Abstract
The purpose of this study was to isolate starch from the tubers of Cyperus esculentus L. and evaluate its physicochemical and binder properties. Extraction of starch using sodium metabisulfite yielded 37 g of starch per 100 g of the tubers. Scanning electron microscopy indicated that Cyperus starch consists of oval to elliptical particles with a smooth surface. Cyperus starch demonstrates a narrow particle size distribution with a mean of 8.25 μm. Cyperus starch conforms well to United States Pharmacopeia standards established for widely used starches like maize and potato. The X-ray powder diffraction pattern and moisture sorption profile of Cyperus starch were comparable to that of maize starch. Cyperus starch had lower swelling power than maize and potato starch, indicative of stronger associative forces within the granules. Carr’s index and Hausner ratio indicate that Cyperus starch should have comparable flow properties with respect to maize and potato starch. Cyperus starch was employed as binder for the formulation of metronidazole tablets. Formulations containing 5%, 7.5%, and 10% Cyperus starch were compared with those containing 10% potato starch. At 10% binder concentration, the tablets containing Cyperus starch exhibited better hardness and negligible friability as compared with those with potato starch. Although the binder concentration had a significant effect on the disintegration time of the tablets, it did not seem to affect the dissolution profile. These results indicate that Cyperus starch provides excellent binding properties without compromising drug release characteristics and should be explored in pharmaceutical formulations.
Key words: Cyperus esculentus, Cyperus starch, pharmaceutical excipient, physicochemical evaluation, starch characterization
INTRODUCTION
Starch, a polysaccharide composed exclusively of d-glucose, is one of the most abundant organic compounds found on earth. Starch can be isolated from leaves, stems, tubers, seeds, and roots of higher plants where it serves as an energy reserve. Chemically, starch is a carbohydrate polymer consisting of anhydroglucose units linked by α-d-(1, 4) glucosidic bonds. It consists of two inherently incompatible molecules: amylose (15–30%), a linear polymer, and amylopectin (85–70%), a branched chain polymer. Starch is used in a variety of industries including food, textiles, cosmetics, plastics, adhesives, paper, and pharmaceuticals. Starch is widely used by the food industry, especially as a thickening agent in processed foods (1). Starch has also gained attention as a biodegradable plastic for the production of disposable items (2). Reports on the use of starch for personal care and cosmetic purposes date back to the seventeenth century (3). In the pharmaceutical industry, starch is employed as a binder, diluent, and disintegrant. Freshly prepared starch paste at a concentration of 5–20% is routinely used during tablet manufacture (4). At 5–15% concentration, starch finds its applicability as a disintegrant in a number of tablet formulations (5). Novel applications of starch have been explored in the area of nasal (6), periodontal (7), and film-based (8) drug delivery systems. However, such diversified applicability demands the modification of the currently available starches or exploitation of novel sources. Although other industries are continuously involved with the discovery of novel starches with special characteristics, reports of similar work by pharmaceutical scientists are rather sporadic (9–13). Studies designed specifically at understanding the properties of starch from a formulation standpoint may provide us with alternatives to currently available excipients.
Cyperus esculentus L. (commonly known as yellow nut-grass or chufa flatsedge) is a perennial from rhizomes and tubers that grows to heights between 24 and 55 cm (14,15). The stems are three-sided and triangular in cross-section while the leaves are yellow to green in color with a distinct ridge. It is found throughout the USA as a common weed in agronomic and horticultural crops. The fast-growing and tuberous habit of yellow nut-grass allows it to out-compete cultivated crops in fields, ornamentals in flower beds, and native species in other areas. Such characteristics make it a difficult plant to eradicate and hence C. esculentus L. has been deemed a noxious weed in the USA (16). It is also found in other parts of the world and has been ranked as no. 16 among the world’s worst weeds (17). However, the tubers of this plant have high starch content (18), and this starch is comparable to cassava and rice starch (19). Radley (20) indicated that the starch from these tubers could be used for many starch-based foods, cosmetic products, and fabric stiffening. Thus, starch obtained from C. esculentus L. or “Cyperus starch” may be an interesting candidate with implications for use as a pharmaceutical excipient.
Since the physicochemical characteristics of any excipient have a direct impact on its functionality, a detailed study in this aspect is mandatory. A comparative assessment of binder characteristics with respect to another tuber starch i.e. potato starch is also informative. The purpose of this study was to isolate starch from the tubers of C. esculentus L. and evaluate its physicochemical and binder properties.
MATERIALS AND METHODS
Materials
Tubers of C. esculentus L. and Solanum tuberosum L. (potato) were procured from an open market in Abuja, Nigeria. Sodium metabisulfite was obtained from Sigma Chemicals Ltd (St. Louis, MO, USA) while metronidazole was brought from NIPCO (Abuja, Nigeria). All reagents were analytical grade and used without further purification.
Methods
Extraction of Cyperus Starch
Fresh tubers (tiger nuts) of C. esculentus were thoroughly washed with water and all bruises pitted out. Petroleum ether was used to extract oil from the tubers with the help of a Soxhelet apparatus. The tubers were then pulverized and soaked overnight in an aqueous solution of sodium metabisulfite (0.075% w/v). This helped ensure proper hydration of the tubers, thus promoting the release of starch. The soaked tubers were wet-milled into a homogenous fine paste, dispersed in copious amounts of aqueous sodium metabisulfite solution (0.075% w/v), and then sifted through a fine muslin cloth. The suspension was allowed to settle, and the supernatant decanted. The resulting starch was profusely washed with distilled water and then dried in a hot air oven at 50°C for 24 h. This was pulverized into fine powder and weighed. Percentage yield was calculated on a dry weight basis of the nuts. To validate the process of extraction, potato starch was also extracted from the tubers of S. tuberosum L. in a similar manner.
Determination of Organoleptic Properties
Two panels consisting of six assessors evaluated the color, odor, taste, and texture of the samples. Individual assessment of each panel member was documented.
Scanning Electron Microscopy
Starch morphology was obtained using a Hitachi S5200 field emission scanning electron microscope (Hitachi High-Technologies Canada, Inc., Ontario, Canada). Images of uncoated samples were obtained at 1.0 kV accelerating voltage.
Particle Size Analysis
The particle size distribution of the samples was obtained using a Beckman Coulter LS 13 320 laser diffraction particle size analyzer (Beckman Coulter, Inc., Fullerton, CA). The LS 13 320 is equipped with an optical bench and a universal liquid module to measure the size distribution of particles suspended in water at 20°C. All measurements were obtained in triplicate.
