Abstract
FSH stimulates testicular growth by increasing Sertoli cell proliferation and elongation of seminiferous cords. Little is known about the peritubular myoid cells in testicular development. In order to investigate the role of peritubular myoid cells in early testicular growth in rodents, two traditional models to induce testicular growth were used: FSH treatment and hemicastration. In order to affect proliferation of peritubular myoid cells, both treatments were combined with imatinib, a tyrosine kinase inhibitor. In addition, effects of imatinib on human testicular peritubular cell proliferation were investigated. Testicular weight, diameter and length of seminiferous cords, numbers of germ, Sertoli and BrdU-positive cells and FSH-levels were measured. FSH treatment and hemicastration increased length of the seminiferous cords and testicular weight by increasing first the early proliferation of peritubular myoid cells and later also the proliferation of the Sertoli cells. Imatinib blocked the FSH and hemicastration -induced testicular hypertrophy and decreased the proliferation of PDGF-stimulated human testicular peritubular cells in vitro. Present results provide new evidence that peritubular myoid cells have an important role in postnatal testicular growth.
Keywords: FSH, hemicastration, imatinib, peritubular myoid cells, postnatal, rat, testis growth
Introduction
Postnatal increase in the number of Sertoli cells is believed to determine final testicular size and later total sperm output. Follicle-stimulating hormone (FSH) is known to stimulate the proliferation of Sertoli cells and to induce testicular growth. Indeed, mice lacking FSH receptor show decreased testicular growth1 and also humans who have non-functional FSH receptor have small testicular size.2 In agreement with these findings Sertoli cell proliferation3-6 and testicular growth7,8 can be stimulated by FSH administration during postnatal period. Similarly, postnatal hemicastration (HCA), which is known to increase pituitary FSH secretion by reducing inhibin B production, stimulates testicular hypertrophy of the contralateral testis.9-11 Adequate postnatal testicular growth is important for future fertility since the final number of Sertoli cells in the adult testis correlates with total sperm output.12,13
Less is known about the role of peritubular myoid cells in testicular growth. Proliferation of the peritubular myoid cells14 coincides with the expansion of the Sertoli cells during postnatal period which ceases at the postnatal day 15 in the rat.15,16 Supporting their close interaction during development, communication between the peritubular myoid and the Sertoli cells is required for the formation of basal lamina during postnatal development.17,18 Thus, peritubular myoid cell proliferation may play a more important role in the regulation of testicular growth than earlier appreciated.
In order to investigate the role of the peritubular myoid cells in testicular growth in rats, we used two traditional models to induce testicular growth: FSH treatment and hemicastration. Since we have earlier shown that the inhibition of peritubular myoid cell proliferation during postnatal period is able to decrease final testicular size19 we combined FSH administration or hemicastration with imatinib mesylate treatment (Glivec®, STI571, Novartis Pharma). FSH treatment and hemicastration increased the length of the seminiferous cords and testicular weight by increasing the early proliferation of peritubular myoid cells and later also the proliferation of the Sertoli cells. Imatinib blocked the FSH and hemicastration -induced testicular hypertrophy and was also able to decrease the proliferation of the human testicular peritubular cells (HTPCs) in vitro.
Results
FSH administration and hemicastration induced testicular growth
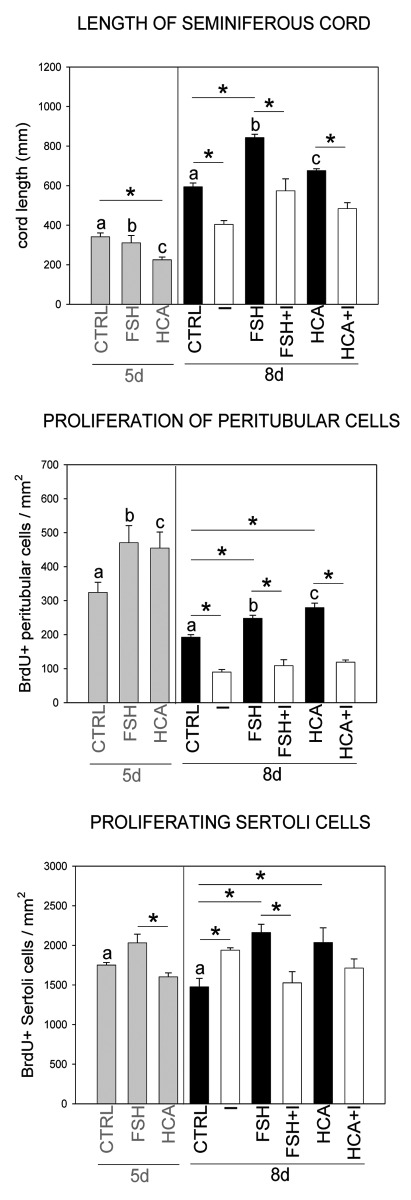
Hemicastration and FSH treatment increased plasma FSH levels, resulting in testicular growth at the postnatal age of 8d (Table 3). FSH treatment caused 45% and hemicastration 9% increase in testicular weight when compared with the control value, but the body weights did not change. Faster longitudinal outgrowth of seminiferous cords and increased proliferation rate of Sertoli cells and peritubular myoid cells (Figs. 1 and 2) were evident in both the hemicastrated group and FSH-treated group. Proliferation of the peritubular myoid cells increased earlier (day 5) than that of the Sertoli cells (day 8), suggesting a role in the FSH-induced testicular growth (Table 3 and Figure 1). No significant changes in germ or Sertoli cell numbers per area, in the proliferation rate of germ cells or in the diameter of seminiferous cords were detected after FSH administration or hemicastration when compared with controls at the postnatal age of 8d indicating that the testicular growth was due to lengthening of the testicular seminiferous cords / tubules.
