ABSTRACT
Repetitive sequences, especially transposon-derived interspersed repetitive elements, account for a large fraction of the genome in most eukaryotes. Despite the repetitive nature, these transposable elements display quantitative and qualitative differences even among species of the same lineage. Although transposable elements contribute greatly as a driving force to the biological diversity during evolution, they can induce embryonic lethality and genetic disorders as a result of insertional mutagenesis and genomic rearrangement. Temporary relaxation of the epigenetic control of retrotransposons during early germline development opens a risky window that can allow retrotransposons to escape from host constraints and to propagate abundantly in the host genome. Because germline mutations caused by retrotransposon activation are heritable and thus can be deleterious to the offspring, an adaptive strategy has evolved in host cells, especially in the germline. In this review, we will attempt to summarize general defense mechanisms deployed by the eukaryotic genome, with an emphasis on pathways utilized by the male germline to confer retrotransposon silencing.
Keywords: epigenetics, genetics, germline, infertility, small noncoding RNAs, spermatogenesis, transposon
A comprehensive review of the currently known and possible mechanisms underlying the control of transposable elements during germline development is presented.
INTRODUCTION
Protein-coding genes make up only a small portion of the genome in most organisms, and the remainder of the genome is largely occupied by repetitive sequences that do not appear to encode mRNAs for protein production and were thus once called junk DNA [1, 2]. These noncoding DNA sequences can be generally categorized into two classes: one is represented by tandem repeats, such as the major satellite repeats, minor satellite repeats, and microsatellite repeats; the other is the interspersed repeats, most of which are derived from the transposable elements (TEs) [3, 4]. Transposable elements, also called selfish elements or parasitic elements, are mobile DNA segments present in the genomes of organisms from prokaryotes to eukaryotes [4, 5]. Transposable elements can move and replicate themselves in the host genome through a process called transposition [5]. To defend the genome integrity, host cells have developed mechanisms that can prevent TEs from propagating and transposing [6]. In general, DNA methylation and heterochromatin formation are the primary mechanisms responsible for TE control at transcriptional levels [7, 8]. Small RNA-mediated degradation is an efficient way of restraining TEs at posttranscriptional levels [9]. Recent discoveries have drawn our attention to the star molecules, PIWI-interacting RNAs (piRNAs; initially named rasiRNAs), which exclusively function in the germline to suppress TEs probably at both transcriptional and posttranscriptional levels [10–12].
Transposable Elements as Main Building Blocks of the Eukaryotic Genome
Eukaryotic genomes with rare exceptions are suffused with tandem repetitive sequences in the centromeric and telomeric regions and interspersed repetitive sequences throughout the genome [13]. Whole genome sequencing analyses reveal that most of these repetitive DNAs result from the activity of TEs [14, 15] and that the proportion of TEs in a genome correlates with the haploid genome size of the organism, for example, the proportion of TEs in the short-tailed opossum is 52% of the genome, 45%∼50% in primates, 39%∼40% in the mouse and rat, 34% in the dog, 10% in the domestic chicken, 7% in the medaka, and 3%∼4% in pufferfish species, including Takifugu rubripes and Tetraodon nigroviridis [13, 16–19], suggesting that TEs are the basic constituents of the genome.
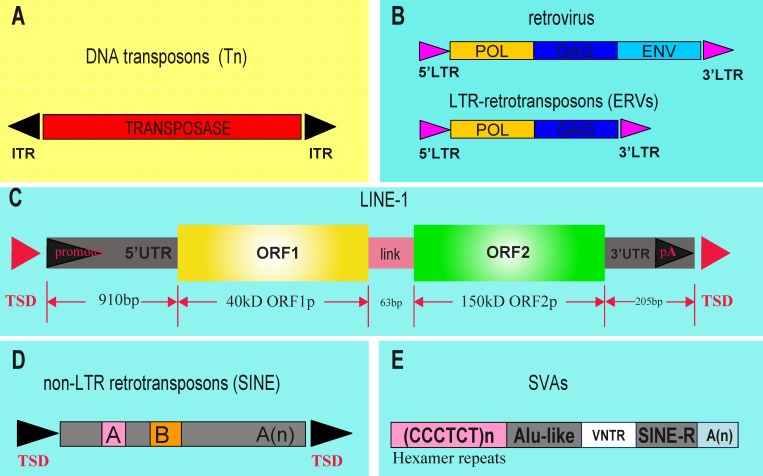
Transposable elements can generally be categorized into two classes according to the mechanisms of transposition: retrotransposons (class I) require an RNA transcript as an intermediate, propagating in a copy-and-paste manner, and DNA transposons (class II) are excised directly, propagating in a cut-and-paste fashion (Fig. 1, A and B) [2, 6]. Most of mammalian genomes are occupied by the retrotransposons that can be further subcategorized into long terminal repeat (LTR) retrotransposons with LTRs at both ends, and non-LTR retrotransposons, including LINEs (long interspersed nucleotide elements), SINEs (short interspersed nucleotide elements) and SVAs (named after SINE, VNTR [variable number of tandem repeats], and Alu) (Fig. 1, C–E) [2, 6, 20]. LTR retrotransposons, also known as the endogenous retrovirus (ERV), can encode two proteins, POL and GAG, but lack the coding sequence of ENV protein that is critical for the full life cycle of the retrovirus [2, 6, 20]. LINE1, with the typical full-length structure being shown in Figure 1C, is capable of translating two ORFs: ORF1, encoding a RNA-binding protein, and ORF2, encoding two enzymes—reverse transcriptase and endonuclease—that are essential for autonomous movement of LINEs [21–23]. The insidious promoter residing in the 5′-untranslated region (UTR) drives the transcription of the non-LTR LINE by DNA polymerase II, whereas SINEs, of which a typical representative is Alu (Fig. 1D), comprise ∼18% of the human genome and are short DNA sequence that cannot encode any functional reverse transcriptase protein and thus rely on other mobile elements for transposition [24–26]. SVAs (Fig. 1E) contain two typical sequence motifs, namely Alu-like and SINE-R, which are separated by a VNTR sequence [27, 28]. Most TEs are kept in a quiescent state, while a small fraction (<0.05% of TEs in humans), however, still remains active in eukaryotic genomes (Table 1) [29–34]. In humans, currently known active TEs include the autonomous LINE1 (L1) element and the nonautonomous SINE and SVAs [32].
FIG. 1. .
Schematic depiction of different classes of transposable elements (TEs) in the mammalian genome. Transposable elements can be divided overall into DNA transposons (class II; A) and retrotransposons (class I; B–E), according to their sequence features and mode of transposition. Most mammalian genomes are occupied by the retrotransposons, which can be further subcategorized into long terminal repeat (LTR) retrotransposons with LTRs at both ends (B) and non-LTR retrotransposons, including LINEs (long interspersed nucleotide elements) (C), SINEs (short interspersed nucleotide element) (D), and SVAs (E). LTR retrotransposons, also known as endogenous retrovirus (ERV), can encode two proteins, POL and GAG, but lack the coding sequence of ENV protein that is critical for the full life cycle of the retrovirus. LINE1, with the typical full-length structure being shown in C, is capable of translating two ORFs: ORF1, encoding a RNA-binding protein, and ORF2, encoding two enzymes—reverse transcriptase and endonuclease—that are essential for autonomous movement of LINEs. The insidious promoter residing in the 5′-UTR drives the transcription of non-LTR LINE by DNA polymerase II, whereas SINEs, of which a typical representative is Alu (D), comprises ∼18% of the human genome and are short DNA sequence that cannot encode any functional reverse transcriptase protein and thus rely on other mobile elements for transposition. SVAs (E) contain two typical sequence motifs, namely, Alu-like and SINE-R, which are separated by a variable number of tandem repeats (VNTR) locus. To date, active TEs currently known include LINEs, SINEs and SVAs. LINEs can transpose through autonomous mobilization, whereas both SINEs and SVAs depend on LINE1 activity for nonautonomous transposition.