Density Measurements
The bulk and tapped density of the samples were obtained using Stampfvolumeter STAV 2003 (J. Engelsmann AG, Ludwigshafen am Rhein, Germany). A 50-g sample of starch powder was transferred to a 200-mL calibrated glass cylinder through a short-stemmed glass funnel, and the volume occupied by the powder was noted. The cylinder was then subjected to 500 taps to calculate the volume change. The true density of the samples was determined using a 50-mL specific gravity bottle with xylene as the displacement liquid. The Carr’s “percent compressibility” and the Hausner ratio were calculated as per the following equations:
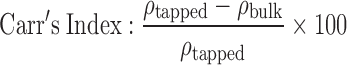 |
1 |
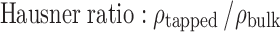 |
2 |
United States Pharmacopeial Specifications
The starch samples were checked for their conformity with United States Pharmacopeial (USP) standards (21). The identification and microbial limits of the samples were tested as described in the USP. The pH of 20% w/v aqueous slurry of starch was determined potentiometrically. The samples were dried at 120°C for 4 h to obtain the percent loss on drying (LOD). A 2-g sample of starch was weighed into a platinum crucible and incinerated. Estimation of ash was done by measurement of the residue left after combustion in a furnace at 575 ± 25°C. A series of tests for oxidizing substances, sulfur dioxide, iron content, and other microelement determination were carried out. All measurements were carried out in triplicate, and the average values are reported.
Estimation of Amylose-to-Amylopectin Ratio
The ratio of amylose to amylopectin was estimated by colorimetric measurement of the of amylose–iodine inclusion complexes formed as a result of the iodine binding capacity of amylose. A 5 g sample of starch was defatted using 75% n-propanol at 85°C in a Soxhlet extractor. The lipid-free starch was air-dried for 12 h followed by oven drying at 30°C for 24 h. Approximately 20 mg of the dried, lipid-free sample was dispersed in 8 mL of 90% dimethylsulfoxide and heated in a water bath at 85°C until a clear solution was obtained. One milliliter of this solution was mixed with 5 mL of freshly prepared iodine reagent (0.0025 M iodine/0.0065 M potassium iodide) and 44 mL of distilled water. The color was allowed to develop over a period of 15 min, and absorbance was measured at 600 nm. Mixtures containing known ratios of pure amylose and pure amylopectin were treated in a similar manner to obtain a calibration curve.
X-ray Powder Diffraction
Structural characterization was carried out using a Siemens D5000 X-ray diffractometer at ambient temperature. Powder samples, packed in rectangular aluminum cells, were illuminated using CuKα radiation (λ = 1.54056 Å) at 45 kV and 40 mA. Samples were scanned between diffraction angles of 5° to 40° 2θ, which is sufficient to cover all significant diffraction peaks of starch crystallites. Scan steps of 0.1 with a dwell time of 15 s were used. A nickel filter was used to reduce the Kβ contribution to the X-ray signal. The d spacing was computed according to Bragg’s law of diffraction.
Determination of Swelling Power
Aqueous dispersions of starch samples were heated at 10°C intervals between 55°C and 95°C. Each sample was then centrifuged at 4,500 rpm for 30 min and analyzed for the weight of sediment per gram of starch dry weight basis. The supernatant liquid obtained at each point was dried in a hot air oven and weighed after cooling in a desiccator. A correction was made for the soluble starch, in order to provide for a measure of swelling of the swollen but undissolved granules.
Gravimetric Moisture Sorption
Moisture sorption isotherms were generated at 25°C and ambient pressure using an SGA-100 Symmetric Vapor Sorption Analyzer (VTI Corp, Hialeah, FL). The SGA-100 is equipped with an electronic microbalance (CI Electronics, Wiltshire, UK) and a dew point analyzer (Edgetech, Milford, MA) for the accurate measurement of weight and relative humidity (RH), respectively. The instrument was calibrated using sodium chloride and PVP K30. Samples were dried at 60°C and 0% RH for a minimum of 6 h and exposed to relative humidity in steps of 10% between 10% and 90% RH. Equilibrium was assumed when the microbalance recorded a change of less than 0.01% over a period of 5 min (dm/dt = 0.002%/min). The samples were equilibrated for a maximum of 24 h.
Preparation and Evaluation of Metronidazole Granules
Granulation was achieved by the wet method of massing and screening. An 80-g sample of metronidazole was wet-massed with predetermined quantities of 5%, 7.5%, and 10% w/w Cyperus starch or 10% w/w potato starch. The wet mass was then kneaded for 15 min, passed through a no. 10 sieve, and dried in a hot air oven at 50°C for 30 min. These granules were then passed through a no. 16 sieve and dried at 50°C for 1 h to obtain the final product. The granule moisture content, density (bulk, tapped, and true), particle size distribution, and friability were determined. To determine the friability, granules retained on a no. 18 sieve were isolated. A 10-g sample of the pre-screened granules was subjected to friability testing at 25 rpm for 4 min using an Erweka TA 220 friability tester (Erweka GmbH, Heusenstamm, Germany). The granules were then sieved again, and the weight of granules retained on the no. 18 sieve was determined. This was expressed as a percentage of the initial weight.
Preparation and Evaluation of Metronidazole Tablets
Various batches of the granules were intimately mixed with 5% w/w of the corresponding dry starch for 4 min and with 1% w/w magnesium stearate for an additional 1 min in a tumbler mixer (JEL-Karl Kolb, Dreieich, Germany). The premixed blends were then compressed using a 12-mm flat punch on a single-station tablet press (Shanghai Tianxiang & Chentai Pharmaceutical, Shanghai, China) such that each tablet weighed around 450 mg. Approximately 200 tablets were compressed for each formulation. Hardness, friability, disintegration, and dissolution of the tablets were determined as per the physical tests specified in the USP (21).
RESULTS AND DISCUSSION
Isolation
The isolation of starch from tubers of C. esculentus L. was relatively straightforward. Starch granules settled easily, and this settling was not hampered by the presence of non-starch materials from the tubers. The non-starch materials remained suspended in the supernatant liquid and were decanted off. The yield of Cyperus starch, after extraction of oil, was found to be approximately 37 g per 100 g of fresh tubers. This was considered satisfactory as yields of approximately 21% w/w basis have been previously reported in the literature (22).