Table 3. The effects of FSH administration and hemicastration (HCA) with or without imatinib treatment (I) on the serum levels of FSH and the morphological findings of postnatal testicular growth in rats.
| |
Parameter |
Age and treatment group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
5d |
|
8d |
|
|||||||
| CTRL | FSH | HCA | CTRL | I | FSH | FSH+I | HCA | HCA+I | |||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Body weight (n = 8) |
|
13.7 ± 0.8 |
11.6a ± 0.2 |
13.5 ± 0.4 |
|
20.3c ± 0.8 |
12.8d ± 0.2 |
21.0c ± 0.4 |
14.1d ± 0.6 |
20.8c ± 0.9 |
14.0d ± 0.9 |
|
| |
FSH (ng/ml) (n = 8) |
|
3.8 ± 0.4 |
26.1a ± 1.3 |
11.4 ± 1.4 |
|
5.3 ± 0.8 |
2.9d ± 0.5 |
15.6bc ± 0.7 |
50.0d ± 6.3 |
16.2bc ± 1.3 |
15.7 ± 1.6 |
|
| |
Testis weight (mg) (n = 8, except in HCA and HCA+I n = 16) |
|
9.4 ± 0.04 |
7.8 ± 0.03 |
8.7 ± 0.04 |
|
17.5c ± 0.05 |
13.7d ± 0.04 |
25.3bc ± 0.08 |
21.0d ± 0.16 |
19.1ce ± 0.05 |
15.9d ± 0.06 |
|
| |
Cord diameter (μm) (n = 4) |
|
54.2 ± 0.9 |
47.1a ± 1.8 |
52.9e ± 0.5 |
|
55.4 ± 0.9 |
58.0 ± 1.4 |
57.1c ± 0.6 |
61.1d ± 1.4 |
56.5c ± 0.9 |
60.4 ± 1.4 |
|
| |
Sertoli cells / mm2 (n = 4) |
|
6689 ± 283 |
6900 ± 430 |
6029 ± 172 |
|
7372 ± 440 |
9428d ± 383 |
8498c ± 354 |
9099 ± 670 |
8512c ± 439 |
9374 ± 576 |
|
| |
Germ cells / mm2 (n = 4) |
|
180 ± 18 |
133 ± 11 |
181 ± 25 |
|
511c ± 91 |
299 ± 42 |
412c ± 77 |
275 ± 27 |
539c ± 116 |
251 ± 67 |
|
| |
BrdU+ germ cells / mm2 (n = 4) |
|
99.8 ± 5.0 |
45.3 ± 19.7 |
50.5 ± 15.8 |
|
219.1c ± 31.3 |
147.6 ± 17.4 |
229.4c ± 43.8 |
149.6 ± 17.2 |
268.3c ± 51.3 |
114.5d ± 33.0 |
|
The values presented are means ± SEM, significant difference (p < 0.05) acompared with control value at the age of 5-d-old, bcompared with control value at the age of 8-d-old, ccompared with value after similar treatment at the age of 5-d old, dcompared with value after similar treatment without imatinib at the age of 8-d-old and eHCA compared with FSH at the same age.
Figure 1. The effects of FSH administration (FSH), hemicastration (HCA) and imatinib treatment (I) on the seminiferous cord length and the number of BrdU-positive Sertoli and peritubular myoid cells. Results are means + SEM * and abc indicate significant differences (p < 0.05) between the experimental groups.
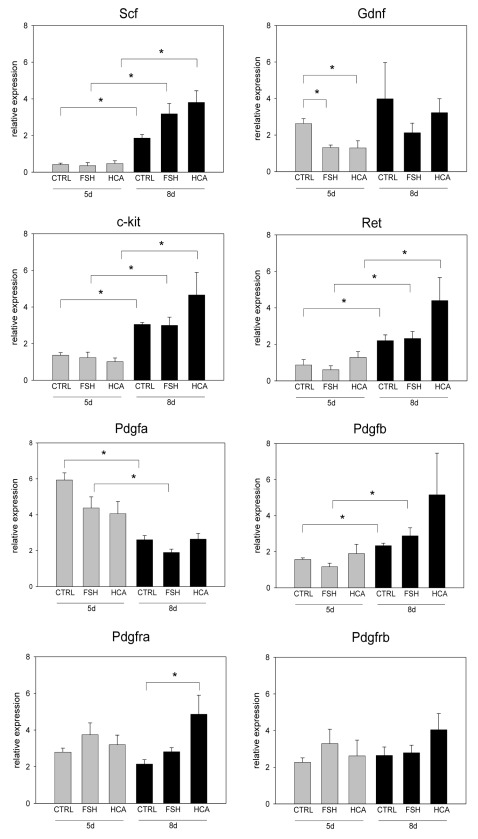
To further study the mechanism behind FSH- and hemicastration-induced testicular hypertrophy, we analyzed transcript levels of SCF, c-kit, GDNF, Ret, PDGF ligands and receptors. Significant ontogenetic changes in the levels of these transcripts were found. Transcript levels of SCF, c-kit, PDGFB and Ret were significantly increased and PDGFA significantly decreased from postnatal age of 5d to postnatal age of 8d (Fig. 3) at these age points. FSH administration and hemicastration did not significantly affect the expression pattern of SCF, c-kit, Ret or PDGF ligands (Fig. 3). Only PDGFR-α expression was significantly increased after hemicastration on postnatal day 8 (Fig. 3) and GDNF expression significantly decreased after FSH-treatment and hemicastration on postnatal day 5 (Fig. 3).
