ABSTRACT
CDH1 is a cell-cell adhesion molecule expressed in the epithelium to coordinate key morphogenetic processes, establish cell polarity, and regulate epithelial differentiation and proliferation. To determine the role of CDH1 in the mouse uterus, Cdh1 was conditionally ablated by crossing Pgr-Cre and Cdh1-flox mice, and the phenotype was characterized. We found that loss of Cdh1 results in a disorganized cellular structure of the epithelium and ablation of endometrial glands in the neonatal uterus. Cdh1d/d mice lost adherens junctions (CTNNB1 and CTNNA1) and tight junctions (claudin, occludin, and ZO-1 proteins) in the neonatal uterus, leading to loss of epithelial cell-cell interaction. Ablation of Cdh1 induced abnormal epithelial proliferation and massive apoptosis, and disrupted Wnt and Hox gene expression in the neonatal uterus. Although the uteri of Cdh1d/d mice did not show any myometrial defects, ablation of Cdh1 inhibited expression of epithelial (cytokeratin 8) and stromal (CD10) markers. Cdh1d/d mice were infertile because of defects during implantation and decidualization. Furthermore, we showed in the model of conditional ablation of both Cdh1 and Trp53 in the uterus that interrupting cell cycle regulation through the loss of Cdh1 leads to abnormal uterine development. The uteri of Cdh1d/d Trp53d/d mice exhibited histological features of endometrial carcinomas with myometrial invasion. Collectively, these findings suggest that CDH1 has an important role in structural and functional development of the uterus as well as adult uterine function. CDH1 has a capacity to control cell fate by altering directional cell proliferation and apoptosis.
Keywords: Cdh1, endometrial differentiation, gland development, implantation, uterus
CDH1 regulates epithelial cell fate during postnatal uterine development and affects adult uterine function.
INTRODUCTION
Uterine endometrium consists of a simple luminal epithelium (LE) supported by stroma cells that contain coiled endometrial glands [1–3]. Although histogenesis of the uterus is initiated in the fetus, uterine development is not completed until after birth in humans, laboratory rodents, and domestic livestock [2, 4–6]. A major developmental event in the neonatal uterus is adenogenesis, which is defined as the differentiation and development of glands in the endometrium [5, 6]. Histologically, the mouse uterus lacks endometrial glands and consists of a simple LE and relatively undifferentiated mesenchyme at birth [1]. Between birth (Postnatal Day 0 or P0) and P3, the three layers of mesenchyme are distinctly segregated into endometrial stroma and the inner circular and prospective outer longitudinal layers of myometrium. By P5–6, epithelial invaginations appear in the LE that represent the formation of glandular epithelium buds [7]. By P10–12, endometrial glands extend from the LE into the surrounding endometrial stroma, and the outer longitudinal layer of the myometrium is fully organized into bundles of smooth muscle cells [1]. The basic adult histoarchitectural configuration of the mouse uterus is established by P15–20 [8, 9]. Uterine morphogenesis is critical because disruption of endometrial adenogenesis and mesenchymal specification and differentiation can cause permanent fertility problems in the adult [5, 10–13].
Complex Wnt and Hox gene networks between epithelium and stroma regulate developmental processes of the uterus [14–17]. Knockout mice studies have shown that mice lacking Wnt7a, Wnt5a, or Foxa2, a member of the forkhead transcription factor family, lack uterine glands and have disrupted uterine adenogenesis [10, 17–19]. Thus, epithelial differentiation and growth are key processes in uterine morphogenesis and are central to the formation of uterine development.
In epithelial tissues, interactions between neighboring cells are mediated by adherens junctions. Adherens junctions play a role in the establishment of cell polarity and maintenance of the epithelial phenotype, and are essential for epithelial development, including cell differentiation and proliferation [20]. CDH1 (E-cadherin) belongs to the cadherin superfamily of cell adhesion molecules [21] and is a single-span transmembrane glycoprotein consisting of five extracellular cadherin-binding domains and a conserved intracellular domain that associates with members of the catenin family. CTNNB1 (β-catenin) is one of the critical intracellular mediators necessary for the maintenance and function of CDH1 in cell-cell interactions. CTNNB1 binds to the cytoplasmic domain of CDH1 [22, 23], and CTNNA1 (α-catenin) bridges the cadherin-catenin adhesion complex with the actin filament network [24]. The intracellular mechanism of cadherin/catenin adhesive activity is critical to regulate architectural development of epithelial differentiation.
In the present study, we investigated the role of CDH1 in uterine function. We generated a mouse model in which Cdh1 was conditionally ablated in the developing uterus because global mutation of Cdh1 causes early embryonic lethality [25, 26]. The ablation of Cdh1 resulted in defects in epithelial gland formation and adult uterine function as a result of alternation of uterine cell fate, disruption of epithelial-stromal interaction, and induction of abnormal epithelial differentiation.
MATERIALS AND METHODS
Animals and Tissue Collection
Mice were maintained in the vivarium at Southern Illinois University according to the institutional guidelines for the care and use of laboratory animals. B6.129-Pgrtm2(cre)Lyd (also known as Pgrcre/+) mice were provided by Drs. Franco DeMayo and John Lydon [27]. B6.129-Cdh1tmKem2/J (also known as Cdh1flox, Jax no. 005319) and FVB.129-Trp53tm1Brn (also known as Tp53flox, no. 01XC2) were obtained from The Jackson Laboratory and the Mouse Models of Human Cancers Consortium, National Institutes of Health, respectively. To determine the effects of loss of Cdh1 or Trp53 on neonatal uterine morphogenesis and gene expression, female pups were necropsied on P5, P10, P15, P20, P35, or P60 (n = 5/day). Two hours before they were euthanized, the pups received subcutaneous injections of 100 mg/kg of bromodeoxyuridine (BrdU) (Sigma) to assess cell proliferation.