TABLE 1. .
Distribution of mobile DNA elements in the genomes of three organisms.
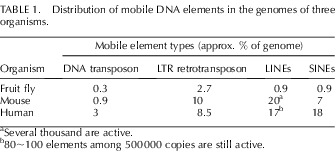
Several thousand are active.
80∼100 elements among 500 000 copies are still active.
Transposable elements have been regarded as parasites of the genome because they benefit at the expense of the host, and thus, TEs are also considered as selfish DNA [5, 35, 36]. Transposable element activity has resulted in broad variations in quantity, diversity, and chromosomal loci that are often found between or sometimes even within species [5, 35, 36]. Despite the selfish and parasitic nature, the movement and accumulation of TEs may have contributed to the creation of new genes and the formation of a complex regulatory network system in the host genome [8]. Transposable elements may also serve as building blocks for the assembly of a variety of regulatory systems to direct and coordinate eukaryotic gene expression or gene silencing under selective pressure during evolution, both at the level of single genes and across larger chromosomal regions [5, 8]. For example, >16% of eutherian-specific conserved noncoding elements are derived from various classes of TEs [37]. As a supply source, TEs can provide regulatory elements to the genome in diverse manners, such as producing a new promoter or a binding site for microRNA (miRNA) or RNA-binding proteins, introducing a new cis-regulatory element or alternative polyadenylation sites, driving antisense transcription, or disrupting the existing regulatory elements [8, 38]. A survey of mammalian genomes has revealed that 25% of human promoter regions consist of sequences that are derived originally from mobile DNA elements for the control of gene expression [36]. Alu, belonging to the SINE family, is the most abundant repetitive element group in the human genome (Table 1). Alu elements are found in >5% of human 3′-UTRs and are probable miRNA targets, suggesting that they have coevolved with their hosts [39, 40].
Although mobile elements have contributed greatly to the evolution of the host genome, they can indeed cause damage, once activated, to the host genome by generating insertional mutations or DNA rearrangement [41, 42]. Above all, if TE-induced genetic disruptions occur in the germline [43], these mutations will be passed onto the next generation and thus the impact becomes transgenerational. Therefore, the activity of mobile elements must be under tight control by the host defense system to maintain the integrity and function of the host genome. Indeed, most of the eukaryotic organisms appear to have evolved elaborate and efficient mechanisms to cope with the presence and activity of mobile DNA elements.
Developmental Reprogramming Opens a Risky Window for TE Escape
Reprogramming refers to the capability of a nucleus to modify epigenetic marks on its chromatin (e.g., DNA methylation and histone modifications) and thus control specific gene expression profiles (e.g., levels of both coding and noncoding RNAs) at a specific developmental stage [44]. There are at least two such events during mammalian development: one occurs during fetal germline development, and the other takes place during preimplantation embryonic development (Fig. 2).
FIG. 2. .
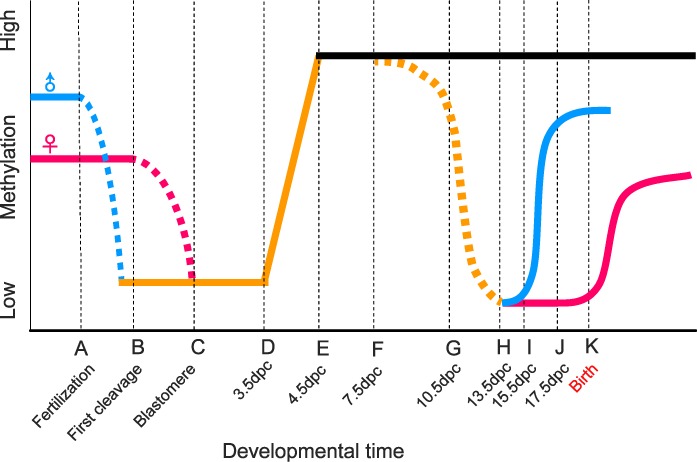
Schematic representation of two genomic reprograming events during murine development. Genomic methylation levels (vertical axis) during preimplantation and fetal germ cell development (horizontal axis) in mice are shown. Blue and red lines represent paternal and maternal genomes, respectively. Dashed lines represent the progression of demethylation. Developmental time points are indicated on the horizontal axis. A) Fertilization: demethylation of the paternal genome initiates immediately after the formation of the pronucleus, while methylation of the maternal genome is unchanged. B) First cleavage: demethylation of the maternal genome commences, whereas the paternal genome demethylation is complete prior to the first cleavage. C) Blastomere stage: there is a notable passive demethylation in the maternal genome concomitant with embryo cleavages between stages B and C. D) At 3.5 dpc: the embryo is at the blastocyst stage. Both paternal and maternal genomes start the remethylation process. E) At ∼4.5 dpc: methylation levels of both paternal and maternal genomes peak in the embryo. F) At 7.5 dpc: PGCs appear in the allantois as a population of ∼40 cells and continue to proliferate and migrate toward the gonad. G) At 10.5–11.5 dpc: PGCs reach the genital ridge, and demethylation occurs in the PGC genome. H) At 13.5 dpc: PGCs cease proliferation, and sex-specific gonad differentiation is being completed. PGCs become prospermatogonia in the testis and oogonia in the ovary. Prospermatogonia continue to proliferate mitotically while oogonia proceed to meiosis. I) At 15.5 dpc: de novo methylation continues in prospermatogonia while oogonia become oocytes that are in prophase I of meiosis. J) At 17.5 dpc: methylation levels of prospermatogonia almost peak at 17.5 dpc. K) Birth: de novo remethylation of maternal genome commences at growing oocyte close to or shortly after birth in females.
In the first case, reprogramming occurs in primordial germ cells (PGCs) during fetal germ cell development. At 7.5 dpc (days postcoitum) in the mouse, PGCs first appear in the epiblast. These cells proceed to proliferate and migrate into the genital ridge between 10.5 and 11.5 dpc, where they continue to multiply to increase the PGC population until 13.5 dpc [45]. Genomewide DNA demethylation commences as early as ∼7.5 dpc and terminates before 13.5 dpc in PGCs of both male and female mice [19, 45]. During this process, most, if not all, DNA methylation marks are removed including those of differentially methylated regions (DMRs) of imprinted genes, TEs, and repetitive satellite DNA repeats [19, 46, 47]. It is unclear to what extent the demethylation takes place in other regions across the genome [48–50]. Once the genome of male or female PGCs has been demethylated, these cells enter mitotic (male) or meiotic (female) arrest. After several days, genomewide DNA remethylation will initiate (Fig. 2). Interestingly, this de novo remethylation commences earlier (∼13.5 dpc) in the male germ cells and proceeds until the reentry of mitosis at Postnatal Day 3 [48, 49]. In the female, however, remethylation in oocytes starts close to or shortly after birth and continues during folliculogenesis after puberty [48–51] (Fig. 2). As the oocyte growth is a lengthy developmental process, remethylation occurs in different chromosomal regions at different stages during folliculogenesis [51].
The second reprogramming takes place during preimplantation embryonic development, in which demethylation commences soon after fertilization through either an active (male) or a passive (female) mechanism [19, 51]. During sperm-egg fusion, the paternal genome undergoes a rapid transformation inside the oocyte cytoplasm in which remodeling of the sperm-derived chromatin takes place via the removal of protamines and the replacement by acetylated histones [19, 51]. During this process, genomewide demethylation occurs and proceeds until DNA replication starts [52]. In contrast, genomewide demethylation of the maternal genome occurs later compared to the paternal genome through a passive mechanism, that is, the number of methylated DNA sites will be reduced with cell proliferation as a result of semiconservative DNA replication [45] (Fig. 2). This differential pattern of demethylation between paternal and maternal genomes may suggest that some paternal gene products are required for early embryonic development because the rapid reprogramming can eliminate repressive epigenetic marks (e.g., methylation) and thus induce gene expression. Intriguingly, most, if not all, of the germline imprinted genes and TEs are not subject to demethylation during this preimplantation reprograming, suggesting that a protective mechanism has evolved to maintain the silenced state of imprinted genes and TEs [45]. Despite these interesting observations, the underlying mechanism remains elusive. Taken together, the two reprograming events during mammalian development open a risky time window for TEs to escape from the control of the host genome, especially when the genome is mostly demethylated and the remethylation has not yet completed.