Figure 3. The effects of FSH administration and hemicastration on the relative levels of testicular SCF, c-kit, GDNF, Ret, PDGF-A, PDGF-B, PDGFR-α and PDGFR-β gene expression in 5- and 8-d-old rats. Results are means + SEM *depicts the statistically significant difference.
Imatinib decreased postnatal testicular growth
Imatinib treatment alone significantly decreased plasma levels of FSH, testicular size, length of the seminiferous cords and proliferation of the peritubular myoid cells on postnatal day 8 (Table 3 and Figure 1). Instead, imatinib increased the number of the proliferating Sertoli cells significantly and led to a significantly increased number of Sertoli cells per area (Table 3 and Figures 1 and 2). Imatinib treatment did not affect the total number or the number of proliferating germ cells on postnatal day 8.
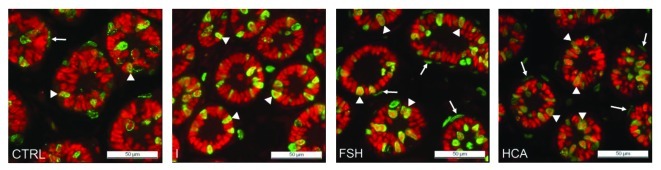
Figure 2. The immunohistochemical detection of BrdU incorporation (green fluorescence) and WT-1 positive Sertoli cells (red fluorescence) in the testis of control (CTRL), imatinib-treated (I) FSH-treated (FSH) and hemicastrated (HCA) rats at the age of 8 d. Proliferating peritubular myoid cells are identified according to BrdU staining and peritubular location (→) and proliferating Sertoli cells by double staining with BrdU and WT-1 (►).
Imatinib abolished testicular growth induced by FSH treatment and hemicastration
Imatinib treatment was able to abolish both the FSH- and hemicastration-induced longitudinal growth of seminiferous cords and significantly decreased peritubular myoid cell proliferation in both of these treatment groups (Fig. 1). Proliferation of the Sertoli cells was significantly decreased in FSH-treated group but the decrease in the hemicastrated group was more modest and did not reach statistical significance. Imatinib treatment in combination with FSH administration or hemicastration had not significant effect on the total number of Sertoli cells per area (Table 3). The diameter of the seminiferous cords increased after combined administration of FSH and imatinib (Table 3). Imatinib treatment in combination with FSH administration or hemicastration did not significantly affect the number of germ cells.
Imatinib inhibited the proliferation of the human testicular peritubular cells (HTPCs) in vitro
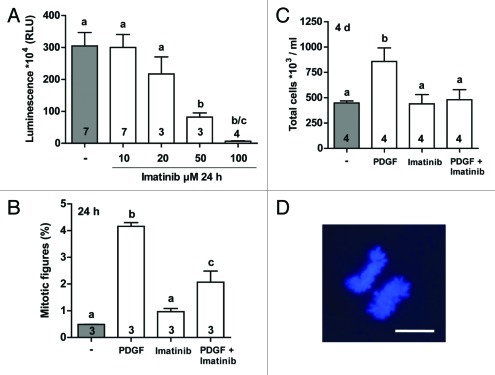
Effects of increasing concentrations of imatinib on viability of human testicular peritubular cells are shown in Figure 4A. Viability of the cells was not affected by the concentrations ≤ 10 µM but was dose dependently reduced with higher concentrations. Imatinib concentration of 10 µM was therefore used for further analysis. Exposure of human peritubular cells to 10 µM imatinib had no effect on their proliferation or number per ml when compared with control values. However, when HTPCs were stimulated with 5 ng/ml of recombinant human PDGF, the natural ligand of the PDGFα and β receptors,31 a significant increase in cell proliferation (Fig. 4D) and cell number/ml (Fig. 4C) was detected. Treatment with 10 µM imatinib effectively blocked this PDGF-induced proliferation and increase in HTPC numbers (Fig. 4B and C).
Figure 4. The effect of imatinib in cultured human testicular peritubular cells (HTPCs) on (A) cell viability (ATP assay), (B) the proportion of proliferating cells showing a mitotic figure (%) and (C) total cell counts. Imatinib blocked the PDGF-induced increase in cell proliferation of HTPCs. (D) Example of a mitotic figure stained with DAPI indicating a proliferating HTPC. Bar: 3 µm. Results are means + SEM. Different letters indicate significant differences. Numbers in columns indicate the number of patients used for cell harvesting. RLU, relative luminescence units.
Discussion
The aim of the present study was to investigate the role of peritubular myoid cells in postnatal testicular growth. Therefore two traditional models to stimulate Sertoli cell proliferation, FSH administration and hemicastration, were compared and combined with imatinib treatment. Our present data indicate that peritubular myoid cells play a more critical role in determination of final testicular size than earlier thought.