Pregnancy samples were obtained by the mating of wild-type C57BL/6 mice, and the day that a vaginal plug was observed was considered Day 0.5 of pregnancy. Artificial decidualization was hormonally induced following previously described methods [28]. The uterine decidual response was observed 5 days after stimulation. At collection, uterine tissues were fixed in fresh 4% paraformaldehyde in PBS at room temperature for 8–12 h and embedded in paraffin, or snap-frozen in liquid nitrogen and stored at −80°C.
Immunohistochemistry
Immunolocalization of CDH1, CK8 (cytokeratin 8; official symbol KRT8), CTNNB1, BrdU, claudin, occludin, ZO-1 (official symbol TJP1), CTNNA1, CD10 (official symbol MME), and αSMA (smooth muscle alpha actin; official symbol ACTA2) was performed in cross-sections (5 μm) of paraffin-embedded uterine sections using specific antibodies and a Vectastain Elite ABC kit (Vector Laboratories). The antibodies used in the analyses were anti-CDH1 (2 μg/ml [final concentration], 610181; BD Biosciences), anti-CK8 (1:2000 dilution, MMS-162P; Covance), anti-CTNNB1 (1.5 μg/ml [final concentration], 610513; BD Biosciences), anti-BrdU (1:200 dilution, 11 170 376 001; Roche), anti-claudin (1.25 μg/ml [final concentration], 51-9000; Invitrogen), anti-occludin (2.5 μg/ml [final concentration], 71-1500; Invitrogen), anti-ZO-1 (5 μg/ml [final concentration], ab2272; Abcam), anti-CTNNA1 (1:100 dilution, ab51032; Abcam), anti-CD10 (1:50 dilution, ms 728S0; Thermo Fisher Scientific), and anti-αSMA (1:500 dilution, ab5694; Abcam); negative controls were performed by substituting the same concentration of normal immunoglobulin G (Sigma) for the primary antibody. Antigen retrieval using a boiling citrate buffer was performed as described previously [29].
Quantitative Real-Time RT-PCR
Total RNA was isolated from mouse uterus using the Trizol reagent (Invitrogen) according to the manufacturer's recommendations. The quantity and quality of total RNA was determined by spectrometry and denaturing agarose gel electrophoresis, respectively. The cDNA was synthesized from total RNA (2 μg) using iScript Select cDNA synthesis kit (BioRad). Real-time PCR analysis of mRNA expression was performed using a MyiQ Single-Color real-time PCR detection system (BioRad) with iQ SYBR Green supermix (BioRad) as the detector according to the manufacturer's recommendations. The primers shown in Supplemental Table S1 (all the supplemental data are available online at www.biolreprod.org) were designed to amplify cDNAs of around 100 bp, and all exhibited similar amplification efficiency (95% ± 3%) as assessed by amplification of cDNA dilution series. PCR cycle parameters were 95°C for 15 sec and 60°C for 1 min for 40 cycles. The threshold line was set in the linear region of the plots above the baseline noise, and threshold cycle (CT) values were determined as the cycle number at which the threshold line crossed the amplification curve. PCR without template or template substituted with total RNA were used as negative controls to verify the experimental results. After amplification, the specificity of the PCR was determined by both melt-curve analysis and gel electrophoresis to verify that only a single product of the correct size was present. Data were normalized against Gapdh and are shown as the average fold increase ± the SEM. The fold changes are equivalent to 2(x – y) where x is the CT value of the control and y is the CT value of ablation of Cdh1.
Statistical Analysis
All the experimental data were subjected to one-way ANOVA, and differences between individual means were tested by a Tukey multiple-range test or a Student t-test using Prism 4.0 (GraphPad). Real-time PCR data were corrected for differences in sample loading using the Gapdh data as a covariate. Tests of significance were performed using the appropriate error terms according to the expectation of the mean squares for error. A P value of 0.05 or less was considered significant. Data are presented as least-square means with the SEM.
RESULTS
Impact of Conditional Ablation of Cdh1 in Neonatal Uterus
Cdh1-null mice show early embryonic lethality [25, 26]. In order to examine the role of CDH1 in the neonatal uterus, conditional ablation of Cdh1 was conducted to circumvent the embryonic lethal phenotype. We utilized the PgrCre/+ mouse line in which Cre recombinase is under the control of the Pgr promoter [27]. PgrCre/+ mice were crossed with Cdh1f/f mice [30] to provide a tissue-specific knockout of Cdh1 (PgrCre/+ Cdh1f/f = Cdh1d/d) in Pgr-expressing cells. Our previous study confirmed that the PgrCre/+ mouse line is an excellent model to ablate epithelial genes in the neonatal uterus [8, 18].