Male Germinal Granules Are Involved in TE Suppression
Most germline cells possess a cytoplasmic structure known as the germinal granule, or nuage, which is probably conserved in many eukaryotic organisms with different names, such as the polar granule in Drosophila [53], the P granule in Caenorhabditis elegans and Xenopus [54], the intermitochondrial cement and the chromatoid body in mice and humans [55]. Germinal granules are rife with membrane-free amorphous ribonucleoprotein (RNP) aggregates, which are localized to the cytoplasmic side of the nuclear envelope [56, 57]. In several lower eukaryotic organisms, whether embryonic cells develop into germ or soma is dependent upon maternally inherited factors during the early stages of embryogenesis [58]. In Drosophila, maternal germline determinants are concentrated in the so-called germ plasm in female oocytes, and further assemble into dense body, known as germinal granules, in the germ plasm [58, 59]. Because of the asymmetric partition of both the germinal granule and germ plasm into daughter cells during cell division, prospective cells that possess germ granules will contribute to the germline specification, indicating that germinal granules are tightly associated with the establishment and differentiation of germline cells [59]. In mammals, PGCs are derived from a population of epiblast cells induced by stimulation signals produced from the surrounding somatic cells [45]. Thus far, no evidence supports a maternal contribution to PGC determination because no accumulation of nuage-like organelles was ever observed in either mature oocytes or zygotes [45]. During male germline development in the mouse, two types of germinal granules have been identified. The first has been termed intermitochondrial cement because these granules are localized between mitochondria in the cytoplasm of prospermatogonia, type A spermatogonia, and late pachytene spermatocytes [55]. The second type of male germinal granules, termed chromatoid body, is found to localize to the perinuclear regions in late pachytene spermatocytes and round spermatids [55] (Fig. 3). Although the precise molecular composition of these two types of granules remains an enigma, the data available so far have found that they indeed share numerous common components, such as RNA helicase Vasa (MVH), RNA decapping enzymes (DCPs), Argonaute family proteins (AGO1-4), Tudor domain-containing proteins (TDRD1–9), Sm (spliceosomal complex protein), and small RNAs [60, 61] (Table 2). Interestingly, these components are all involved in RNA fate control [62, 63]. Recent studies on components of the germinal granule link its function to the repression of retrotransposons based on observations that some RNA-silencing pathway components are colocalized and concentrated in the nuage [59] and that knockout (KO) mice lacking components of the RNA interference (RNAi) pathway, for example, Mvh [64] and maelstrom (Mael) [65], display TE derepression. In addition, it has been reported that targeting ORF1p and ORF2p proteins as well as L1 RNA of LINE1 retrotransposon to the stress granule, which shares components similar to the germinal granule, is one of the proposed mechanisms of controlling retrotransposons [66–68].
FIG. 3. .
Male germinal granules, including the intermitochondrial cement and the chromatoid body, are present in the cytoplasm during male germline development in the mouse. Spermatogenic cells containing the intermitochondrial cement are framed with a blue dashed line, whereas the red dashed-line frame indicates spermatogenic cells possessing the chromatoid body. Note that both the intermitochondrial cement and the chromatoid body are present in late pachytene spermatocytes.
TABLE 2. .
Summary of murine germinal granule- and piRNA pathway-associated components that are involved in TE repression.
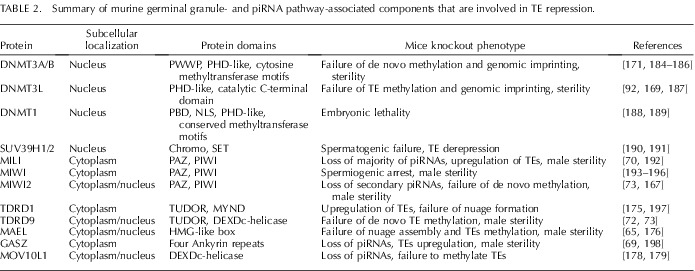
In male mice, the accumulating evidence points to the pivotal role of the germinal granule in the repression of transposable elements. GASZ, a germ cell protein with Ankyrin repeats, colocalizes in the intermitochondrial cement in the prospermatogonia with the Argonaute subfamily protein MILI (PIWIL2) during early germ line development [69]. Gasz-null testes show increased hypomethylation and elevated expression of retrotransposons that is similar to the MILI KO testes [70]. Spermatogenesis is blocked at the zygotene to pachytene transition, resulting in male sterility [69]. Maelstrom is a highly conserved protein found predominantly in female germline nuage of Drosophila [71]. While expressed in the male germline in the mouse, maelstrom accumulates throughout the cytoplasm and in prominent perinuclear nuage in late pachytene and diplotene spermatocytes and colocalizes with other germinal granule components: MIWI (PIWIL1), TDRD1, and MVH [65, 68]. Mael-null testes display severe LINE1 derepression, and spermatocytes exhibit massive DNA damage. L1 RNP aggregates at the onset of meiosis, indicating that MAEL is a component of the germinal granule in the male germline and is indispensable for TE silencing [65, 68] (Table 2). MILI (PIWIL2) and MIWI2 (PIWIL4) localize to two distinct cytoplasmic foci in fetal prospermatogonia in mice [72]. The first type of granule, termed pi-bodies, constitutes the MILI-TDRD1 module of the piRNA pathway and is likely analogous to intermitochondrial cement present in early fetal development. The second type, containing the MIWI2-TDRD9-MAEL complex, has been shown to correspond to a subpopulation of processing bodies (P-body) as verified by immunostaining using P-body markers DCP1A, DDX6, XRN1, and TNRC6A (GW182); this granule has subsequently been named piP-bodies [72, 73]. Because the PIWI-piRNA pathway is critical to the establishment and maintenance of transposon silencing, the colocalization of both distinct complexes to the germinal granules suggests a close relationship between the nuage and TE silencing [72–74]. Given the complex molecular composition of germinal granules during germ cell development, analyses of the transcriptome and proteome of these male germinal granules will help us gain more insights into the function of this enigmatic structure [75, 76].
PATHWAYS INVOLVED IN THE MALE GERMLINE CONTROL OF TEs
Transposable elements, especially LINE1 retroelements, are interspersed throughout the host genome, and TE derepression can compromise the integrity of the host genome by insertional mutations, alternative splicing driven from different promoters, antisense transcripts that can trigger RNAi or interferon response, and dysregulation of gene expression as a result of nearby abnormal chromatin context [30]. Transposable elements have the inherent ability to replicate and propagate within the host genome so as to make their fitness better than that of their host. Given that TEs can be relieved as a result of germline reprogramming and TE-inflicted deleterious effects can be transmitted to and thus affect the next generation, germline cells are particularly under selective pressure to curb TE activity by coevolving complex strategies, which we will discuss.
DNA Methylation
To date, it has been well accepted that DNA methylation represents the primary mechanism of transposon suppression in the host genome [7, 8]. The majority of cytosine methylations reside in transposons that are in a silenced state in mice. In turn, demethylation will recover TE activity [7, 8]. Methylation of cytosine-5 at the CpG dinucleotide has been commonly found in eukaryotic organisms. DNA methylation, the most basic and frequent epigenetic modification, often leads to gene silencing, which is apparent for genes subject to X-chromosome inactivation [77], for imprinted genes [7, 8], and for a large number of TEs that belong to interspersed repetitive elements occupying up to 50% of the human genome [78]. Promoters of retrotransposons are silenced in prospermatogonia in the male and meiotic dictyotene oocytes in the female [79].