Interestingly, both FSH administration and hemicastration increased first peritubular myoid cell proliferation, already on the postnatal day 5. Three days later an increase in the number proliferating Sertoli cells was seen and accordingly the length of the seminiferous cords and testis weight increased. Since Sertoli cells are the only cells expressing FSH receptors in the testis,32,33 FSH administration and hemicastration most probably affected the proliferation of peritubular myoid cells through paracrine factors like PDGF ligands. Increased expression level of PDGF receptor α was detected after hemicastration, and there was a tendency to higher PDGFβ, suggesting that hemicastration and FSH treatments may affect the PDGF signal cascade. Our finding is not in agreement with the previous study by Gnessi and coworkers where they showed that FSH dose dependently inhibits PDGF production.34 Possible explanation is that our work was done in vivo, and Gnessi and coworkers investigated the effects of FSH on PDGF production in in vitro cultures.
To further study the mechanism behind FSH- and hemicastration-induced testicular hypertrophy, we analyzed also transcript levels of SCF, c-kit, GDNF and Ret. However, pathways analyzed here seemed not to be similarly affected. An obvious limitation of the present study is that the measurements of the steady-state mRNA levels were done in the whole testes and therefore may not properly reflect changes at the cellular level.
Since we had earlier shown that tyrosine kinase inhibitor imatinib caused inhibition of peritubular myoid cell proliferation during the postnatal period and decreased final testicular size,19 we used imatinib to test, whether it could interfere in the communication of Sertoli cells and peritubular myoid cells, particularly PDGF signaling. Sertoli cells produce PDGFs that act on myoid cells harboring PDGF receptors.34,35 Imatinib is a small molecular analog of ATP that inhibits the Bcr-Abl, PDGFR-α, PDGFR-β, c-Fms, Arg and c-kit tyrosine kinases.36-39 PDGFs stimulate gonocyte and peritubular myoid cell proliferation.40-42 C-kit receptor, in turn is known to be expressed on differentiating spermatogonia and Leydig cells43-45, and its ligand SCF is produced by Sertoli cells.44,46 Treatment with imatinib alone led to a significant decrease in the proliferation of the peritubular myoid cells, without decreasing the number of proliferating Sertoli cells, which are known to be c-kit and PDGF receptor-negative. Present data confirms our earlier observations showing that a short 3-d postnatal imatinib treatment and inhibition of peritubular myoid cell proliferation is able to affect the final testicular size in adult rats.19
The present study demonstrates that imatinib treatment is able to block the FSH-stimulated testicular growth and hemicastration-induced compensatory hypertrophy of the contralateral testis. When imatinib was combined with FSH administration or hemicastration, especially the early proliferation of peritubular myoid cells, which were shown to initiate the testicular hypertrophy, decreased significantly. This blocked the FSH-induced accelerated tubular outgrowth and prevented the hypertrophy of the testis. Observation suggests that peritubular myoid cell proliferation is a significant determinant of longitudinal growth of the seminiferous cords and the final testicular size.
The inhibition of myoid cell proliferation was seen also in human peritubular cells. Imatinib affected the cells that were actively induced to proliferate by PDGF stimulation. The observation confirms that imatinib exerts its function in peritubular cells by interfering with active protein tyrosine kinase cascade. Thus, activity of imatinib in a particular cell should be selective in terms of how active this cascade is in the cell. During postnatal testicular development such ligand stimulation and cascade activation is known to occur in peritubular myoid precursor and germ cells.
In conclusion, the present study provides new evidence that peritubular myoid cells have an important role in the longitudinal growth of seminiferous cords in the postnatal rat testis. Thus, Sertoli cell proliferation is not the only determinant of postnatal testicular growth but peritubular myoid cells participate in this regulation and affect the growth of basement membrane lined by Sertoli cells.
Materials and Methods
Animals and treatments
Sprague-Dawley rats were housed in plastic cages (Tecniplast) in a climate-controlled room at 21 ± 3°C with a relative humidity of 55 ± 15% at the Animal Center of Turku University. Aspen chips (Tapvei Co.) were used as bedding material and animals were maintained on a 12 h light/ 12 h dark cycle (lighted from 07 to 19 h). Animals had free access to tap water and standard laboratory animal feed [Commercial RM3 (E) SQC, Special Diet Service].
The day of birth was designed as day 0. Two days after birth, the size of each litter was reduced by random selection to 8. Litters were divided into nine treatment groups (Table 1) and every group included pups from two or more litters.
Table 1. The experimental groups and the time-line for treatment of immature male rats.