Physicochemical Characterization
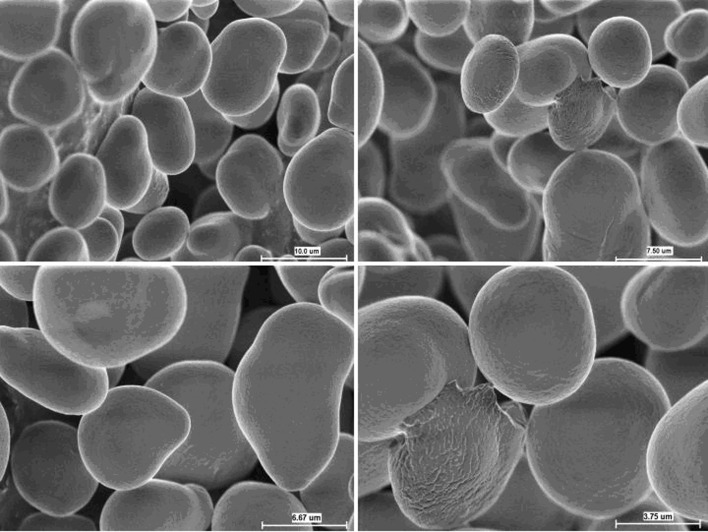
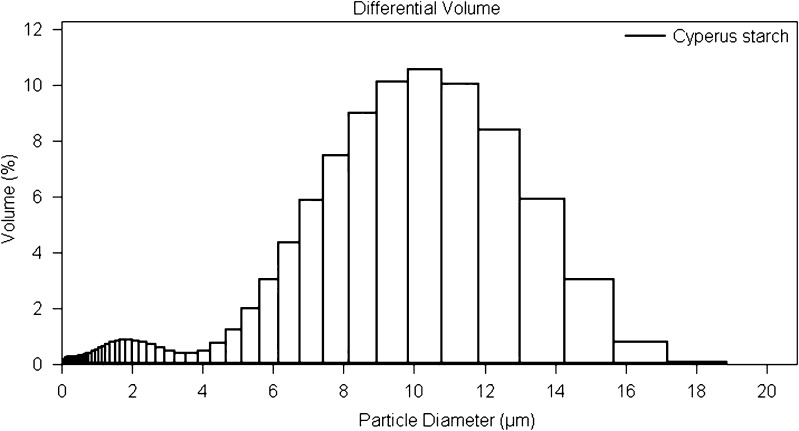
Starch occurs in plants as granules that are characteristic in size, shape, and morphology. As these characteristics are determined by the biological origin, a thorough investigation of the physicochemical properties is warranted. Cyperus starch is a brilliant white, odorless powder with a warm bland taste and smooth texture. As is evident from the micrographs in Fig. 1, Cyperus starch exhibits elliptical to spherical granules with a relatively smooth surface. Scanning electron microscopy indicates that Cyperus starch has uniform granular size, shape, and morphology. Size distribution of a potential excipient has been shown to affect various formulation characteristics like flowability, compactibility, water binding capacity, and drug release (23–25). Although no precise categorization of granule size is documented in the literature, starch granules have been arbitrarily classified as large (>25 μm), medium (10–25 μm), small (5–10 μm), and very small (<5 μm) (26). Cyperus starch predominantly consists of small- to medium-sized granules (percent volume basis) with a mean particle size of 8.25 μm. The particle size distribution of Cyperus starch, shown in Fig. 2, depicts granules ranging from 2 to 17 μm and is fairly consistent with the previously reported range of 3–12 μm (22). Typical particle size distributions for commercially used starches include maize starch (2–32 μm), potato starch (10–100 μm), rice starch (2–20 μm), tapioca starch (5–35 μm), and wheat starch (2–45 μm) (4).
Fig. 1.
Scanning electron micrographs of starch granules obtained from C. esculentus
Fig. 2.
Particle size distribution of Cyperus starch granules
Native starches are known to contain varying amounts of monovalent and divalent cations inherited from the parent material. Incinerating the starch sample and analyzing the residue (known as ash) can be used to estimate the metal ions present in the sample (27,28). Cyperus starch samples show a predominance of divalent cations as compared with maize starch, which has been shown to primarily contain monovalent cations like sodium and potassium (28). The ash value of Cyperus starch is slightly higher than the value recommended in the USP (21) for starch samples and may be due to the presence of magnesium and calcium. Although Cyperus starch was found to contain iron, magnesium, and calcium as indicated in Table I, no traces of heavy metals were detected. Some starches contain small amounts of lipid materials (e.g., maize starch ~0.6–0.7%), and hence require pretreatment for removal of fatty materials (29). Tubers of C. esculentus L. have been shown to contain as much as 27% fixed oils (30). Although most of this oil was removed using Soxhelet extraction, Cyperus starch retains about 0.3% of the lipid content and is probably distributed endogenously. Since fatty substances appear to be complexed with the linear fractions of native starches, their presence significantly alters properties like granule swelling, enzyme conversion, and iodine affinity (31).
Table I.
Evaluation of Cyperus Starch Properties with Respect to USP Specifications
| Test | Cyperus starch | USP specifications for starch |
|---|---|---|
| Identification | Conforms | + |
| Microbial limits | Conforms | + |
| pH (20% slurry) | 6.5–6.9 | 4.5–7.0 |
| Loss on drying | 12.5% | ≤14.0% |
| Residue on ignition | 1.0% | ≤0.5% |
| Oxidizing substances | 0.001% | ≤0.002% |
| Sulfur dioxide | 0.005% | ≤0.008% |
| Iron content | 0.4000 ppm | ≤20 ppm |
| Magnesium content | 0.0014 ppm | – |
| Calcium content | 0.7440 ppm | – |
| Lipid content | 0.3% | – |
Pharmacopeial Specifications
Cyperus starch conforms well to USP standards established for widely used starches like maize and potato (21). The results of the pharmacopeial specifications are indicated in Table I. A sample of Cyperus starch produced a translucent, whitish jelly in boiling water and was colored reddish violet to deep blue as indicated by the identification test in the USP (21). The total aerobic microbial count was well within the limits specified in the compendium. As indicated in Table I, Cyperus starch samples meet the specific requirement for pH values. Although starch rarely contains volatiles other than water, LOD is a good technique to determine the volatile components present in the sample. The 12.5% LOD for Cyperus starch is within the limits set by the USP (21) and is attributed to the presence of moisture.
Swelling Power
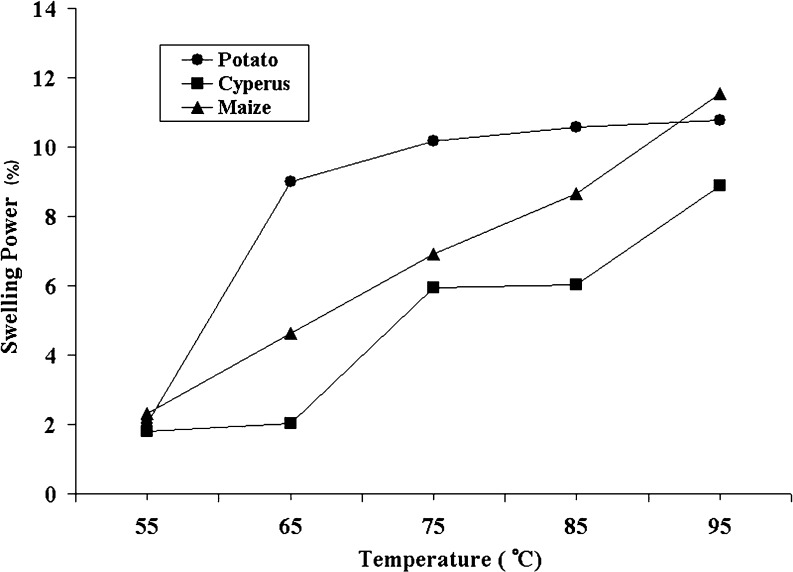
To be potentially useful as a pharmaceutical excipient, the ability of starch granules to swell has important implications. Swelling power is not only a measure of the hydration capacity of the sample but is also indicative of the associative forces in the granules. The swelling profile of maize, potato, and Cyperus starch is shown in Fig. 3. As compared with potato and maize starch, Cyperus starch demonstrates the highest resistance to swelling. This lower swelling power could be due to the interplay of various factors: particle size and lipid content being the prominent ones. The small granule size of Cyperus starch already tends to confer compact packing, resulting in a greater degree of association and hence lower swelling. This property of Cyperus starch could be explored for its potential use as a binder in pharmaceutical formulations.