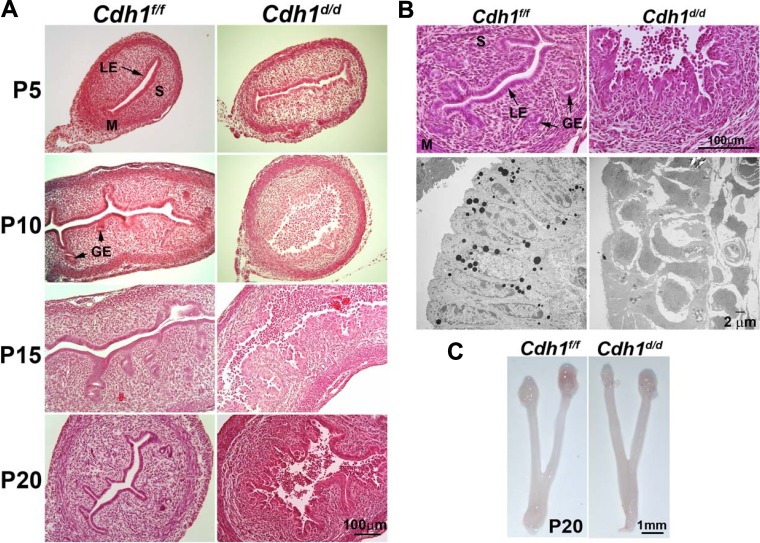
Histological analysis of neonatal uteri was conducted in Cdh1f/f and Cdh1d/d mice on P5, P10, P15, and P20 (Fig. 1, A and B). Uterine histology revealed that ablation of Cdh1 in the neonatal uterus resulted in a disorganized cellular structure of the epithelium and disrupted endometrial adenogenesis. Although there were no developing endometrial glands in control uteri on P5, LE in the uteri of Cdh1d/d mice already exhibited disorganization. On P10, we did not see a formed, single epithelial cell layer, and the epithelial cells present were extremely abnormal and rounded in shape. The majority of cells had lost cell-cell connection, were detached from the uterus, and gathered in the uterine lumen. On P15 and P20, epithelial cells were distinguishable; however, each cell was disorganized lacking any normal epithelial cellular structure. High-resolution examination of the uteri on P20 using transmission electron microscopy revealed that the uterine epithelium of Cdh1d/d mice had neither columnar epithelium nor cell adhesion between neighbor cells (Fig. 1B). However, we did not see any difference in total uterine size between Cdh1f/f and Cdh1d/d mice on P20 (Fig. 1C).
FIG. 1.
Ablation of Cdh1 disrupts mouse uterine gland development. A) Uterine histology of Cdh1f/f and Cdh1d/d mice. Tissues were stained using hematoxylin and eosin. GE, glandular epithelium; LE, luminal epithelium; M, myometrium; P, postnatal; S, stroma. B) Uterine histology and transmission electron microscopy of Cdh1f/f and Cdh1d/d mice on P20. C) Gross anatomy of ovary and uterus of Cdh1f/f and Cdh1d/d mice on P20.
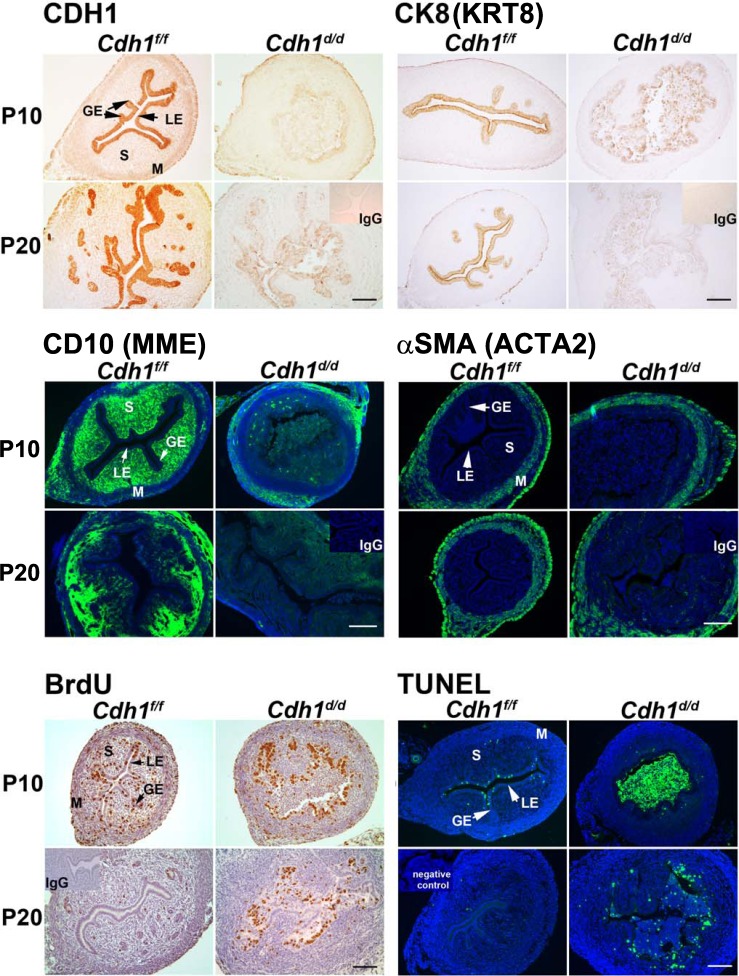
To validate CDH1 ablation, CDH1 immunoreactivity was determined in the neonatal uterus (Fig. 2). Immunohistochemical analysis confirmed that epithelial CDH1 was ablated by Pgr-driven Cre activity on P10 and P20. CK8, an epithelial cell-specific marker, was decreased but still detectable in the uteri of Cdh1d/d mice on P10; however, it was completely lost on P20, suggesting that disorganized and abnormal cells were losing epithelial characteristics during endometrial adenogenesis. CD10 is a known marker of normal endometrial stromal cells [31]. Because epithelial-stromal interaction is a key component of uterine development, immunoreactivity toward CD10 was also examined. While we observed stromal-specific CD10 in Cdh1f/f mice, CD10 was completely absent in the uteri of Cdh1d/d mice, suggesting that ablation of Cdh1 causes abnormal stromal differentiation. In contrast, there was no difference in the localization of αSMA, which is a marker of smooth muscle, in the uteri of Cdh1f/f and Cdh1d/d mice, indicating that the loss of Cdh1 in neonatal uterus does not have any gross effect on the myometrium (Fig. 2).