| Age |
Group |
Treatment |
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | 5 d | ||||||
| 5 d |
CTRL |
Control |
|
|
|
|
DEATH |
|
|
|
| FSH |
FSH |
x |
x |
x |
x |
|
|
|
||
| HCA |
Hemicastration |
x |
|
|
|
|
|
|
||
| 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | 8 d | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 d | CTRL |
control |
|
|
|
|
|
|
|
DEATH |
| I |
imatinib |
|
|
|
|
x |
x |
x |
||
| FSH |
FSH |
x |
x |
x |
x |
x |
x |
x |
||
| FSH+I |
FSH |
x |
x |
x |
x |
x |
x |
x |
||
| imatinib |
|
|
|
|
x |
x |
x |
|||
| HCA |
hemicastration |
x |
|
|
|
|
|
|
||
| HCA+I | Hemicastration |
x |
|
|
|
|
|
|
||
| imatinib | x | x | x |
In order to induce the growth of the contralateral testis, one group of animals were unilaterally castrated, i.e., hemicastrated (HCA) a day after birth (day 1) using cooling as analgesia.20 Another group of male pups was exposed with lyophilized recombinant human FSH (Puregon) dissolved in water (200 IU/kg, s.c.) daily from day 1 onwards to stimulate testicular hypertrophy. Part of the hemicastrated and FSH-exposed pups and one group of intact pups were treated with imatinib mesylate (150 mg/kg; Glivec®, STI571, Novartis Pharmaceuticals Corporation) dissolved in water and injected (150 µl) intracavitally into the stomach once daily on postnatal days 5–7 as described earlier.19 Control animals were injected in the same manner with water alone. The experimental groups and the timeline of the experiment are presented in Table 1. In 30% of experimental animals combined administration of FSH and imatinib caused lethal reaction up to 24 h after treatment. Animals became lethargic and did not survive the planned experiment. The reason for mortality remained unsolved. Substitute animals were injected to maintain the group size large enough.
The animals were sacrificed by decapitation at the age of 5 or 8 d. The weight of the body and testis were recorded and blood and testicular samples were collected. All animal experiments were approved by the Turku University Committee on the Ethics of Animal Experimentation.
Analysis of the diameter and length of seminiferous cords
In order to calculate the diameter and the length of the seminiferous cords, Bouin-fixed 4-µm thick testicular sections (n = 4 per group) were stained with hematoxylin and eosin. Morphometric analysis involved the measurement of cord diameter and the proportion of testicular tissue made by interstitial tissue. Cord length was calculated by using a formula21: length of the cord (m) = {weight of the testis (mg) x cord area (%)}/{π x [diameter of the cord (µm)/2)]2}. All analyses were performed utilizing a morphometric program (Leica IM500 Version: 4.0; Leica Microsystems Imaging solutions Ltd., Cambridge, UK).
The immunohistochemical detection of BrdU incorporation and the double staining with WT-1
BrdU (Roche Applied Science) (50 mg/kg) was injected intra peritoneally three hours prior to sacrificing the animal. The samples of testicular tissue were fixed overnight with 4% paraformaldehyde-fixative, embedded in paraffin and cut to 4-µm thick sections. To detect BrdU-positive peritubular myoid cells, immunohistochemistry was performed for the testis samples following manufacturer's instructions (Roche Diagnostics Corp.), as described earlier.19 Shortly, monoclonal anti-BrdU (6 µl/100 µl PBS, Roche Diagnostics Corp.) was used as the primary antibody and the secondary antibody treatment and visualization were performed utilizing the PowerVision immunohistochemistry kit (PowerVision+TM Poly-HRP IHC Kit Biotin-free, anti-mouse/rabbit, ImmunoVision Technologies, Co.), according to the manufacturer’s instructions.
Proliferating Sertoli and germ cells were detected using double fluorescense immunohistochemistry with antibodies against BrdU and WT-1 as described earlier.22,23 A rabbit polyclonal antibody against WT-1 (180) (Santa Cruz; 1:100 dilution) was used for identifying Sertoli cells and an anti-BrdU antibody (a mouse monoclonal antibody, Roche Diagnostics Corp., 6 µl / 100 µl TBS) was utilized to label proliferating cells. Cell nuclei were stained with DAPI (0.5 ng/ml, Sigma). After secondary antibody incubation the slides were examined under fluorescence microscope.
All Sertoli and germ cells as well as BrdU-positive peritubular myoid, Sertoli and germ were counted from the minimum area of 300,000 µm2 per testis section (n = 4 per group).
Hormone assays
Plasma levels of FSH were measured employing immunofluorometric assay as described earlier.24 For the FSH assay, the sensitivity was 0.1 µg/L and the intra- and interassay coefficients of variation 4.4% and 10.4%, respectively, at a level of 4.8 µg/L.
The FSH levels of eight animals in each experimental group were measured.
Extraction of RNA and performance of quantitative RT-PCR
Total RNA was extracted from testicular tissue using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions and treated with the DNase I Amplification Grade Kit (Invitrogen) as described earlier.25 Briefly, quantitative RT-PCR was performed employing the DyNAmo SYBR Green 2-Step qRT-PCR Kit (Finnzymes), with the inclusion of controls without reverse transcriptase. For each set of conditions four independent samples were analyzed in triplicate. The mRNA levels of platelet derived growth factors Pdgfa and Pdgfb and their receptors Pdgfra and Pdgfrb, c-kit, Stem cell factor (Scf), Glial cell line-derived neurotropic factor (Gdnf) and its receptor Ret were analyzed relative to the levels of glyceraldehyde-3-phosphate dehydrogenase (Gapdh), hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) and ribosomal protein S26 (S26) mRNAs. The primers and annealing temperatures used in this regard are presented in Table 2.