Fig. 3.
Comparative evaluation of the swelling power of potato, Cyperus, and maize starch
Amylose-to-Amylopectin Ratio
It is well accepted that starches contain two polysaccharide components: the linear fraction (amylose) and the branched fraction (amylopectin). Aqueous dispersions of amylose are unstable due to intermolecular attraction and association with neighboring molecules. Hence, the amylose content significantly affects many fundamental properties like pasting temperature, viscosity, gel stability, water solubility, and the degree of resistance of starch granules to digestion by amylases. Moreover, amylose plays a role in disrupting the crystalline nature of the starch granules (32). The amount of amylose tends to differ among starches from different sources, and thus, a quantitative estimation of amylose content is necessary. As indicated in Table II, the amylose content of Cyperus and maize starch are comparable, a fair indication of the similarity in some of their functional characteristics.
Table II.
Comparative Elucidation of Some Physicochemical Properties of Potato, Cyperus, and Maize Starch
| Test | Potato starch | Cyperus starch | Maize starch |
|---|---|---|---|
| Bulk density (g/cm3) | 0.833 | 0.601 | 0.462 |
| Tapped density (g/cm3) | 1.071 | 0.753 | 0.658 |
| True density (g/cm3) | 1.798 | 1.660 | 1.478 |
| Carr’s index (%) | 22.22 | 20.19 | 29.79 |
| Hausner ratio | 1.28 | 1.25 | 1.42 |
| Particle size range (μm) | 10.0–100.0 | 2.0–17.0 | 2.0–32.0 |
| Swelling temperature (°C) | 65 | 65 | 64 |
| Pasting temperature (°C) | 66–72 | 75–79 | 76–81 |
| Browning temperature (°C) | 214–223 | 220–229 | 222–228 |
| Charring temperature (°C) | 258–263 | 260–264 | 260–264 |
| Amylose/amylopectin | 26.5:73.5 | 11.5:88.5 | 11.5:88.5 |
Density Measurements
The density of starch plays an important role for technological and engineering purposes. Several trends have been proposed that relate the bulk density of excipients to functional properties like compactibility and powder flow (33). The Carr’s index and the Hausner ratio are simple methods that use density values to predict the flow properties of powders (34,35). Carr’s index, also known as percent compressibility, describes the flow properties of powders as excellent (5–15%), good (12–16%), fair (18–21%), and poor (23–28%). Similarly, a Hausner ratio value of less than 1.20 is indicative of good flow while a value greater than 1.50 indicates poor flow properties (36). The compressibility index has also been used as measures of interparticulate friction and the likelihood of arch formation (37). The Carr’s index and the Hausner ratio for Cyperus, potato, and maize starch are given in Table II. Based on these values, it is evident that Cyperus starch has fairly comparable flow with respect to the other commonly used starches like maize and potato.
X-ray Powder Diffraction Analysis
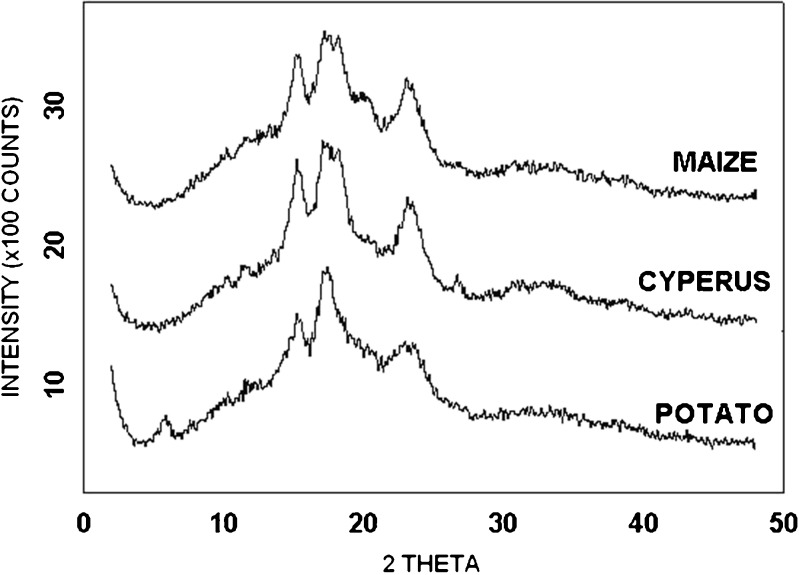
Typically starch granules are found to exhibit diffraction patterns that have been classified as A, B, or C. The type of diffraction pattern primarily depends upon the arrangement of the double-helical amylopectin chains. The A pattern is obtained as a result of a close-packed arrangement with a water molecule between each double helix, while a more open hexagonal packing, with water molecules in the central cavity, exhibits a B pattern (38,39). The C pattern is very similar to the A pattern, except for the appearance of a peak around 5° 2θ. Although the biological origin of starch plays an important role in determining the diffraction pattern, other factors like amylopectin chain length, amylase, and moisture content are known to affect the pattern within the same parent source (40,41). Zobel noted that powder patterns may become weak at moisture contents below 10% and the discrete line pattern of B-type structure completely disappears below 1% moisture content (42). Therefore, diffractograms of all samples were obtained at appropriate moisture content (~10%). Powder X-ray diffractograms of Cyperus, potato, and maize starch are shown in Fig. 4. The Braggs reflection angle, 2θ, along with the interplanar spacing, d, and the relative intensity of the peaks are listed in Table III. The interplanar spacing has been calculated using Bragg’s equation given as nλ = 2 d sinθ, where θ is one half the angle read from the diffractogram.
Fig. 4.
Powder X-ray diffraction patterns of the potato, Cyperus, and maize starch
Table III.