In mammals, five members of the DNA cytosine-5 methyltransferase family (DNMT1, DNMT2, DNMT3A, DNMT3B, and DNMT3L), which share similar sequence motifs, are known to be responsible for de novo methylation or the maintenance of methylation of corresponding DNA substrates [80, 81] (Table 2). However, only three members (DNMT1, DNMT3A, and DNMT3B) have been identified as active methyltransferase in vivo and in vitro through biochemical and genetic studies [78]. DNMT1 contains a carboxyl-terminal domain that is closely related to the bacterial cytosine-5 restriction methyltransferases and an amino-terminal domain that possesses multiple regulatory functions, including maintenance of methylation, import into the nuclei, inhibition of de novo methylation, and regulation of cell cycle-dependent protein degradation [78]. Dnmt1-deficient mouse embryos died at the head-fold stage, with severely demethylated genomes, reduced monoallelic expression at most imprinted loci, and elevated levels of intracisternal A particle (IAP) retrotransposon transcripts [82, 83]. DNMT1 appears to be most active against hemimethylated DNA that is produced from semiconservative DNA replication and appears to predominantly function as a maintenance methyltransferase [84]. However, there is also evidence showing overlapping functions in de novo methylation between DNMT1 and DNMT3 [85, 86]. In the male, DNMT1 is detected in spermatogonial stem cells (SSCs), spermatogonia, preleptotene, and leptotene spermatocytes, but not in pachytene spermatocytes, despite the fact that meiotic germ cells at all stages express Dnmt1 mRNA, suggesting that the translation of Dnmt1 must be subjected to posttranscriptional modification [87] (Table 2). DNMT1 can silence postmigratory germ cell-specific genes by DNA methylation in both premigratory germ cells and somatic cells [88]. Meanwhile, Dnmt1 knockdown can induce rapid apoptosis of germline stem cells [89]. All of these lines of evidence are consistent with the role of DNMT1 in establishing and maintaining the methylation pattern in the male germline cells and responsible for the silencing of repetitive elements [83].
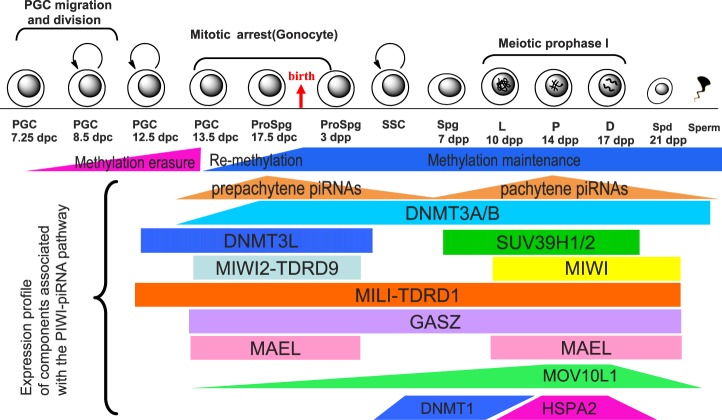
DNMT3L, a truncated form strikingly distinct from other two DNMT3 family members, lacks motifs conserved in the DNA methyltransferase family and is thus deficient in enzymatic activity [90]. It is required for de novo methylation of imprinted genes in oocytes and for transposon repression in male germ cells [90]. In male germ cells, full-length DNMT3L protein is encoded by a transcript expressed at a stage where retrotransposons undergo de novo methylation (Fig. 4). A truncated form of DNMT3L is produced from a second promoter residing in intron 9 of Dnmt3l gene in late spermatocytes, whereas only one full-length, oocyte-specific promoter-derived DNMT3L has been found in growing oocytes [90, 91]. The peak of expression levels of DNMT3L and DNMT3A overlaps during a critical time window (E15.5–E17.5) when genomewide de novo DNA methylation commences, supporting the interplay of these two methyltransferases during prenatal male germline development [86] (Table 2 and Fig. 4).
FIG. 4. .
Schematic summary of expression profiles of the PIWI-piRNAs pathway components and currently known male germ cell-associated epigenetic modifiers during male germline development. Reprogramming occurs in fetal male germ cells (primordial germ cells and prospermatogonia) between 7.5 dpc and birth. DNMT3L is responsible for de novo methylation, while DNMT3A/B serve as the major maintenance methyltransferases. PIWI family proteins associate with respective TDRD members, such as MILI-TDRD1and MIWI2-TDRD9, and localize to different subcellular compartments. Moreover, these proteins most probably complex with other cofactors, such as MOV10L1, MAEL, GASZ, and HSPA2 (HSP70-2), to fulfill their functions in piRNA production and TE suppression. The height of the thick lines represents the relative expression levels of a protein at the stages indicated. Prospg, prospermatogonia; SSC, spermatogonial stem cell; Spg, spermatogonia; L, leptotene spermatocytes; P, pachytene spermatocytes; D, diplotene spermatocytes; Spd, spermatids.
Dnmt3l-deficient postnatal spermatogonia showed hypomethylated patterns not only in the paternal DMRs but also in the repetitive elements such as IAP, LINE1, and SINEB1 [92]. DNMT3L can interact with DNMT3A and DNMT3B in vitro and is assumed to target them to the DMRs of imprinted genes, thereby facilitating genomewide de novo methylation during germline development [85]. DNMT3A and DNMT3B are regarded as de novo methyltransferases in the establishment of the genomewide methylation patterns and both proteins are localized to germ cell nuclei at the stage when de novo methylation is ongoing [92]. Mice with germline conditional deletion of Dnmt3a show an impaired spermatogenesis and a lack of methylation, similar to the phenotype of Dnmt3l-null mice, suggesting both DNMT3A and DNMT3L are essential for the establishment of methylation patterns (Table 2). Interestingly, DNMT2 has long been regarded as a conserved member of methyltransferases family, and it appears to play a role in RNA methylation in zebrafish because Dnmt2-null zebrafish display reduced RNA methylation levels as well as developmental defects [93, 94]. However, the exact function of Dnmt2 in the mammals remains to be elucidated [95].
Heterochromatin Formation
Similar to the immune defense system, safeguarding the genome from invaders like TEs is essential for the survival of the host. One of the defense mechanisms to counteract and silence those genomic parasites is to package DNA sequence containing repetitive elements into transcriptionally inactive heterochromatin.
The genome of eukaryotes can be roughly partitioned into regions composed of euchromatin that are rich in active coding genes and regions composed of heterochromatin that remain condensed and are much less transcriptionally active [96]. Heterochromatin is associated with telomeres and centromeric regions of chromosomes in which large segments of the genome are packaged in a permanently inactive form known as the constitutive chromatin [96]. Further characterization later identified another kind of heterochromatin, which is represented by genomic regions that exhibit a heterochromatin form of packaging associated with cellular development and differentiation termed facultative heterochromatin [97, 98]. Heterochromatin regions are predominantly comprised of repetitive DNA, including highly tandem satellite DNA and interspersed repetitive sequences including TEs [7, 99].
It has long been known that heterochromatin silencing is mostly dependent on the covalent modifications present on either DNA or the conserved core histones. Various combinations of modification patterns on histones are viewed as the information code or the histone code, and still remains poorly understood. Some previous data have unveiled the relationship between histone modifications and the epigenetic state. Histone H3 methylation on lys-4, lys-36, and lys-79 has been implicated in active gene expression, whereas methylation of histone H3 on lys-9 and lys-27 and histone H4 on lys-20 are closely related to gene silencing [100]. Acetylation of histones H3/4 by histone acetyltransferase loses the chromatin structure and promotes polymerase II-mediated gene activation. In turn, deacetylation by histone deacetylase leads to gene silencing [101–104]. Histone phosphorylation takes place at the serine residues of all core histones and usually correlates with up-regulated gene expression [101–104]. The impact of histone ubiquitination varies and appears to be dependent on each core histone that is modified [100–104].