FIG. 2.
Analysis of conditional ablation of Cdh1 on markers of epithelium, stroma, myometrium, cell proliferation, and apoptosis in the neonatal mouse uterus. CDH1, cytokeratin 8 (CK8; official symbol KRT8), CD10 (official symbol MME), smooth muscle alpha actin (αSMA; official symbol ACTA2), and incorporated BrdU were detected by immunohistochemistry. Cellular apoptosis was determined by TUNEL analysis. GE, glandular epithelium; LE, luminal epithelium; M, myometrium; P, postnatal; S, stroma. Bars = 100 μm.
Previous [8] and current results (Fig. 2) show that proliferative cells are present in the endometrial and myometrial layers of the neonatal uterus. Specifically, active cell proliferation is obvious in the developing glands on P10 but ceases by P20 when uterine morphogenesis is structurally completed. In Cdh1d/d mice, we observed that the majority of abnormal epithelial cells were BrdU positive on P10 and P20. Further, TUNEL analysis revealed that detached cells observed in the uterine lumen of Cdh1d/d mice were apoptotic on P10, whereas fewer cells were TUNEL positive in the LE of Cdh1f/f mice. On P20, apoptotic cells were still observed in disorganized epithelium of the uteri of Cdh1d/d mice but not in the uteri of Cdh1f/f mice. Thus, ablation of Cdh1 affected the critical period of endometrial adenogenesis by changing epithelial cell fate.
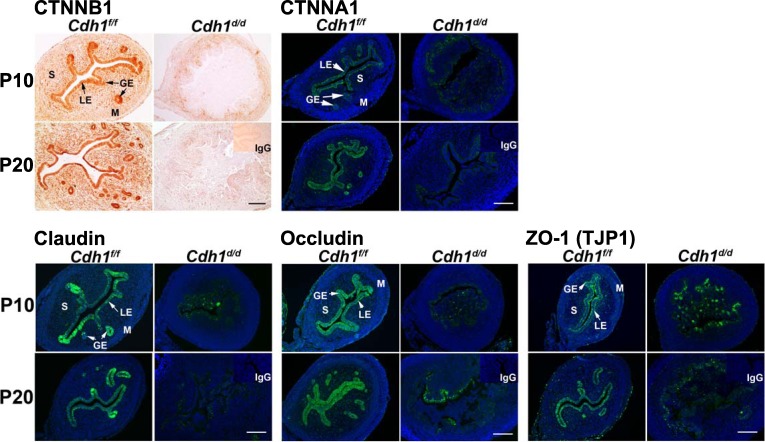
Because epithelial cells exhibit loss of cell-cell connection in the uteri of Cdh1d/d mice, we further surveyed epithelial cell adhesion markers (Fig. 3). Upon establishing cell-cell adhesion, cadherins cluster in specialized cell adherens junctions that associate with the actin cytoskeleton [32]. CDH1 creates a transcellular network that enables groups of cells or tissues to coordinate their function [32, 33]. Therefore, we examined the localization of adherens junction molecules (CTNNB1 and CTNNA1) associated with CDH1 in the cytoplasm. The critical intracellular mediator CTNNB1 was detected in most of the uterine cell types in uteri of Cdh1f/f mice but was absent in the epithelium and decreased in the stroma following ablation of Cdh1 in the uterus. CTNNA1 was detected in endometrial epithelia of Cdh1f/f mice and was inhibited in the epithelium by ablation of Cdh1. Next, we studied the tight junction molecules claudin, occludin, and ZO-1 (Fig. 3), which are associated with the adherens junctions and are epithelial-specific molecules that support epithelial structure. Ablation of Cdh1 inhibited claudin, occludin, and ZO-1 in the epithelium. The results indicate that abnormal epithelial structure was induced by the loss of adherens junction and tight junction molecules in the uteri of Cdh1d/d mice.
FIG. 3.
Analysis of conditional ablation of Cdh1 on adherens junction and tight junction molecules in the neonatal mouse uterus. Immunoreactivity toward CTNNB1, CTNNA1, claudin, occludin, and ZO-1 (TJP1) was detected. GE, glandular epithelium; LE, luminal epithelium; M, myometrium; P, postnatal; S, stroma. Bars = 100 μm.
At 5-wk old, examination of the uteri of Cdh1f/f and Cdh1d/d mice clearly showed loss of uterine glands upon ablation of Cdh1 (Supplemental Fig. S1). We also observed rounded and noncolumnar epithelium in Cdh1d/d mice. The number of uterine glands per uterine section was significantly reduced (P < 0.001) in the uteri of Cdh1d/d mice (∼6% of controls). This result was supported by decreased levels of Foxa2, which is a glandular marker in the uterus. Both the ovary and oviduct of 5-wk-old Cdh1d/d mice appeared normal (Supplemental Fig. S2). Furthermore, we also observed normal vaginal opening and estrous cycle of adult Cdh1d/d mice (data not shown), suggesting that the ovarian function in Cdh1d/d mice is normal.