Table 2. The primers used in qRT-PCR analysis.
| Primers for quantitative RT-PCR | ||||
|---|---|---|---|---|
| Gene | Primer sequence | Annealing temperature | Product length | GenBank accession number |
|
Gapdh |
5′-AGACAGCCGCATCTTCTTGT-3′ 5′-CTTGCCGTGGGTAGAGTCAT-3′ |
55°C |
207 bp |
NM_017008 |
|
Hbrt1 |
5′-AAGCTTGCTGGTGAAAAGGA-3′ 5′-CCGCTGTCTTTTAGGCTTTG-3′ |
54°C |
185 bp |
NM_012583 |
|
S26 |
5′-AAGGAGAAACAACGGTCGTG-3′ 5′-GCAGGTCTGAATCGTGGTG-3′ |
57°C |
300 bp |
XM_001066146 |
|
c-kit |
5′-GGCCTAGCCAGAGACATCAG-3′ 5′-CATTCGGAAACCTTCCTTGA-3′ |
59°C |
234 bp |
D12524 |
|
Scf |
5′-CAAAACTGGTGGCGAATCTT-3′ 5′-GCCACGAGGTCATCCACTAT-3′ |
61°C |
217 bp |
NM_021843 |
|
Pdgfra |
5′-ACGTTCAAGACCAGCGAGTT-3′ 5′-CAGTTTGATGGACGGGAGTT-3′ |
64°C |
225 bp |
XM_214030 |
|
Pdgfrb |
5′-ACCTGGTGGACTACCTGCAC-3′ 5′-TGTCCGCGTATTTGATGTGT-3′ |
63°C |
234 bp |
NM_031525 |
|
Pdgfa |
5′-GAGATACCCCGGGAGTTGAT-3′ 5′-AAATGACCGTCCTGGTCTTG-3′ |
63°C |
244 bp |
NM_012801 |
|
Pdgfb |
5′-GTCGAGTCGGAAAGCTCATC-3′ 5′-CACTGCACATTGCGGTTATT-3′ |
60.5°C |
212 bp |
XM_001075973 |
|
Ret |
5′-ACAGCCTTCCGTCTGAAAGA-3′ 5′-AAGCCCCGTACAACTTGATG-3′ |
58°C |
157 bp |
NM_012643 |
| Gdnf | 5′-GCCGAGACAATGTACGACAA-3′ 5′-CTGGAGCCAGGGTCAGATAC-3′ |
57°C | 206 bp | NM_019139 |
Culture of human peritubular cells
Isolation of human testicular peritubular cells (HTPC) was performed as previously described.26-28 HTPCs originate from patients displaying normal spermatogenesis. The cells, passages 3–10, were maintained in DMEM supplemented with 10% fetal calf serum (FCS; both from PAA GmbH). All participants granted written informed consent. The local ethics committee of Technical University of Munich approved the study.
To determine viability of cells in culture the CellTiter-Glo® Luminescent Cell Viability Assay (Promega GmbH) was used as described before.29 Cells were seeded in quadruplicates in 24-well tissue culture plates and incubated for 24h with/without imatinib (10, 20, 50 and 100 µM). The kit reagents were added directly to the cells and luminescence, as a marker of cell viability, was measured with FLUOstar OPTIMA (BMG LABTECH GmbH).
For further analysis cells were seeded on 60-mm dishes and treated with 5 ng/ml PDGF-BB (human recombinant; Sigma) or/and 10 µM imatinib for 4 d. As the PDGF stock was prepared in 4 mM HCl containing 0.1% BSA, a 1:1.000 dilution of this stock without PDGF was added to the media as a control. Cells were trypsinized and numbers determined with an automated cell counting device (CASY-system, Casy, Schärfe Systems.28
Cell proliferation was evaluated by counting anaphase, metaphase and telophase mitotic figures in HTPCs, stained with DAPI (4′,6-diamidino-2.phenylindole; 1,5 µg/ml; Vectashield mounting medium, Vector laboratories). Labeling of nuclei was done as described previously.30 Mitotic events were expressed as percentage of DAPI stained nuclei. At least 200 nuclei per slide were counted.
Statistical analysis of the data
The means and SEM values obtained in independent experiments were calculated. The Mann-Whitney Rank Sum test or t-test (pairwise comparison) was used for single statistical comparison of independent groups of samples. ANOVA followed by the Tukey’s test was employed for multiple comparisons. A p value of less than 0.05 was considered to indicate a statistically significant difference between groups.
Acknowledgments
We thank Prof. U. Schwarzer, Freising for providing human samples and Taija Leinonen, Jonna Palmu, Taina Kirjonen and Astrid Tiefenbacher for skilful technical assistance.
Glossary
Abbreviations:
- ATP
adenosine triphosphate
- BrdU
5-bromo-2-deoxyuridine
- DAPI
4′, 6′-Diamidino-2′-phenylindole
- FCS
fetal calf serum
- FSH
follicle stimulating hormone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GDNF
Glial cell line-derived neurotropic factor
- HCA
hemicastration
- HPRT1
hypoxanthine guanine phosphoribosyl transferase 1
- HTPCs
human testicular peritubular cells
- PDGF
platelet-derived growth factor
- PFA
paraformaldehyde
- PBS
phosphate-buffered saline
- SCF
stem cell factor
- S26
ribosomal protein S26
- WT1
Wilms’ tumor suppressor gene 1
Disclosure of Potential Conflicts of Interest
The authors have nothing to declare. The part of this study dealing with human peritubular cells was done in partial fulfilment of the requirements of a Dr. rer. nat. thesis at LMU of Marion Adam.