The Bragg Reflection Angle (2θ), the Interplanar Spacing (d), and the Relative Intensity of the Peaks for Diffraction Pattern of Maize, Cyperus, and Potato Starch
| Maize starch | Cyperus starch | Potato starch | ||||||
|---|---|---|---|---|---|---|---|---|
| 2θ | d spacing (Å) | Intensity | 2θ | d spacing (Å) | Intensity | 2θ | d spacing (Å) | Intensity |
| 10.1 | 8.69 | W | 10.3 | 8.63 | W | 5.5 | 16.07 | M |
| 11.4 | 7.65 | W | 11.4 | 7.78 | W | 9.8 | 9.05 | W |
| 15.3 | 5.79 | S | 15.4 | 5.82 | S | 10.9 | 8.15 | W |
| 17.4 | 5.09 | S | 17.3 | 5.17 | S | 14.5 | 6.14 | M |
| 18.2 | 4.87 | S | 18.7 | 4.86 | S | 17.5 | 5.12 | S |
| 20.1 | 4.41 | M | 20.2 | 4.39 | W | 19.3 | 4.66 | W |
| 23.2 | 3.83 | S | 23.6 | 3.84 | S | 22.1 | 4.09 | M |
| 26.9 | 3.24 | W | 27.2 | 3.37 | W | 23.9 | 3.81 | M |
| 30.8 | 2.90 | W | 34.4 | 2.72 | W | 26.3 | 3.48 | W |
W weak, M medium, S strong
Interplanar spacing and relative intensities of characteristic peaks by which the three patterns can be differentiated are well documented in the literature (42). Visual inspection of the diffraction data obtained for the three samples (Fig. 4) indicates that Cyperus and maize starch have their strongest peaks at approximately 15°, 17°, and 23° 2θ. Moreover, the peak at 18° 2θ is unresolved and has been converted into a shoulder, a feature characteristic of an A-type pattern. Potato starch has its strongest diffraction peak at around 17° 2θ, relatively medium peaks at around 5°, 15°, 22°, and 24° 2θ and a couple of weak peaks scattered around 10° and 19° 2θ. The pattern of peaks seen in the diffractogram of potato starch is characteristic of a B-type crystalline structure (42). Although tuber starches usually give a B-type powder pattern, the distinguishing peak for B pattern at 5° 2θ is not seen for Cyperus starch. Thus, it was concluded that the crystalline structure of Cyperus starch is very similar to that of maize starch. Irrespective of the powder pattern, it is generally accepted that a starch granule involves alternating regions of amorphous and crystalline lamellae. The degree of crystallinity was estimated utilizing the technique described by Nara and Komiya (43). The degree of crystallinity of the three samples was in the order of Cyperus starch > maize starch > potato starch with the numerical values being 39.4%, 38.3%, and 20.9%, respectively. Potato starch with the highest amylose content has the lowest degree of crystallinity, thus providing more evidence that amylose seems to disrupt the crystalline nature of starch granules. Cyperus and maize starch have similar amylose content and correspondingly exhibit similar crystallinity.
Moisture Sorption Analysis
Moisture sorption by starch has been attributed to the interaction between the hydroxyl groups of the hexose moiety and water molecules (44). Water molecules form hydrogen bonds with both amylose and amylopectin, but the amylopectin structure has been shown to physically trap water molecules. On this basis, it was hypothesized that starch granules high in amylopectin would have a higher moisture sorption potential (45). However, another factor that seems to supersede this hypothesis is the crystallinity of the polymer. Crystalline polymers have been proposed to have extensive secondary intermolecular bonding. This secondary bonding causes the hydroxyl groups on adjacent glucose units to interact with each other and hence reduces the available sites for adsorption of water molecules (46). As a result, the higher degree of crystallinity could reduce the moisture sorption, as seen in Fig. 5. No significant difference is noted in the ability of Cyperus and maize starch to interact with moisture. This is further confirmed by fitting the Guggenheim, Anderson, and de Boer (GAB) equation (47) to the moisture sorption data. It has also been shown that most of the water associated with polymers is localized in the amorphous region (48). Thus, the value of the so-called monolayer coverage obtained from the GAB equation should be corrected by dividing the Wm value by the fraction of the non-crystalline region and then referred to as the Wcorrm. The GAB parameters for the three starch samples are given in Table IV. It should be of interest to note that, upon accounting for the degree of crystallinity, potato starch has the lowest value of monolayer coverage. The high equilibrium moisture content values exhibited by potato starch are primarily due the localization of moisture in the amorphous region at high relative humidity.
Fig. 5.
Equilibrium moisture content of maize, Cyperus, and potato starch across the relative humidity scan
Table IV.
The Interaction Parameters C G and K, and the Monolayer Coverage W m According to the Equations Proposed by Guggenheim, Anderson, and de Boer (GAB)
| Sample | C G | K | W m (g/g) | Crystallinity | W Corrm (g/g) |
|---|---|---|---|---|---|
| Maize | 15.62 | 0.69 | 0.0858 | 39.4% | 0.1412 |
| Cyperus | 17.38 | 0.66 | 0.0927 | 38.3% | 0.1502 |
| Potato | 10.96 | 0.72 | 0.0957 | 20.9% | 0.1209 |
W Corrm is the monolayer coverage after taking into account the crystallinity of the polymer
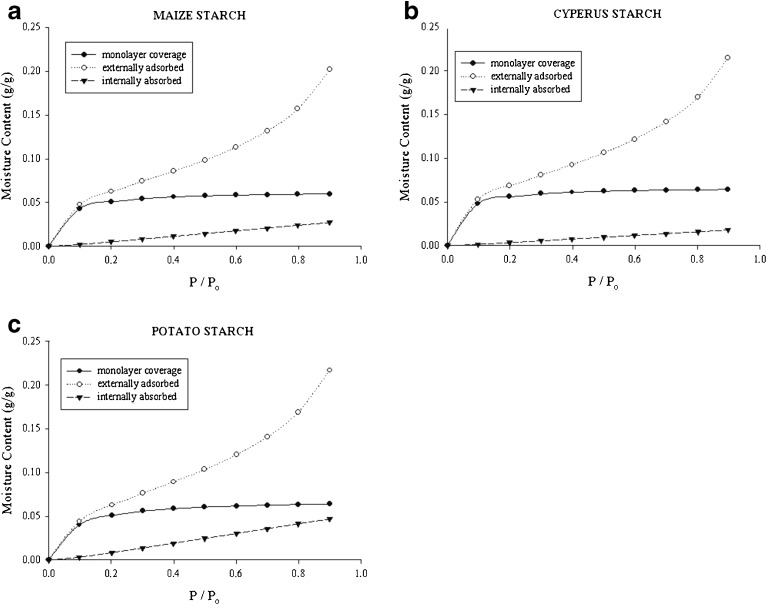
Diffraction studies performed to elucidate the structural characteristics of starch granules have clearly indicated the effect of water content on powder pattern. It was further shown that granules exhibiting a B-type pattern (e.g., potato) can hold more water molecules as compared with an A-type pattern (e.g., maize and Cyperus) (38,39). These studies clearly designate that water associated with starch is not merely adsorbed at the surface but also penetrates the granules. Young and Nelson derived a set of equations that differentiate the moisture associated with biological materials as “bound” or adsorbed monolayer on the surface, “normally condensed” or external moisture, and “absorbed” or internal moisture (49–51). The amount of internally absorbed moisture tends to decrease the “free” water and thus alter the potential of an excipient to participate in bond formation. The analysis of moisture sorption data according to the Young and Nelson equations is illustrated in Fig. 6a–c. As explicated by this analysis, Cyperus starch has the lowest amount of absorbed water and most of the water associated with Cyperus starch is externally adsorbed. The high amount of internally absorbed water for potato starch is in good agreement with its internal structure, i.e., more open and hydrated hexagonal crystallites. The amount of externally adsorbed water is important as it can assist in the formation of liquid bridges between particles. Soluble components present in the formulation tend to associate with these liquid bridges and upon drying; they convert to solid bridges that help maintain the binding efficiency. In cases where the internally absorbed moisture is high, the amount of water available for bridge formation decreases and may result in formulation problems.