Gene Expression in the Uteri of Cdh1d/d Mice
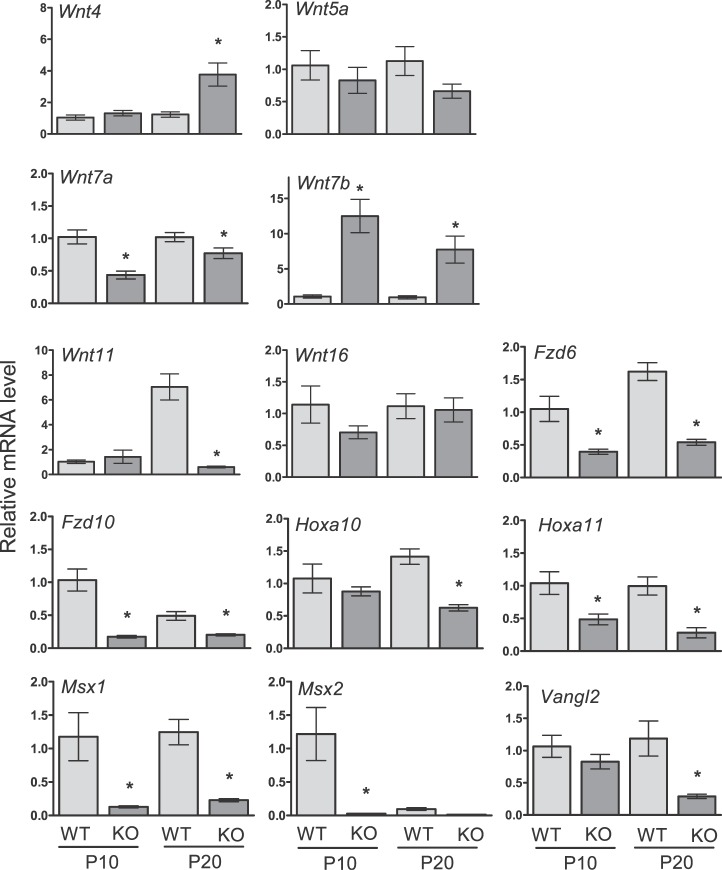
To examine the effect of Cdh1 ablation on Wnt and Hox genes, known to be critical for uterine development [8], real-time RT-PCR analysis was conducted (Fig. 4). Ablation of Cdh1 decreased epithelial Wnt genes (Wnt7a and Wnt11), Wnt receptors (Fzd6 and Fzd10), and epithelial and stromal homeobox genes (Hoxa10, Hoxa11, Msx1, and Msx2) on P10 and/or P20 (P < 0.05). The expression of stromal genes, Wnt5a and Wnt16, was not altered by the loss of Cdh1 in the neonatal uterus. Interestingly, stromal Wnt4 and epithelial Wnt7b were elevated in the uteri of Cdh1d/d mice (P < 0.05). Vangl2, which is required for planar cell polarity, was reduced in the uteri of Cdh1d/d mice (P < 0.05).
FIG. 4.
Analysis of conditional ablation of Cdh1 in the neonatal mouse uterus. The level of gene expression was measured in the uteri of Cdh1f/f (wild type, WT) and Cdh1d/d (knockout, KO) mice on Postnatal Day (P) 10 and P20 by real-time RT-PCR. The results represent the mean ± SEM (WT vs. KO, *P < 0.05).
Fertility and Implantation Defect in Cdh1d/d Mice
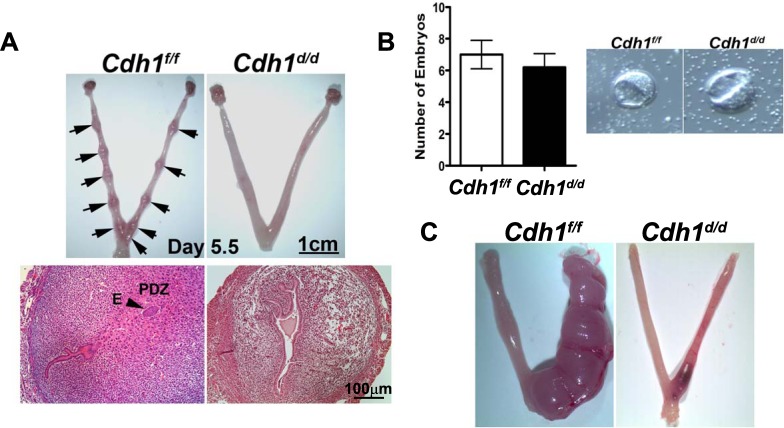
To determine the role of Cdh1 in adult uterine function, Cdh1f/f and Cdh1d/d mice were mated to wild-type male mice for 6 mo. Cdh1f/f mice exhibited normal fecundity over the period, whereas Cdh1d/d mice were found to be infertile (Table 1). To determine the cause of the infertility, we examined whether blastocysts were able to undergo successful implantation. On the morning of Day 5.5 of pregnancy, implantation sites were observed in the uteri of Cdh1f/f but not in the uteri of Cdh1d/d mice (Fig. 5A). Histological examination revealed no blastocysts attached to the uterine lumen and no decidual response in the uteri of Cdh1d/d mice. We confirmed that no difference was found in the number and morphology of embryos recovered from the uterus of Cdh1f/f and Cdh1d/d mice when the uterus was flushed on Day 3.5 (Fig. 5B), suggesting that matured embryos are not able to attach to or invade the uterine lumen of Cdh1d/d mice.
TABLE 1.
Fertility defect of Cdh1d/d mice.
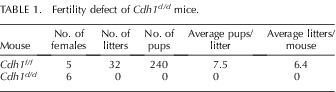
FIG. 5.
