhou D, Thies RS, et al. Monitoring early differentiation events in human embryonic stem cells by massively parallel signature sequencing and expressed sequence tag scan. Stem Cells Dev. 2004;13:694–715. doi: 10.1089/scd.2004.13.694. [DOI] [PubMed] [Google Scholar]
- 118.Assou S, Le Carrour T, Tondeur S, Ström S, Gabelle A, Marty S, et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25:961–73. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miyazaki T, Futaki S, Hasegawa K, Kawasaki M, Sanzen N, Hayashi M, et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem Biophys Res Commun. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- 120.Vuoristo S, Virtanen I, Takkunen M, Palgi J, Kikkawa Y, Rousselle P, et al. Laminin isoforms in human embryonic stem cells: synthesis, receptor usage and growth support. J Cell Mol Med. 2009;13(8B):2622–33. doi: 10.1111/j.1582-4934.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–78. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meng Y, Eshghi S, Li YJ, Schmidt R, Schaffer DV, Healy KE. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J. 2010;24:1056–65. doi: 10.1096/fj.08-126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–65. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 124.Prowse AB, Doran MR, Cooper-White JJ, Chong F, Munro TP, Fitzpatrick J, et al. Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials. 2010;31:8281–8. doi: 10.1016/j.biomaterials.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 125.Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 126.Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–41. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 127.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 128.Forsten-Williams K, Chua CC, Nugent MA. The kinetics of FGF-2 binding to heparan sulfate proteoglycans and MAP kinase signaling. J Theor Biol. 2005;233:483–99. doi: 10.1016/j.jtbi.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 129.Paine-Saunders S, Viviano BL, Zupicich J, Skarnes WC, Saunders S. glypican-3 controls cellular responses to Bmp4 in limb patterning and skeletal development. Dev Biol. 2000;225:179–87. doi: 10.1006/dbio.2000.9831. [DOI] [PubMed] [Google Scholar]
- 130.Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, et al. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 2003;130:1515–22. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- 131.Koziel L, Kunath M, Kelly OG, Vortkamp A. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev Cell. 2004;6:801–13. doi: 10.1016/j.devcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697–706. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–32. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- 134.Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–6. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- 135.Shimokawa K, Kimura-Yoshida C, Nagai N, Mukai K, Matsubara K, Watanabe H, et al. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev Cell. 2011;21:257–72. doi: 10.1016/j.devcel.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 136.Levenstein ME, Berggren WT, Lee JE, Conard KR, Llanas RA, Wagner RJ, et al. Secreted proteoglycans directly mediate human embryonic stem cell-basic fibroblast growth factor 2 interactions critical for proliferation. Stem Cells. 2008;26:3099–107. doi: 10.1634/stemcells.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sasaki N, Okishio K, Ui-Tei K, Saigo K, Kinoshita-Toyoda A, Toyoda H, et al. Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem. 2008;283:3594–606. doi: 10.1074/jbc.M705621200. [DOI] [PubMed] [Google Scholar]