Financial Support
This work was supported by the Sigrid Juselius Foundation, The Academy of Finland, the Cancer Society of Southwestern Finland, The Swedish Child Cancer Fund, the Finnish Cancer Society, the Finnish Pediatric Research Foundation, the Swedish Barncancerfonden, the Nona and Kullervo Väre Foundation, Orion Research Foundation, DFG MA1080/16–3 and DAAD.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/20067
References
- 1.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–7. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapanainen JS, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–6. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- 3.Griswold MD, Solari A, Tung PS, Fritz IB. Stimulation by follicle-stimulating hormone of DNA synthesis and of mitosis in cultured Sertoli cells prepared from testes of immature rats. Mol Cell Endocrinol. 1977;7:151–65. doi: 10.1016/0303-7207(77)90064-8. [DOI] [PubMed] [Google Scholar]
- 4.Meachem SJ, McLachlan RI, de Kretser DM, Robertson DM, Wreford NG. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod. 1996;54:36–44. doi: 10.1095/biolreprod54.1.36. [DOI] [PubMed] [Google Scholar]
- 5.Buzzard JJ, Wreford NG, Morrison JR. Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH. Reproduction. 2002;124:633–41. doi: 10.1530/rep.0.1240633. [DOI] [PubMed] [Google Scholar]
- 6.Meachem SJ, Ruwanpura SM, Ziolkowski J, Ague JM, Skinner MK, Loveland KL. Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation. J Endocrinol. 2005;186:429–46. doi: 10.1677/joe.1.06121. [DOI] [PubMed] [Google Scholar]
- 7.Bentley MJ, Gass GH, Leidl W. Effects of FSH and LH administration on the testes and seminal vesicles. Andrologia. 1978;10:357–61. doi: 10.1111/j.1439-0272.1978.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 8.Ultee-van Gessel AM, Timmerman MA, de Jong FH. Effects of treatment of neonatal rats with highly purified FSH alone and in combination with LH on testicular function and endogenous hormone levels at various ages. J Endocrinol. 1988;116:413–20. doi: 10.1677/joe.0.1160413. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham GR, Tindall DJ, Huckins C, Means AR. Mechanisms for the testicular hypertrophy which follows hemicastration. Endocrinology. 1978;102:16–23. doi: 10.1210/endo-102-1-16. [DOI] [PubMed] [Google Scholar]
- 10.Klaij IA, Timmerman MA, Kramer P, Meijs-Roelofs HM, de Jong FH. Testicular and serum levels of inhibin and expression of inhibin subunit mRNAs are differentially affected by hemicastration in rats of various ages. J Endocrinol. 1994;141:143–51. doi: 10.1677/joe.0.1410143. [DOI] [PubMed] [Google Scholar]
- 11.Brown JL, Chakraborty PK. Comparison of compensatory pituitary and testicular responses to hemicastration between prepubertal and mature rats. J Androl. 1991;12:119–25. [PubMed] [Google Scholar]
- 12.Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod. 1984;31:785–95. doi: 10.1095/biolreprod31.4.785. [DOI] [PubMed] [Google Scholar]
- 13.Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–94. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- 14.Palombi F, Farini D, Salanova M, de Grossi S, Stefanini M. Development and cytodifferentiation of peritubular myoid cells in the rat testis. Anat Rec. 1992;233:32–40. doi: 10.1002/ar.1092330106. [DOI] [PubMed] [Google Scholar]
- 15.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–92. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 16.Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod. 1971;4:84–7. doi: 10.1093/biolreprod/4.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Tung PS, Skinner MK, Fritz IB. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann N Y Acad Sci. 1984;438:435–46. doi: 10.1111/j.1749-6632.1984.tb38304.x. [DOI] [PubMed] [Google Scholar]
- 18.Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–7. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurmio M, Toppari J, Zaman F, Andersson AM, Paranko J, Söder O, et al. Inhibition of tyrosine kinases PDGFR and C-Kit by imatinib mesylate interferes with postnatal testicular development in the rat. Int J Androl. 2007;30:366–76, discussion 376. doi: 10.1111/j.1365-2605.2007.00755.x. [DOI] [PubMed] [Google Scholar]
- 20.Orth JM, Higginbotham CA, Salisbury RL. Hemicastration causes and testosterone prevents enhanced uptake of [3H] thymidine by Sertoli cells in testes of immature rats. Biol Reprod. 1984;30:263–70. doi: 10.1095/biolreprod30.1.263. [DOI] [PubMed] [Google Scholar]
- 21.Leidl W, Bentley MI, Gass GH. Longitudinal growth of the seminiferous tubules in LH and FSH treated rats. Andrologia. 1976;8:131–6. doi: 10.1111/j.1439-0272.1976.tb02121.x. [DOI] [PubMed] [Google Scholar]
- 22.Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S. Irradiation causes acute and long-term spermatogonial depletion in cultured and xenotransplanted testicular tissue from juvenile nonhuman primates. Endocrinology. 2007;148:5541–8. doi: 10.1210/en.2007-0809. [DOI] [PubMed] [Google Scholar]