Implantation and decidualization defect in Cdh1d/d mice. A) Gross anatomy shows no implantation sites in the uteri of Cdh1d/d mice on Day 5.5. Implanted blastocysts (arrows) were observed in the uteri of Cdh1f/f mice on Day 5.5 but not in Cdh1d/d mice. E, embryo; PZD, primary decidual zone. B) Embryos recovered from the uteri of Cdh1f/f and Cdh1d/d mice on Day 3.5. C) Gross anatomy showed a significant decrease in size of the decidual horn in Cdh1d/d mice.
Defect of Decidualization in Cdh1d/d Mice
To determine whether ablation of Cdh1 affects the ability of stromal cells to undergo decidualization, the reaction to hormonally induced artificial decidualization was observed in the uteri of Cdh1f/f and Cdh1d/d mice (Fig. 5C). As we expected, the right uterine horn of Cdh1f/f mice exhibited a robust decidual response 5 days after artificial stimulation. In contrast, Cdh1d/d mice displayed significant reduction in decidual response. These results indicate that the uteri of Cdh1d/d mice do not retain any functions required for the onset of implantation and stromal differentiation to undergo decidualization.
Ablation of Cdh1 Leads to Abnormal Uterine Development with Loss of Trp53
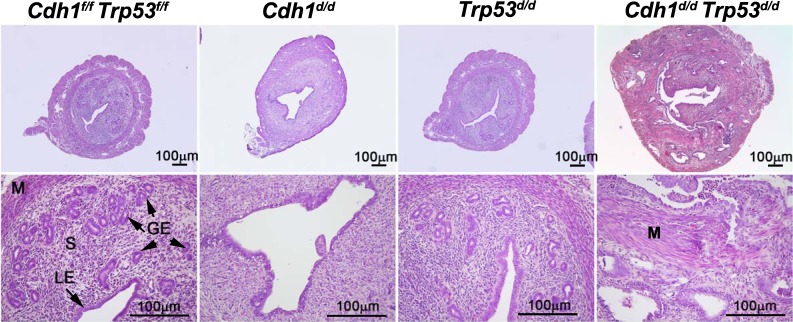
Because our results revealed that Cdh1 ablation alters epithelial cell fate following abnormal epithelial proliferation and apoptosis, we expected that these cells undergo abnormal cell cycle. Therefore, we interrupted the uterine cell cycle by ablating Trp53, which regulates cell cycle arrest, DNA repair, and programmed cell death [34], in addition to ablation of Cdh1. Conditional ablation of Trp53 alone in the uterus using PgrCre/+ mice (PgrCre/+ Trp53f/f = Trp53d/d) does not show any significant differences of uterine histology and function [35]. In this study, we deleted both Cdh1 and Trp53 in the uterus (PgrCre/+ Cdh1f/f Trp53f/f = Cdh1d/d Trp53d/d) (Fig. 6). At 8-wk old, ablation of Cdh1 still exhibited no endometrial glands, but ablation of Trp53 did not show any differences in the uterus, as expected. The size of the uteri in Cdh1f/f Trp53f/f, Cdh1d/d, and Trp53d/d mice were similar. In contrast, the uteri of Cdh1d/d Trp53d/d mice showed abnormal uterine development, including disorganized glandularlike adenosis into stroma and myometrium similar to histological features of neoplastic transformation, which is mixed endometrioid and serous adenocarcinomas with myometrial invasion. The size of the uteri appeared larger in the uteri of Cdh1d/d Trp53d/d mice.
FIG. 6.
Uterine histology of Cdh1f/f Trp53f/f, Cdh1d/d, Trp53d/d, and Cdh1d/d Trp53d/d mice at 8-wk old. Tissues were stained using hematoxylin and eosin. Ablation of Cdh1 and Trp53 displayed histological features of endometrial carcinomas with myometrial invasion. GE, glandular epithelium; LE, luminal epithelium; M, myometrium; S, stroma.
DISCUSSION
CDH1 is critical to regulate architectural development of epithelial differentiation because CDH1 forms cell-cell adhesion structures known as adherens junctions that mediate intercellular adhesion through dynamic interactions with the actin cytoskeleton [36]. In fact, embryos with global mutation of Cdh1 exhibit severe abnormalities at the transition from compacted morula to blastocyst because of the incorrect establishment of adhesion junctions in the trophectoderm and die around the peri-implantation period [25, 26]. Mice lacking Cdh1 fail to produce milk proteins as a result of undifferentiated alveolar epithelium [30]. CDH1 also regulates ductal lumen formation during branching morphogenesis of the salivary gland [37]. In the present study, we demonstrate that CDH1 is one of the critical regulators of uterine formation and development. First, we found that loss of Cdh1 in the neonatal uterus ablates endometrial glands and causes infertility due to defects of implantation and decidualization. Second, we also found that CDH1 has a capacity to control cell fate by altering directional cell proliferation and apoptosis. Third, we showed in the model of conditional ablation of both Cdh1 and Trp53 in the uterus that interrupting cell cycle regulation under the loss of Cdh1 leads to abnormal uterine development. Taken together, these findings suggest that CDH1 has an important role in structural and functional development of the uterus as well as adult uterine function.