In the germline, histone modification patterns in PGCs fluctuate concomitantly with developmental reprogramming. For example, levels of H3K9me reach their highest level at E7.5 and gradually decrease thereafter. From E7.5 onward, however, both the repressive marks of histone modification (e.g., H3K27me and H3K9me) and the activating forms of modification (e.g., H3K4me and H3K9ac) start to emerge simultaneously [52, 105–107]. In addition, previous data show that histone modification can be influenced by the preexisting epigenetic marks of the histone, for example, H3K9me and H3K4me are mutually exclusively expressed. In addition, some effector complexes can recognize dual histone modification marks [108]. Given that global demethylation occurs during reprogramming when higher risks are posed by the repetitive elements, a diverse combination of histone modifications and the cross-talk among various epigenetic marks may be responsible for the repression of the target DNA segment in the genome through distinct pathways [108–111]. During mouse spermatogenesis, the dynamic distribution of distinct histone-carried covalent modifications has been well characterized. Levels of both H3K4me (H3K4 methylation at lys-4) and H3/4ac (acetylated H3/4) peak in spermatogonial stem cells with a sharp decline when meiosis initiates [112–114], suggesting that these histone modifications direct the expression of meiosis-associated genes. At the stage of spermatocytes, the increase in expression levels of H3K9me and H3K27me is reminiscent of the silencing mechanisms required for the meiotic division [112–114]. While meiosis is completed, levels of H3K4me and H3/4ac go up again, presumably ensuring the supply of proteins essential for the proper histone to protamine exchange during spermiogenesis [115].
Two interesting phenomena are worth mentioning. First, repetitiveness within TEs alone is sufficient for heterochromatin formation. In Drosophila, bulk repetitive DNA sequences that apparently share nothing in common are packaged into heterochromatin, giving rise to the notion that the features themselves residing in the repeats could initiate the formation of heterochromatin [116]. Previous studies have demonstrated that as few as three copies of P elements in the fly genome can suffice to induce position-effect variegation (PEV) silencing, a phenomenon occurring when a gene is inserted juxtaposed to regions of heterochromatin [116]. PEV-induced gene silencing can encompass genes that are hundreds of kilobases away from the heterochromatin/euchromatin boundary and can be explained as the local formation of heterochromatin around the genes through the recruitment of a large number of modifier components [117, 118]. Increases in copy number of P elements result in enhanced silencing, while reversing a transposon within a transgene array would cause the strengthening of silencing, suggesting that the highly repetitive elements and inverted repeats produced by the neighboring inverted repeat-containing transposons are more likely to trigger the assembly of the heterochromatin state [119].
Second, the feature buried within some repetitive elements can bind specific DNA-interacting proteins (trans-acting factors) to guide the heterochromatin formation. L1 elements are dispersed throughout the genome with high contents of CpG islands in the promoter region [120]. Retinoblastoma (Rb) family members can bind CpG-rich regions in the genome with high affinity and mediate gene silencing through the recruitment of corepressor proteins that modify the chromatin epigenetically [121]. Recent data also demonstrate that Rb protein can bind the L1 element promoter in vivo and in vitro, while Rb-null cells exhibit aberrant histone methylation patterns in pericentromeric heterochromatin regions [120]. Ultimately, it has been postulated that L1 elements may function as a master regulator of the chromatin structure through heterochromatin silencing of discrete chromosomal regions where structural genes that are regulated in a cell cycle-dependent fashion reside [120]. It is possible that Rb functions as a regulator of epigenetic silencer of L1 elements in wild-type cells through the recruitment of corepressors (e.g., histone deacetylase or histone methyltransferase) to perform the function of histone deacetylation/methylation and/or DNA methylation [122, 123].
The RNA Interference Pathway
RNAi is a highly conserved gene-silencing mechanism triggered by processing double-stranded RNAs (dsRNAs) into single stranded RNAs (ssRNAs) that are subsequently loaded into effector complexes to modulate gene expression in a sequence-specific fashion [124]. In mammals, three distinct classes of small RNAs, including miRNAs, endogenous small interfering RNAs (endo-siRNAs), and piRNAs, have been identified based upon differences in size, source of biogenesis, and mode of action [125, 126]. Here in this section, we mainly focus on the TE control function of siRNAs and miRNAs, both of which exploit DICER to produce mature ssRNAs, whereas piRNAs, the most intriguing class of small RNAs confined to the germline, will be discussed separately in the section to follow.
Small interfering RNAs refers to synthetic siRNAs or those derived from synthetic dsRNAs introduced into the host cells. These exogenous siRNAs bind to their complementary sequences in target mRNAs and induce degradation or repress translation. Recent reports demonstrate that many eukaryotes can generate endo-siRNAs, which are generally derived from the retrotransposons, pseudogenes, and other genomic regions that can produce transcripts capable of forming dsRNA structures [127–132]. In plants and lower animals, endo-siRNAs have been hypothesized to protect host genome from invading TEs mainly through two mechanisms in eukaryotic organisms: one is RNA-directed DNA demethylation (RdDM) [133–135], and the other is RNA-mediated heterochromatin formation [136–141]. In both situations, the siRNAs function as guide molecules to direct associated protein factors to target specific genomic regions by DNA cytosine methylation or promoting the formation of heterochromatin. In the first instance, initially discovered in viroid-infected tobacco, dsRNA-harboring sequences homologous to promoter regions can elicit promoter methylation and gene silencing by methylation of not only symmetrical CpG dinucleotides, but also non-CpG nucleotides [133]. In plants, the proposed model involving RdDM points to a positive feedback mechanism through which RNAi is amplified by the synthesis of additional dsRNAs using the target as a template and catalyzed by RNA-directed RNA polymerase (RdRP), which is conserved in fission yeast, Neurospora crassa, C. elegans, and plants, but not found in flies and mammals [134, 135]. This is a stepwise process in which site-specific DNA methyltransferase (MET1) can be recruited to the target-specific DNA regions directed by RNAi-induced transcriptional-silencing complex (RITS) for de novo cytosine methylation, and then histone modification helps maintain the silencing state, which can otherwise be lost by a passive or active demethylation process [136].
How do siRNAs recognize target-specific regions in the genome? Currently, two potential pathways have been proposed. The first model states that an siRNA incorporated into a RITS can base pair with its complementary strand in the genome, implying the necessity of unwinding DNA helix to facilitate the interaction. In the second model, the RITS complex can be recruited to specific DNA by base pairing of an siRNA with nascent transcript RNA [137, 138]. This pathway highlights the role of RdRP, but it remains unknown whether this pathway operates in mammals. Recent data show that some of the known components of RdDM are present in mammals as well [139, 140], suggesting the significance and generality of this mechanism in genome defense and transposon silencing.
The role of the RNAi pathway has been linked to heterochromatin formation with the discovery that defects in the RNAi pathway components can cause aberrant assembly of heterochromatin. First identified in fission yeast, RNAi mutants deficient in components of the RNAi pathway, such as Argonaute, Dicer, and RdRP, display the derepression of centromeric outer–transposon repeats, suggesting the role of siRNAs in targeting chromatin modification for heterochromatin formation [130, 142, 143]. Further evidence came from the observation in which a transgene inserted in the centromeric repeats, which normally exhibit a silent state, was activated in RNAi mutant Drosophila [144]. These data led to the proposed model for RNAi-mediated heterochromatin formation: the inverted repeats located in the centromeric region generate dsRNA molecules that are then processed into effector siRNAs by DICER. Such siRNAs might be amplified by synthetic activity of RdRP, and subsequently guide the histone methyltransferases (HMTs) to the respective sites of the chromosome to catalyze methylation of H3K9, a typical hallmark of heterochromatin [145]. The modified form of H3 can recruit HP1 and other associated corepressors to maintain the heterochromatin state [143]. Furthermore, apart from the centromeric heterochromatin silencing, RNAi has also been implicated in the targeting of interstitial sites of euchromatin (noncentromeric repeats) for silencing in a polycomb-dependent manner through methylation of H3K27 rather than H3K9 [142, 146]. The transcription of an introduced hairpin RNA can trigger silencing of homologous sequences throughout the host genome, and retrotransposons with LTRs have been demonstrated to participate in this pathway [147, 148]. Commonly, RNAi-mediated methylation of cytosine is confined to the region of RNA-DNA homology, with a minimum target DNA size of ∼30 bp [133], whereas in RNAi-directed heterochromatin assembly, it can spread over 7∼8 kilobases from the siRNA targeted sites [149]. It should be noted that these two pathways might interconnect to some extent based upon some common components. But the notion that the DNA methylation always precedes histone modification is inconclusive because this sequence of events is reversed in N. crassa, in which methylation of H3K9 is a prerequisite for all cytosine methylation [150].