Fig. 6.
Analysis of moisture sorption data according to the equations developed by Young and Nelson
Evaluation of Binding Efficiency
Cyperus starch was employed as a binder in concentrations of 5%, 7.5%, and 10% w/w while a similar formulation containing 10% w/w potato starch as binder was used for comparison. To study the effect of binder concentration, all other factors, i.e., the amount of drug, lubricant, and endo-disintegrant were maintained constant across formulations. The details of the four formulations namely C1, C2, and C3 for Cyperus starch and P1 for potato starch are provided in Table V. Both granules and subsequently tablets, obtained using the technique of wet granulation and compression, were characterized. The moisture content, density, friability, porosity, and compressibility data for the granules are presented in Table VI. The average particle size of granules from all formulations ranged between 1,050 and 1,080 μm. Particle size is an important parameter since particle size distribution affects the average tablet weight, disintegration time, granule friability, and granule flowability. Similarly, the moisture content of the granules did not show significant differences. For Cyperus starch, the concentration of binder seemed to have a direct effect on the compressibility index. Increasing the concentration of Cyperus starch as binder seemed to lower the compressibility index of the granules. This indicated that the flow properties of the granules improved significantly with an increase in binder concentration. A similar trend was also observed for the porosity and the friability of the granules. A comparison of formulations containing the same amount of Cyperus and potato starch as binder (10% w/w) indicates that granules obtained using Cyperus starch have better flow properties, lower porosity, and lower friability.
Table V.
Formulation for Metronidazole Granules with Varying Concentration of Cyperus Starch as a Binder
| Formulation | Starch | Binder concentration (w/w) | Metronidazole (mg) | Magnesium stearate (mg) | Endo-disintegrant (mg) |
|---|---|---|---|---|---|
| C1 | Cyperus | 5% | 400 | 4.5 | 22.5 |
| C2 | Cyperus | 7.5% | 400 | 4.5 | 22.5 |
| C3 | Cyperus | 10% | 400 | 4.5 | 22.5 |
| P1 | Potato | 10% | 400 | 4.5 | 22.5 |
Table VI.
Some Properties of Granules Obtained Using Cyperus and Potato Starch as a Binders
| Formulation | Average particle size (μm) | Moisture content (%) | Compressibility index (%) | Porosity (%) | Friability (%) |
|---|---|---|---|---|---|
| C1 | 1,050 | 1.2 | 18.44 | 21.15 | 5.29 0± 0.51 |
| C2 | 1,070 | 1.2 | 14.95 | 20.46 | 1.75 ± 0.13 |
| C3 | 1,080 | 1.2 | 11.24 | 15.50 | 1.25 ± 0.38 |
| P1 | 1,060 | 1.4 | 15.53 | 25.56 | 8.88 ± 0.53 |
Selected properties of metronidazole tablets are listed in Table VII. For formulations with 5% and 7.5% w/w Cyperus starch (formulations C1 and C2, respectively), no significant difference in the hardness of the tablets was noted, but the disintegration time increased significantly. Increasing the concentration of Cyperus starch paste to 10% w/w (formulation C3) leads to a considerable increase in both hardness and disintegration time. It is interesting to note that the hardness of the tablets employing 5% w/w of Cyperus starch as binder is similar to that obtained using twice the amount of potato starch (10% w/w). When Cyperus starch is used at 10% w/w (formulation C3), the hardness of the tablets was found to be significantly higher while the friability appeared negligible with respect to the potato starch formulation at the same binder concentration (formulation P1). The disintegration time, however, did increase with increasing concentration of Cyperus starch. At a similar binder concentration (10% w/w), the disintegration time for tablets containing Cyperus starch (formulation C3) was twice that of tablets containing potato starch (formulation P1). The increase in disintegration time can be attributed to two factors, viz., the lower porosity of the granules formulated using Cyperus starch and the inherent ability of the two starches to swell. Porosity values reflect the network of pores through which water can penetrate a compact, causing it to swell and consequently disintegrate. Additionally, as discussed earlier, the swelling power of Cyperus starch is inherently lower than that of potato and maize starch (Fig. 3). Interplay between the lower porosity of formulated granules and the intrinsic resistance to swelling appears to manifest itself in the form of increased disintegration time for tablets containing Cyperus starch as binder.
Table VII.