Ablation of Cdh1 disrupted epithelial cellular structure in the developing uterus. The result from immunohistochemical analysis confirmed that uterine epithelium in Cdh1d/d mice does not have cell adhesion because of the loss of adherens junction and tight junction molecules. The epithelial marker CK8 disappeared in the epithelium of Cdh1d/d mice, resulting in loss of epithelial character in these cells in the developing uterus. Loss of Cdh1 altered expression of a number of epithelial Wnt genes as well as an epithelial cell polarity regulator, Vangl2. Thus, our results indicate that a novel mechanism promoting uterine adenogenesis was perturbed by ablation of Cdh1 in the neonatal uterus, suggesting that the uteri of Cdh1d/d mice are structurally and functionally abnormal. It is well known that epithelial-stromal interaction is critical for uterine morphogenesis [6, 8, 16, 17]. Stromal Wnt and Hox genes, as well as growth factors and receptors, regulate endometrial adenogenesis [6, 8, 16, 17, 38]. While the stromal layer looks mostly intact, ablation of Cdh1 inhibited the stromal marker CD10 in the uteri of Cdh1d/d mice, suggesting that stromal function is potentially abnormal. Currently, we are performing microarray analyses to identify additional genes regulated by loss of Cdh1 in the stroma.
Cdh1d/d mice lost adult uterine function and were infertile with an inability of their uteri to support embryo attachment and/or invasion and decidualization. The uteri of adult Cdh1d/d mice do not contain any endometrial glands. Endometrial glands and their secretions are critical regulators of peri-implantation embryo survival, implantation, and establishment of uterine receptivity and decidualization [12, 39–41]. Ablation of Wnt7a and Foxa2 results in defects of both implantation and decidualization due to absence of endometrial glands [10, 18, 42]. Lif mutant mice also fail at blastocyst implantation and induction of decidualization because LIF is an implantation critical factor secreted from endometrial glands [43]. Therefore, the lack of uterine glands in Cdh1d/d mice leads to the key defect that underlies their infertility and defect of implantation. In addition, we have confirmed that infertility was caused by the uterine defect because no histological and functional defects of the ovary, oviduct, or pituitary were observed in Cdh1d/d mice.
The process of implantation involves a series of cell-cell communications between the two epithelial tissues, trophectoderm derived from the embryo and maternal LE. Prior to implantation, they are two separate entities and are polarized with a continuous seal of junctional complexes and cell adhesion molecules. With the onset of embryo attachment, the trophectoderm and maternal LE make first contact at their apical borders, at which point the trophoblast cells become invasive [44, 45]. The trophoblast cells permeate the LE to anchor the implanting embryo to the decidualizing stroma, a process achieved by a series of membrane-mediated events occurring between embryonic and maternal cells. CDH1 is abundantly expressed in the LE prior to implantation [46, 47], while CDH1 is lost from the LE before invasion of the blastocyst into the stroma [48]. This downregulation is controlled by calcitonin, which is a critical regulator of implantation, and suggests that remodeling the adherens junctions between epithelial cells is a critical event during implantation of the embryo [48]. In the present study, our maternal LE does not express any CDH1 in Cdh1d/d mice. Epithelium of adult uteri in Cdh1d/d mice shows abnormal columnar structure, indicating that adult epithelial cells are not able to remodel the adherens junctions for implantation even though the embryonic trophectoderm is normal. Thus, these results suggest that the embryo is not able to attach to and invade the uterine lumen as a result of not only the absence of endometrial glands but abnormally differentiated epithelium in Cdh1d/d mice as well.
Abnormal epithelial differentiation occurred in the neonatal uterus of Cdh1d/d mice. We observed the majority of proliferative cells in the epithelium of Cdh1d/d mice. In the developing uterus, cell fate is regulated by the interaction of epithelial and stromal factors (i.e., Wnt and Hox genes). Proliferating cells are primarily localized to the tip of the endometrial glands and stroma on P10 and are absent on P20 when the uterus is structurally completed. Apoptotic cells are also observed in the epithelium on P10 as a result of active cell cycling in the developing uterus. Thus, the present results indicate that the ablation of Cdh1 alters uterine cell fate, especially epithelial cells, leading to unregulated or uncontrolled cell types. Abnormal Wnt and Hox gene expression is probably one of the reasons. Interestingly, the ablation or inhibition of Cdh1 in the mammary gland [30] and salivary gland [37] induces massive apoptosis in the epithelium. We assume that abnormal cells are not able to keep cell-cell interaction due to loss of cell adhesion, supporting the result of massive apoptotic cells in the uterine lumen.
In support of the role of CDH1 in cell differentiation, we found a difference in ablation of Cdh1 compared to loss of Wnt7a in the developing uterus. Conditional ablation of Wnt7a in the neonatal uterus disrupts endometrial gland development but does not affect cell proliferation in any uterine cell types [18]. Thus, Wnt7a is a critical factor for gland development, but loss of Wnt7a does not lead to further abnormal cell differentiation. To further examine alternation of epithelial cell fate by Cdh1 ablation, we interrupted the uterine cell cycle by ablating Trp53. TRP53 mutation and loss of CDH1 occur in 90% of type II endometrial carcinomas [49, 50]. In the present study, we found that loss of the cell cycle regulator Trp53 in addition to the ablation of Cdh1 induces unexpected abnormal uterine development. Conditional ablation of Trp53 does not show any defect in the uterus [35], and loss of Cdh1 causes loss of endometrial glands. However, ablation of both Trp53 and Cdh1 induces histological features of endometrial carcinomas with myometrial invasion. Thus, these results suggest that cell fate in the uteri of Cdh1d/d mice has been altered, and these cells are able to differentiate abnormally because of an interruption of cell cycle regulation, leading to neoplastic transformation. However, the abnormal uterine phenotype due to loss of Trp53 and Cdh1 in these mice needs to be further characterized.