MicroRNAs, with a size between 18 to 25 nucleotides, represent a highly conserved small regulatory RNA family present from prokaryotes to eukaryotes [151]. Biogenesis of miRNAs is a multi-step process through which the stem-loop hairpin RNA precursors encoded by the host genome are cleaved by DICER into mature miRNAs, which are subsequently incorporated into their effector complexes [152]. Expression of miRNAs is subject to tight spatiotemporal regulation and has been linked to suppressing gene expression by completely or partially complementary base pairing to the target mRNAs in most organisms at the posttranscriptional level [151]. Abnormal expression of miRNAs has been demonstrated to be a cause of developmental deformity and tumorigenesis in humans and animals [153, 154]. In mice, a set of miRNAs, such as miR-17-92 cluster (MIRC1) and miR-290-295 cluster (MIRC5), are highly expressed in primordial germ cells and subject to developmental regulation [155, 156]. Previously, it has been demonstrated that disruption of Dicer result in the up-regulation of retrotransposons in preimplantation embryos and embryonic stem (ES) cells, indicating that miRNAs play significant roles in the repression of retrotransposons [157, 158]. In the germline, however, Dicer-null PGCs unexpectedly exhibit down-regulated expression of LINE1 and IAP to levels that are comparable to those seen in Dicer-null neonatal prospermatogonia, suggesting that DICER is dispensable for the suppression of retrotransposons in male germ cells [155]. Given that many miRNAs are derived from transposons and the prevalence of retrotransposon sequence in the UTR regions of mammalian mRNAs is high [152, 159, 160], miRNAs might have a function similar to that of other small RNA species (e.g., siRNA and piRNAs) in suppressing transposable elements and regulating mRNAs at posttranscriptional levels.
Compared to the high degree of evolutionary conservation of miRNAs that are present from prokaryotes to eukaryotes, endo-siRNAs have so far only been identified in a few organisms. For example, endo-siRNAs exist not only in somatic cells but also in the germline in Drosophila [129, 132], whereas in the mouse only spermatogenic cells [132], oocytes [128, 161], and ES cells [131] have been shown to express endo-siRNAs, although no RdRP activity has been found in these cell types [128, 161]. The lack of endo-siRNA expression among diverse organisms and tissues may signify overlapping functions between endo-siRNAs and other small RNA pathways.
The PIWI-piRNA Pathway
Increasing lines of evidence point to an indispensable role of the PIWI-piRNA pathway in the regulation of germline development in most organisms, from fission yeasts to humans [10]. Argonaute proteins, also known as PAZ-PIWI domain proteins, consist of two main subfamilies, namely AGO and PIWI, and the latter is expressed exclusively in the germline and associates with a diverse class of germline-specific small RNAs, called piRNAs, to control the activity of mobile elements from invertebrates to mammals [10–12]. In Drosophila, there are three members in the PIWI family associated with piRNAs, including AGO3, which tightly binds sense strand piRNAs, AUB, and PIWI, which binds antisense strand piRNAs [74, 162, 163]. This binding preference was proposed to produce distinct piRNAs through a ping-pong amplification model based on the sequence characteristic of the piRNAs that they bind, most of which start with a 5′-end uridine and have an adenine at the 10th position [74, 162, 163]. This amplification mechanism highlights the continuing recycling of PIWI components in recognition and excision of target RNAs into mature piRNAs. However, it remains unclear how the preexisting primary piRNAs are transcribed from the host cells and initiate the amplification cycle. A recent study reports that mice MITOPLD (official symbol PLD6 and ortholog of Drosophila Zucchini), a conserved member of the phospholipase D superfamily, is important for primary piRNA biogenesis [164, 165]. Pld6 KO mice display notable demethylation and derepression of retroelements concurrent with meiotic arrest and severe defects in primary piRNA generation [164, 165]. In the mouse, there are three PIWI subfamily members: MIWI, MILI, and MIWI2, alternatively named PIWIL1, PIWIL2, and PIWIL4, respectively, in the official nomenclature. The expression of MILI and MIWI2 overlaps in prospermatogonia during the early germline development from 13.5 dpc to 3 days postpartum [166–168] (Fig. 4). The timing coincides with the temporal window of de novo genomewide remethylation in the nondividing prospermatogonia of the male germline, whereas MIWI is expressed predominantly in the meiotic spermatocytes and round spermatids [166–168] (Table 2). Both Piwil2 and Piwl4 KO mice display elevated expression of retrotransposons (IAP and LINE1) and spermatogenetic arrest at meiotic prophase similar to Dnmt3l KO mice, causing male sterility [70, 73]. MILI and MIWI2 have been, therefore, proposed to direct de novo methylation by recruiting other protein factors, including DNMT3L, via an unidentified mechanism [92, 169]. Interestingly, fetal mouse testes also exhibit high proportion of piRNAs with uridine at the first position and adenine at the 10th [166–168]. In addition, piRNAs expressed in the fetal gonad display sequence complementarity between MILI- and MIWI2-bound piRNA populations, suggesting a similar ping-pong amplification cycle may function in the male germline [166]. However, piRNAs expressed in postnatal testes/ovaries do not appear to result from the ping-pong mechanism [166–168], suggesting the existence of other mechanisms in piRNAs biogenesis.
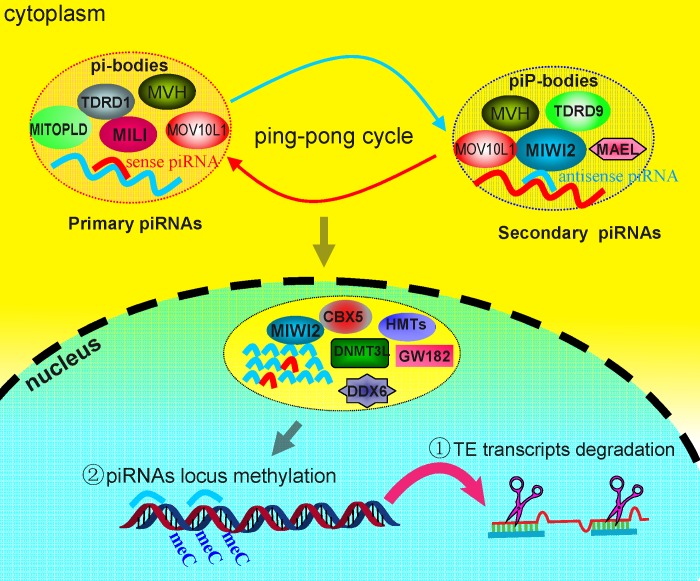
It appears that piRNAs not only participate in the degradation of cytoplasmic repetitive elements-derived RNA at posttranscriptional levels in a manner similar to miRNAs and siRNAs, but also are involved in the recruitment of repressive machinery to guide DNA methylation and heterochromatin formation and thus regulate gene expression at transcriptional levels in the germline [10] (Fig. 5). In Drosophila, large piRNA clusters act as master regulators of transposon silencing [170], whereas in mammals, transposon copies interspersed in the genome initiate the cycling of piRNAs biogenesis. This produces primary piRNAs corresponding to antisense strand of DNA, which predominantly join the MILI complex in the cytoplasm. MIWI2-associated piRNAs have a bias for sequences matching to the sense strand of transposons; hence MIWI2 has been suggested to function in a fashion similar to AGO3 in flies. Furthermore, additional evidence suggests that there is sequence complementarity at the first 10 nucleotides between MILI- and MIWI2- associated piRNAs. Taking together, these data raise the possibility of a ping-pong mechanism being adopted in mammals [167, 168]. Once mature piRNAs are produced, different sets of piRNAs are subsequently sorted into respective effector complexes wherein PIWI proteins reside. Recent reports reveal that both MILI and MIWI2 localize to distinct germinal granules, cytoplasmic RNA, and protein aggregates lacking membrane that are present in close proximity to the nuclear envelope of germ cells [72]. Sense strand piRNAs encoded by the genomic piRNA cluster often bind to the MILI-TDRD1 complex and localize within intermitochondrial cement in prospermatogonia known as pi-bodies, whereas antisense piRNAs recognize the MIWI-TDRD9 complex and have been shown to constitute the granular structure together with other related proteins including Maelstrom in piP-bodies, which also contain signature proteins of RNA-processing bodies (or P bodies) enriched in translationally inactive mRNAs in somatic cells (Fig. 5) [72]. Furthermore, contrary to pi-bodies, which mainly reside in cytoplasm, piP-bodies pathway components can transport between the cytoplasm and nucleus, and hence may be able to target the genome DNA inside the nucleus. To date, it has been demonstrated that both granules share some common components, and different sets of piRNAs emanating from respective granules can interchange if needed at specific timing or stage [72]. The antisense piRNAs that form complexes with piP-bodies translocating from the cytoplasmic compartment to the nucleus could facilitate de novo methylation and the construction of repressive chromatin states through recruitment of other epigenetic modifiers as discussed above. The PIWI-piRNA pathway thus appears to be able to convert a posttranscriptional response to TE reanimation into a silent state of transcriptional suppression. But it remains unclear what other significant effectors or cofactors are involved in this process, how the different piRNAs pathway components are recruited to corresponding complexes and cooperate to function, and finally how the piRNAs direct the core effector complex to specific genomic regions for DNA methylation and/or histone modification.