Some Properties of Tablets Formulated Using Cyperus and Potato Starch as Binders
| Formulation | Hardness (kgF) | Disintegration time (s) | Friability (%) |
|---|---|---|---|
| C1 | 6.38 ± 0.12 | 120 | 4.34 ± 0.56 |
| C2 | 6.63 ± 0.28 | 210 | 1.56 ± 0.08 |
| C3 | 10.75 ± 0.54 | 300 | 0.07 ± 0.03 |
| P1 | 6.33 ± 0.21 | 150 | 3.24 ± 0.27 |
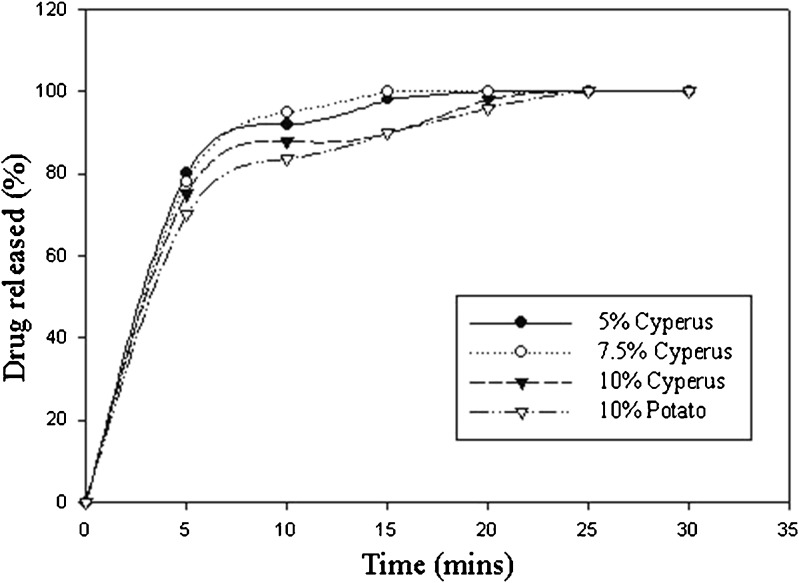
Although differences were noted in the disintegration times of the various formulations, tablets from all four formulations disintegrated within 5 min or less. Hence, a significant impact of binder concentration on the dissolution profile of either formulation was not expected. The dissolution profiles of all four formulations are illustrated in Fig. 7. Visual inspection of the dissolution profiles does not indicate considerable differences between them, and all formulations release 85% or more of the drug in less than 15 min. To further verify this, a model independent mathematical approach to compare the dissolution profiles proposed by Moore and Flanner (52) was used. Using the mean dissolution values from curves at each time point, the mathematical treatment calculates the difference factor (f1) and the similarity factor (f2). The f1 value is 0 when the mean profiles are identical and increases proportionately as the difference between the mean profiles increases. Similarly, the f2 value is 100 when the profiles are identical, and an average difference of 10% at all measured time points results in a f2 value of 50. A value of f1 between 0 and 15 while a value of f2 between 50 and 100 implies sameness or equivalence of the dissolution profiles (53). This method has been widely adopted by the Food and Drug Administration in various guidance documents, with more emphasis on the use of f2 values. It should be noted that, for products which are rapidly dissolving, i.e., more than 85% in 15 min or less, a profile comparison is not necessary. As expected, the f2 values used to measure closeness between the various dissolution profiles of the four formulations were greater than 75, clearly establishing similarity. It is noteworthy that insignificant difference is noted between the dissolution profiles of formulation C3 and formulation P1 (f2 = 92.4), despite the fact that the disintegration time for tablets from formulation C3 is almost twice that of those from formulation P1. These results insinuate that Cyperus starch may provide relatively better binding efficiency without compromising drug release characteristics.
Fig. 7.
Dissolution profile of metronidazole tablets using varying amounts and types of binders
CONCLUSIONS
This study explores the physicochemical properties of starch isolated from the tubers of C. esculentus L. as they relate to its use as an excipient for solid dosage forms. The widespread availability, high yield, and the simplicity of extraction serve as an impetus for its commercial exploitation. The controlled particle size and morphology imply its usefulness in a variety of carrier-based formulations. The moisture sorption and distribution within the Cyperus starch granules make it a good candidate for wet-massing techniques. Of importance could be its binding efficacy as Cyperus starch is more effective than potato starch at lower binder concentrations. This fact is further corroborated by the inherent swelling ability, wherein it shows the highest resistance to swelling. To satisfy the ever-increasing demand for highly specific and functional excipients, Cyperus starch may provide a viable alternative.
REFERENCES
- 1.Moorthy SN. Starch and starch derivatives in food. Trends Carbohydr Chem. 1996;2:133–139. [Google Scholar]
- 2.Sun XS. Biodegradable plastics from renewable biomaterials. Adv Agric Sci Tech. 2002;1:149–167. doi: 10.1142/9789812706584_0006. [DOI] [Google Scholar]
- 3.Martino GT, Solarek DB. Personal care applications of starch polymers. In: Schlossman ML, editor. Chemistry and manufacture of cosmetics. Carol Stream: Allured Publishing Corp; 2002. [Google Scholar]
- 4.Rowe CR, Sheskey PJ, Weller PJ, editors. Handbook of pharmaceutical excipients. 4. London: Pharmaceutical Press; 2003. [Google Scholar]
- 5.Patel NR, Hopponen RE. Mechanism of action of starch as a tablet disintegrant I: factors affecting swelling of starch granules at 37 C. J Pharm Sci. 1996;55:614–617. doi: 10.1002/jps.2600550617. [DOI] [PubMed] [Google Scholar]
- 6.Illum L, Fisher AN, Jabbal-Gill I, Davis SS. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int J Pharm. 2001;222:109–119. doi: 10.1016/S0378-5173(01)00708-6. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg LE, Buxton DK, Friden PM. Novel peridontal drug delivery systems for treatment of periodontitis. J Control Release. 2001;75:93–102. doi: 10.1016/S0168-3659(01)00366-2. [DOI] [PubMed] [Google Scholar]
- 8.Krogars K. Department of Pharmacy. Helsinki: University of Helsinki; 2003. Aqueous-based amylose-rich maize starch solution and dispersion: a study on free films and coatings. [Google Scholar]
- 9.Garr GSM, Bangudu AB. Evaluation of sorghum starch as a tablet excipient. Drug Dev Ind Pharm. 1991;17:1–6. doi: 10.3109/03639049109043805. [DOI] [Google Scholar]
- 10.Nasipuri RN. Evaluation of cocoyam starch as a tablet binder and disintegrant. Pharm Acta Helv. 1979;54:48–53. [PubMed] [Google Scholar]
- 11.Gebre-Mariam T, Nikolayev AS. Evaluation of starch obtained from Ensete ventricosum as a binder and disintegrant for compressed tablets. J Pharm Pharmacol. 1993;45:317–320. doi: 10.1111/j.2042-7158.1993.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande AV, Panya LB. Evaluation of sorghum starch as a tablet disintegrant and binder. J Pharm Pharmacol. 1987;39:495–496. doi: 10.1111/j.2042-7158.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 13.Akande OF, Deshpande AV, Bangudu AB. An evaluation of starch obtained from pearl millet—Pennisetum typhoides as a binder and disintegrant for compressed tablets. Drug Dev Ind Pharm. 1991;17:451–455. doi: 10.3109/03639049109043838. [DOI] [Google Scholar]
- 14.Lowe J, Stanfield DP. The flora of Nigeria Sedge (Family Cyperaceae) Ibadan: University Press, Ibadan; 1974. [Google Scholar]
- 15.Swift HW. The Encyclopaedia Americana. International ed. Danbury: Grolier Incorporated; 1989. Sedge. [Google Scholar]
- 16.USDA(NRCS). The PLANTS database, version 3.5 (http://plants.usda.gov). In: Baton Rouge, LA: The National Plant Data Center; 2004.