Collectively, the results of the present study indicate that CDH1 regulates endometrial differentiation, gland development, and adult uterine function. Although ablation of epithelial Cdh1 causes abnormal stromal differentiation, we are unsure of the mechanism of this disruption. Further investigation of epithelial-mesenchymal interaction and/or transition between epithelium and stroma, and characterization of loss of Trp53 and Cdh1 in the uterus are warranted. Nevertheless, our study shows the functional and structural importance of CDH1 in the uterus.
Supplementary Material
Footnotes
Supported by NIH/NICHD HD0588222, ACS-IL 139038, and SIU-SOM CRC (to K.H.).
REFERENCES
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am J Anat 1989; 186: 21 42 [DOI] [PubMed] [Google Scholar]
- Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol 1976; 47: 137 194 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ 1985; 17: 137 148 [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE. Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl 1999; 54: 287 302 [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311 1323 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005; 68: 85 122 [DOI] [PubMed] [Google Scholar]
- Branham WS, Sheehan DM, Zehr DR, Ridlon E, Nelson CJ. The postnatal ontogeny of rat uterine glands and age-related effects of 17 beta-estradiol. Endocrinology 1985; 117: 2229 2237 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Yoshioka S, Reardon SN, Rucker EB, III, Spencer TE, Demayo FJ, Lydon JP, Maclean JA., II WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 2011; 84: 308 319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biol Reprod 2004; 70: 1870 1876 [DOI] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, Demayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod 2010; 83: 396 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Rubel CA, Creighton CJ, White LD, Broaddus RR, Lewis MT, Lydon JP, Jeong JW, DeMayo FJ. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod 2010; 82: 991 999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124: 289 300 [PubMed] [Google Scholar]
- Cooke PS, Ekman GC, Kaur J, Davila J, Bagchi IC, Clark SG, Dziuk PJ, Hayashi K, Bartol FF. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod 2012; 86 (3):63, 1 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Benson GV, Lim H, Dey SK, Maas RL, Abdominal B. (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES). Dev Biol 1998; 197: 141 154 [DOI] [PubMed] [Google Scholar]
- Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat Genet 1998; 20: 228 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol 2001; 238: 224 238 [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 2004; 131: 2061 2072 [DOI] [PubMed] [Google Scholar]
- Dunlap KA, Filant J, Hayashi K, Rucker EB, III, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, Spencer TE. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod 2011; 85: 386 396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Pavlova A, Sassoon DA. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 1998; 76: 91 99 [DOI] [PubMed] [Google Scholar]
- Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 1999; 21: 211 220 [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 1995; 7: 619 627 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul 1989; 1: 37 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989; 8: 1711 1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 1994; 107 (Pt 12): 3655 3663 [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A 1994; 91: 8263 8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci U S A 1995; 92: 855 859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58 66 [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod 1972; 7: 82 86 [DOI] [PubMed] [Google Scholar]
- Taylor KM, Gray CA, Joyce MM, Stewart MD, Bazer FW, Spencer TE. Neonatal ovine uterine development involves alterations in expression of receptors for estrogen, progesterone, and prolactin. Biol Reprod 2000; 63: 1192 1204 [DOI] [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 2002; 115: 53 62 [DOI] [PubMed] [Google Scholar]
- McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology 2001; 39: 273 278 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol 2000; 148: 399 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sato K, Kaibuchi K. Cadherin-mediated intercellular adhesion and signaling cascades involving small GTPases. Cold Spring Harb Perspect Biol 2009; 1: a003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike BT, Wahl GM. p53, stem cells, and reprogramming: tumor suppression beyond guarding the genome. Genes Cancer 2011; 2: 404 419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res 2008; 68: 5619 5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol 1987; 105: 2501 2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Menko AS, Khalil S, Rebustini I, Hoffman MP, Kreidberg JA, Kukuruzinska MA. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Dev Dyn 2008; 237: 3128 3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Carpenter KD, Spencer TE. Neonatal estrogen exposure disrupts uterine development in the postnatal sheep. Endocrinology 2004; 145: 3247 3257 [DOI] [PubMed] [Google Scholar]
- Bagchi IC, Cheon YP, Li Q, Bagchi MK. Progesterone receptor-regulated gene networks in implantation. Front Biosci 2003; 8: s852 s861 [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002; 87: 2954 2959 [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol 2000; 223: 217 237 [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 1998; 125: 3201 3211 [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992; 359: 76 79 [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science 1994; 266: 1508 1518 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006; 7: 185 199 [DOI] [PubMed] [Google Scholar]
- Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol 1999; 208: 488 501 [DOI] [PubMed] [Google Scholar]
- Jha RK, Titus S, Saxena D, Kumar PG, Laloraya M. Profiling of E-cadherin, beta-catenin and Ca(2+) in embryo-uterine interactions at implantation. FEBS Lett 2006; 580: 5653 5660 [DOI] [PubMed] [Google Scholar]
- Li Q, Wang J, Armant DR, Bagchi MK, Bagchi IC. Calcitonin down-regulates E-cadherin expression in rodent uterine epithelium during implantation. J Biol Chem 2002; 277: 46447 46455 [DOI] [PubMed] [Google Scholar]
- Llobet D, Pallares J, Yeramian A, Santacana M, Eritja N, Velasco A, Dolcet X, Matias-Guiu X. Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. J Clin Pathol 2009; 62: 777 785 [DOI] [PubMed] [Google Scholar]
- Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int 2010; 2010: 162363, 1 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.