- 23.Nurmio M, Toppari J, Kallio J, Hou M, Söder O, Jahnukainen K. Functional in vitro model to examine cancer therapy cytotoxicity in maturing rat testis. Reprod Toxicol. 2009;27:28–34. doi: 10.1016/j.reprotox.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 24.van Casteren JI, Schoonen WG, Kloosterboer HJ. Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol Reprod. 2000;62:886–94. doi: 10.1095/biolreprod62.4.886. [DOI] [PubMed] [Google Scholar]
- 25.Nurmio M, Kallio J, Toppari J, Jahnukainen K. Adult reproductive functions after early postnatal inhibition by imatinib of the two receptor tyrosine kinases, c-kit and PDGFR, in the rat testis. Reprod Toxicol. 2008;25:442–6. doi: 10.1016/j.reprotox.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht M, Rämsch R, Köhn FM, Schwarzer JU, Mayerhofer A. Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocrinol Metab. 2006;91:1956–60. doi: 10.1210/jc.2005-2169. [DOI] [PubMed] [Google Scholar]
- 27.Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB, Mayerhofer A. Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology. 2008;149:1678–86. doi: 10.1210/en.2007-1064. [DOI] [PubMed] [Google Scholar]
- 28.Schell C, Albrecht M, Spillner S, Mayer C, Kunz L, Köhn FM, et al. 15-Deoxy-delta 12-14-prostaglandin-J2 induces hypertrophy and loss of contractility in human testicular peritubular cells: implications for human male fertility. Endocrinology. 2010;151:1257–68. doi: 10.1210/en.2009-1325. [DOI] [PubMed] [Google Scholar]
- 29.Rey-Ares V, Lazarov N, Berg D, Berg U, Kunz L, Mayerhofer A. Dopamine receptor repertoire of human granulosa cells. Reprod Biol Endocrinol. 2007;5:40. doi: 10.1186/1477-7827-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunz L, Rämsch R, Krieger A, Young KA, Dissen GA, Stouffer RL, et al. Voltage-dependent K+ channel acts as sex steroid sensor in endocrine cells of the human ovary. J Cell Physiol. 2006;206:167–74. doi: 10.1002/jcp.20453. [DOI] [PubMed] [Google Scholar]
- 31.Adam M, Schwarzer JU, Köhn FM, Strauss L, Poutanen M, Mayerhofer A. Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: possible role of decorin in male infertility by interfering with growth factor signaling. Hum Reprod. 2011;26:2613–25. doi: 10.1093/humrep/der245. [DOI] [PubMed] [Google Scholar]
- 32.Kliesch S, Penttilä TL, Gromoll J, Saunders PT, Nieschlag E, Parvinen M. FSH receptor mRNA is expressed stage-dependently during rat spermatogenesis. Mol Cell Endocrinol. 1992;84:R45–9. doi: 10.1016/0303-7207(92)90039-9. [DOI] [PubMed] [Google Scholar]
- 33.Heckert LL, Griswold MD. Expression of follicle-stimulating hormone receptor mRNA in rat testes and Sertoli cells. Mol Endocrinol. 1991;5:670–7. doi: 10.1210/mend-5-5-670. [DOI] [PubMed] [Google Scholar]
- 34.Gnessi L, Emidi A, Jannini EA, Carosa E, Maroder M, Arizzi M, et al. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J Cell Biol. 1995;131:1105–21. doi: 10.1083/jcb.131.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basciani S, De Luca G, Dolci S, Brama M, Arizzi M, Mariani S, et al. Platelet-derived growth factor receptor beta-subtype regulates proliferation and migration of gonocytes. Endocrinology. 2008;149:6226–35. doi: 10.1210/en.2008-0349. [DOI] [PubMed] [Google Scholar]
- 36.Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–45. [PubMed] [Google Scholar]
- 37.Nishimura N, Furukawa Y, Sutheesophon K, Nakamura M, Kishi K, Okuda K, et al. Suppression of ARG kinase activity by STI571 induces cell cycle arrest through up-regulation of CDK inhibitor p18/INK4c. Oncogene. 2003;22:4074–82. doi: 10.1038/sj.onc.1206498. [DOI] [PubMed] [Google Scholar]
- 38.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–87. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 39.Dewar AL, Zannettino AC, Hughes TP, Lyons AB. Inhibition of c-fms by imatinib: expanding the spectrum of treatment. Cell Cycle. 2005;4:851–3. doi: 10.4161/cc.4.7.1788. [DOI] [PubMed] [Google Scholar]
- 40.Gnessi L, Emidi A, Scarpa S, Palleschi S, Ragano-Caracciolo M, Silvestroni L, et al. Platelet-derived growth factor effects on purified testicular peritubular myoid cells: binding, cytosolic Ca2+ increase, mitogenic activity, and extracellular matrix production enhancement. Endocrinology. 1993;133:1880–90. doi: 10.1210/en.133.4.1880. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138:1289–98. doi: 10.1210/en.138.3.1289. [DOI] [PubMed] [Google Scholar]
- 42.Basciani S, De Luca G, Dolci S, Brama M, Arizzi M, Mariani S, et al. PDGFR-{beta} regulates proliferation and migration of gonocytes. Endocrinology. 2008;149:6226–35. doi: 10.1210/en.2008-0349. [DOI] [PubMed] [Google Scholar]
- 43.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–99. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 44.Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, et al. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol. 1993;157:85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- 45.Yan W, Kero J, Huhtaniemi I, Toppari J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol. 2000;227:169–82. doi: 10.1006/dbio.2000.9885. [DOI] [PubMed] [Google Scholar]
- 46.Rossi P, Albanesi C, Grimaldi P, Geremia R. Expression of the mRNA for the ligand of c-kit in mouse Sertoli cells. Biochem Biophys Res Commun. 1991;176:910–4. doi: 10.1016/S0006-291X(05)80272-4. [DOI] [PubMed] [Google Scholar]