- 17.Holm DL, Plucknett DL, Pancho JV, Herberger JE. The world’s worst weeds, distribution and biology. Honolulu: University of Hawaii; 1977. [Google Scholar]
- 18.Anon . The New Encyclopaedia Britannica. 15. Chicago: Encyclopaedia Britannica; 1992. Cyperales. [Google Scholar]
- 19.Trease GE, Evans WC. Pharmacognosy. 11. London: Bailliere Tindall; 1978. Carbohydrates. [Google Scholar]
- 20.Radley JA. Chamber’s Encyclopaedia. New revised ed. London: International Learning System Corporation; 1970. Starch; pp. 134–135. [Google Scholar]
- 21.USP25/NF20 . The United States Pharmacopeia. Rockville: United States Pharmacopeial Convention, Inc; 2002. [Google Scholar]
- 22.Umerie SC, Obi NAN, Okafor EO. Isolation and characterization of starch obtained from Cyperus esculentus tubers. Bioresour Technol. 1997;62:63–65. doi: 10.1016/S0960-8524(97)00040-0. [DOI] [Google Scholar]
- 23.Agrawal AM, Manek RV, Kolling WM, Neau SH. Studies on the interaction of water with ethylcellulose: effect of polymer particle size. AAPS PharmSciTech. 2003;4:E60. doi: 10.1208/pt040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal AM, Neau SH, Bonate PL. Wet granulation fine particle ethylcellulose tablets: effect of production variables and mathematical modeling of drug release. AAPS J. 2003;5:E13. doi: 10.1208/ps050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Rudolph V, Weigl B, Earl A. Interparticle van der Waals force in powder flowability and compactibility. Int J Pharm. 2004;280:77–93. doi: 10.1016/j.ijpharm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Lindeboom N, Chang PR, Tyler RT. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch-Starke. 2004;56:89–99. doi: 10.1002/star.200300218. [DOI] [Google Scholar]
- 27.Smith RL. Determination of ash. In: Whistler RL, editor. Methods in carbohydrate chemistry. New York: Academic; 1964. pp. 41–42. [Google Scholar]
- 28.Bultosa G, Taylor JRN. Chemical and physical characterization of grain tef [Eragrotis tef (Zucc.) Trotter] starch granule composition. Starch-Starke. 2003;55:304–312. doi: 10.1002/star.200390065. [DOI] [Google Scholar]
- 29.Schoch TJ. Non-carbohydrate substances in cereal starches. J Am Oil Chem Soc. 1942;64:2954–2956. [Google Scholar]
- 30.Eteshola E, Oraedu AC. Fatty acid composition of tigernut tubers (Cyperus esculentus L.), baobab seeds (Adansonia digitata L.), and their mixture. J Am Oil Chem Soc. 1996;73:255–257. doi: 10.1007/BF02523905. [DOI] [Google Scholar]
- 31.Schoch TJ. Fatty substances in starch. In: Whistler RL, editor. Methods in carbohydrate chemistry. New York: Academic; 1964. pp. 56–61. [Google Scholar]
- 32.Cheetam NWH, Tao L. Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohydr Res. 1998;36:277–284. [Google Scholar]
- 33.Moreton RC. Excipient functionality. Pharm Technol. 2004;28:98–99. [Google Scholar]
- 34.Carr RL. Classifying flow properties of solids. Chem Eng. 1965;72:69–72. [Google Scholar]
- 35.Hausner HH. Friction conditions in a mass of metal powders. Int J Powder Metall. 1967;3:7–13. [Google Scholar]
- 36.Kumar V, Kothari SH, Banker GS. Compression, compaction, and disintegration properties of low crystallinity celluloses produced using different agitation rates during their regeneration from phosphoric acid solutions. AAPS PharmSciTech. 2001;2:E7. doi: 10.1208/pt020207. [DOI] [PubMed] [Google Scholar]
- 37.Amidon GE. Mechanical characterization of powders. In: Brittain HG, editor. Physical characterization of pharmaceutical solids. New York: Marcel Dekker; 1995. pp. 281–319. [Google Scholar]
- 38.Imberty A, Chanzy H, Perez S, Buleon A, Tran V. The double-helical nature of the crystalline part of A-starch. J Mol Biol. 1988;201:365–378. doi: 10.1016/0022-2836(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 39.Imberty A, Perez S. A revisit to the three dimensional structure of B-type starch. Biopolymers. 1988;27:1205–1221. doi: 10.1002/bip.360270803. [DOI] [Google Scholar]
- 40.Cheetam NWH, Tao L. The effects of amylose content on the amylose molecular size and on the average chain length of amylopectin in maize starches. Carbohydr Res. 1997;33:251–261. [Google Scholar]
- 41.Hizukuri S, Kaneko T, Takeda Y. Relationship between the distribution of the chain length of amylopectin and the crystalline structure of starch granules. Carbohydr Res. 1985;141:295–306. doi: 10.1016/S0008-6215(00)90461-0. [DOI] [Google Scholar]
- 42.Zobel HF. X-ray analysis of starch granules. In: Whistler RL, editor. Methods in carbohydrate chemistry. New York: Academic; 1964. pp. 109–113. [Google Scholar]
- 43.Nara S, Komiya T. Studies on the relationship between water saturated state and crystallinity by the diffraction method for moistened potato starch. Starch-Starke. 1983;35:407–410. doi: 10.1002/star.19830351202. [DOI] [Google Scholar]
- 44.Sair L, Fetzer WR. Water sorption by starches. Ind Eng Chem. 1944;36:205–208. doi: 10.1021/ie50411a004. [DOI] [Google Scholar]
- 45.Rebar V, Fischbach ER, Apostopoulos D, Kokini JL. Thermodynamics of water and ethanol adsorption on four starches as model biomass separation systems. Biotechnol Bioeng. 1984;26:513–517. doi: 10.1002/bit.260260517. [DOI] [PubMed] [Google Scholar]
- 46.Beery KE, Ladisch MR. Chemistry and properties of starch based desiccants. Enzym Microb Technol. 2001;28:573–581. doi: 10.1016/S0141-0229(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RB. Modifications of the Brunauer, Emmett and Teller equations. J Am Chem Soc. 1946;68:686–691. doi: 10.1021/ja01208a049. [DOI] [PubMed] [Google Scholar]
- 48.Zografi G, Kontny MJ, Yang AYS, Brenner GS. Water vapor interactions with pharmaceutical cellulose powders. Drug Dev Ind Pharm. 1984;12:2171–2192. [Google Scholar]
- 49.York P. Analysis of moisture sorption hysteresis in hard gelatin capsules, maize starch, and maize starch: drug powder mixtures. J Pharm Pharmacol. 1981;33:269–273. doi: 10.1111/j.2042-7158.1981.tb13779.x. [DOI] [PubMed] [Google Scholar]
- 50.Young JH, Nelson GL. Theory and hysteresis between sorption and desorption isotherms in biological materials. Trans Am Soc Agric Eng. 1967;10:260–263. [Google Scholar]
- 51.Young JH, Nelson GL. Research and hysteresis between sorption and desorption isotherms of wheat. Trans Am Soc Agric Eng. 1967;10:756–761. [Google Scholar]
- 52.Moore JW, Flanner HH. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm Tech. 1996;20:64–74. [Google Scholar]
- 53.O’Hara T, Dunne A, Bulter J, Devane J. A review of methods used to compare dissolution profile data. PSTT. 1998;1:214–223. [Google Scholar]