FIG. 5. .
Subcellular localization of components of the PIWI-piRNA pathway and the proposed mechanism of TE suppression by this pathway. MILI associates with TDRD1 and other cofactors localized to pi-bodies, whereas MIWI2 binds TDRD9 and several other P body components localized to piP-bodies. Sense and antisense piRNAs produced from both granules can exchange through the presumptive ping-pong cycle to enhance piRNAs production. MIWI2 can enter the nucleus by recruiting other epigenetic modifiers such as DNMT3L, CBX5 (HP1a), and HMTs (histone methyltransferases). The resulting secondary antisense piRNAs can presumably function to suppress transposons via two mechanisms: 1) degradation of the retrotranscripts by recognizing complementary RNA in an RNA:RNA hybrid at the posttranscriptional level and 2) recruiting epigenetic repressors responsible for DNA methylation and heterochromatin formation via an unidentified mechanism.
It is noteworthy that several Tudor domain family proteins have been demonstrated to tightly interact with specific PIWI family proteins by recognizing the symmetrical dimethyl arginines and have therefore been suggested as the docking sites for PIWI-like proteins [171, 172]. Supporting this notion, KO mice deficient in these Tudor domain-containing proteins display enhanced retrotransposon activation and male sterility [73, 173, 174]. There are considerable differences among these members although all of them possess at least one Tudor domain. For example, TDRD1 contains four Tudor domains and a zinc finger motif, whereas TDRD9 contains only one Tudor domain combined with an additional ATPase/DExH-type helicase domain, indicating that TDRDs serve not only as scaffolds whereby PIWI assemble but also contain an additional function through an unidentified mechanism in combination with other protein factors [168, 175]. A good example is MAEL, a murine homolog of a Drosophila nuage protein maelstrom; MAEL has recently been shown to localize to the piP-bodies [72]. Mael KO mice exhibit LINE1 derepression and spermatogenic defects as seen in Piwil4 (Miwi2)- and Tdrd9-null mice, with the exception that DNA methylation at LINE1 loci is only moderately impaired in prospermatogonia at 16.5 dpc, suggesting that MAEL facilitates MIWI2-TDRD9-mediated piRNAs pathway [65, 176]. TDRD7, a member of the TUDOR family [62], has recently been found essential for nuage/chromatoid body formation and LINE1 suppression, and interestingly, these actions are independent of piRNA biogenesis [177]. Furthermore, in mice, another requisite component in the piRNA pathway is MOV10L1. Two recent reports demonstrate that MOV10L1 is able to substantially restrict the activity of mouse IAP and LINE1 by interacting with MILI and MIWI2 [178, 179] (Table 2).
Taken together, there are generally two views on the mechanism by which piRNAs modulate TE activities (Fig. 5). First, piRNAs could target complementary retrotranscripts encoded by the host retrotransposons in an RNA: RNA hybrid manner and thus degrade the aberrant TE transcripts. Second, the antisense piRNAs also can recognize the partner DNA strand via an RNA: DNA hybrid to guide the recruitment of repressive chromatic modifiers such as DNA methyltransferases and histone deacetylases. Some recent reports appear to support the latter view [180, 181]. Given the dynamic changes and cooperation among different germinal granules in the germline, deciphering the complete proteomic profile, including core effector proteins and possibly the small RNA composition, will help shed light on the hidden side of the PIWI-piRNA pathway and also on the nuage function in transposon suppression and germline specification.
PERSPECTIVES
The main theme of the male germline defense system is to suppress TEs using the mechanism reviewed above. However, two waves of TE activation during the male germline development have been observed [19, 46, 182]. The first one coincides with the reprograming event in PGCs in the fetal testes [19, 46, 182], whereas the second wave is observed in meiotic male germ cells in the adult mouse testes [19, 46, 182, 183]. The global demethylation may explain the transient increase in TE activity in PGCs. However, a functional role of this TE activation cannot be completely excluded. This transiently elevated TE activity is quickly suppressed in the subsequent de novo remethylation. Although we now know that the PIWI-piRNA pathway is required for the remethylation process [70, 73, 167], the molecular processes through which piRNAs induce targeted remethylation of TEs remain elusive. How do piRNAs attract and guide the methylation machineries to the TE loci? Do those demethylated and transcribed TEs produce any proteins products during the transient activation? What is the fate of those TE-derived piRNAs? Are those TE-derived piRNAs required for the maintenance of the hypermethylated status of TEs? Further study is needed to answer these critical questions.
Reprograming events that occur in PGCs and in preimplantation embryos have been linked to the germline fate determination and the acquisition of pluripotency, respectively [19, 45, 50]. The PGC reprograming wipes off all DNA methylation marks, including those of TEs [19, 45]. In contrast, the preimplantation embryonic reprograming erases most of the DNA methylation marks except those on imprinted genes and TEs [19]. This may explain why the PIWI-piRNA pathway does not function during preimplantation embryonic development [12, 73]. However, the molecular mechanism underlying the difference in these two global de novo demethylation and remethylation processes remains unknown. It would be interesting to investigate how TEs escape the global demethylation and maintain their hypermethylated status in preimplantation embryos.
The second wave of TE activation in the male germline has been observed in the meiotic phase of spermatogenesis in adult testes [182, 183]. The physiological significance of this phenomenon remains unknown. Theoretically, TEs should be entirely suppressed at this stage of germ cell development because disruption of the genome will jeopardize fertility and TE-inflicted genetic defects could be transmitted to the next generation, exerting deleterious effects on the offspring. A mild activation of TEs in meiotic male germ cells may imply an essential role of TEs in meiotic cellular events (e.g., formation of the synaptonemal complex or crossover/homologous recombination). However, because TEs exist in large numbers interspersed throughout the genome with variations in their sequences, it is very difficult to identify which ones are activated and function to contribute to the cellular functions. For the same reason, it is close to impossible to inactivate those activated TEs and then study the physiological effects. Given that the PIWI-piRNA pathway can produce TE-derived piRNAs that induce TE suppression, it may be feasible to introduce the PIWI-piRNA pathway components into the meiotic phase of spermatogenesis and then observe the effects on meiosis, assuming that the transient TE activation is suppressed.
In summary, the male germline suppression of TE activity is achieved through both the epigenetic mechanism that includes DNA methylation and heterochromatin formation as well as small RNAs-mediated posttranscriptional regulation. In addition, the temporal TE activation during the meiotic phase of spermatogenesis suggests a role of TEs in this specific stage of germ cell development, which may warrant further study to elucidate its physiological significance.
ACKNOWLEDGMENT
We would like to thank Keegan R. Idler for editing the text. We apologize to colleagues whose relevant work is not cited here because of the page limitation.
Footnotes
Research in the Yan Laboratory is supported by National Institutes of Health grants HD050281 and HD060858.
REFERENCES
- Biemont C, Vieira C. Genetics: junk DNA as an evolutionary force. Nature 2006; 443: 521 524 [DOI] [PubMed] [Google Scholar]
- Kidwell MG, Lisch DR. Perspective: transposable elements, parasitic DNA, and genome evolution. Evolution 2001; 55: 1 24 [DOI] [PubMed] [Google Scholar]
- Gregory TR, Hebert PD. The modulation of DNA content: proximate causes and ultimate consequences. Genome Res 1999; 9: 317 324 [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J. et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002; 420: 520 562 [DOI] [PubMed] [Google Scholar]
- Kazazian HH., Jr Mobile elements: drivers of genome evolution. Science 2004; 303: 1626 1632 [DOI] [PubMed] [Google Scholar]
- Brookfield JF. The ecology of the genome—mobile DNA elements and their hosts. Nat Rev Genet 2005; 6: 128 136 [DOI] [PubMed] [Google Scholar]
- Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 2009; 10: 295 304 [DOI] [PubMed] [Google Scholar]
- Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet 2008; 9: 397 405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstiel JP. Evolutionary dynamics of transposable elements in a small RNA world. Trends Genet 2011; 27: 23 31 [DOI] [PubMed] [Google Scholar]
- Saxe JP, Lin H. Small noncoding RNAs in the germline. Cold Spring Harb Perspect Biol 2011; 3: a002717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda J, Genzor P. Bortvin A. piRNAs, transposon silencing, and germline genome integrity. Mutat Res 2011; 714: 95 104 [DOI] [PubMed] [Google Scholar]
- Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol 2011; 12: 246 258 [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S. et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004; 428: 493 521 [DOI] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, Paux E, SanMiguel P. et al. A unified classification system for eukaryotic transposable elements. Nat Rev Genet 2007; 8: 973 982 [DOI] [PubMed] [Google Scholar]
- Seberg O, Petersen G. A unified classification system for eukaryotic transposable elements should reflect their phylogeny. Nat Rev Genet 2009; 10: 276 [DOI] [PubMed] [Google Scholar]
- Bohne A, Brunet F, Galiana-Arnoux D, Schultheis C, Volff JN. Transposable elements as drivers of genomic and biological diversity in vertebrates. Chromosome Res 2008; 16: 203 215 [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Barton N. Genome size: does bigger mean worse? Curr Biol 2004; 14: R233 R235 [DOI] [PubMed] [Google Scholar]
- Gentles AJ, Wakefield MJ, Kohany O, Gu W, Batzer MA, Pollock DD, Jurka J. Evolutionary dynamics of transposable elements in the short-tailed opossum Monodelphis domestica. Genome Res 2007; 17: 992 1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 2001; 293: 1089 1093 [DOI] [PubMed] [Google Scholar]
- Levin HL, Moran JV. Dynamic interactions between transposable elements and their hosts. Nat Rev Genet 2011; 12: 615 627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Jiang P, Shen S, Sato S, Davidson BL, Xing Y. Large-scale analysis of exonized mammalian-wide interspersed repeats in primate genomes. Hum Mol Genet 2009; 18: 2204 2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala SH, Zhang L, Kazazian HH., Jr Many LINE1 elements contribute to the transcriptome of human somatic cells. Genome Biol 2009; 10: R100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Lin L, Chen B, Dai J. L1 elements, processed pseudogenes and retrogenes in mammalian genomes. IUBMB Life 2006; 58: 677 685 [DOI] [PubMed] [Google Scholar]
- Deininger P. Alu elements: know the SINEs. Genome Biol 2011; 12: 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Engel AM. LINEs, SINEs and other retroelements: do birds of a feather flock together? Front Biosci 2012; 17: 1345 1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M, Auletta F, Bernardi G. The distributions of “new” and “old” Alu sequences in the human genome: the solution of a “mystery”. Mol Biol Evol 2012; 29: 421 427 [DOI] [PubMed] [Google Scholar]
- Konkel MK, Batzer MA. A mobile threat to genome stability: the impact of non-LTR retrotransposons upon the human genome. Semin Cancer Biol 2010; 20: 211 221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag EM, Goodier JL, Zhang Y, Kazazian HH., Jr SVA elements are nonautonomous retrotransposons that cause disease in humans. Am J Hum Genet 2003; 73: 1444 1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D. et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860 921 [DOI] [PubMed] [Google Scholar]
- Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A 2003; 100: 5280 5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RE, Bennett EA, Iskow RC, Luttig CT, Tsui C, Pittard WS, Devine SE. Recently mobilized transposons in the human and chimpanzee genomes. Am J Hum Genet 2006; 78: 671 679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RE, Bennett EA, Iskow RC, Devine SE. Which transposable elements are active in the human genome? Trends Genet 2007; 23: 183 191 [DOI] [PubMed] [Google Scholar]
- Xing J, Zhang Y, Han K, Salem AH, Sen SK, Huff CD, Zhou Q, Kirkness EF, Levy S, Batzer MA, Jorde LB. Mobile elements create structural variation: analysis of a complete human genome. Genome Res 2009; 19: 1516 1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler J, Strub K. Alu elements as regulators of gene expression. Nucleic Acids Res 2006; 34: 5491 5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschotte C, Pritham EJ. DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 2007; 41: 331 368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan IK, Rogozin IB, Glazko GV, Koonin EV. Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet 2003; 19: 68 72 [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S, Garber M, Gentles AJ, Goodstadt L, Heger A, Jurka J, Kamal M. et al. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature 2007; 447: 167 177 [DOI] [PubMed] [Google Scholar]
- Muotri AR, Marchetto MC, Coufal NG, Gage FH. The necessary junk: new functions for transposable elements. Hum Mol Genet 2007; 16 (suppl 2): R159 R167 [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Torvik VI. Alu elements within human mRNAs are probable microRNA targets. Trends Genet 2006; 22: 532 536 [DOI] [PubMed] [Google Scholar]
- Lehnert S, Van Loo P, Thilakarathne PJ, Marynen P, Verbeke G, Schuit FC. Evidence for co-evolution between human microRNAs and Alu-repeats. PLoS One 2009; 4: e4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DJ, Deininger PL. Inviting instability: transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat Res 2007; 616: 46 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace NA, Belancio VP, Deininger PL. L1 mobile element expression causes multiple types of toxicity. Gene 2008; 419: 75 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Lange J, Keeney S. Genome destabilization by homologous recombination in the germ line. Nature reviews. Mol Cell Biol 2010; 11: 182 195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet 2008; 9: 129 140 [DOI] [PubMed] [Google Scholar]
- Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev 2002; 117: 15 23 [DOI] [PubMed] [Google Scholar]
- Lees-Murdock DJ, De Felici M, Walsh CP. Methylation dynamics of repetitive DNA elements in the mouse germ cell lineage. Genomics 2003; 82: 230 237 [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang YK, Koo DB, Kang MJ, Moon SJ, Lee KK, Han YM. Differential DNA methylation reprogramming of various repetitive sequences in mouse preimplantation embryos. Biochem Biophys Res Commun 2004; 324: 58 63 [DOI] [PubMed] [Google Scholar]
- Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet 2012; 28: 33 42 [DOI] [PubMed] [Google Scholar]
- Kota SK, Feil R. Epigenetic transitions in germ cell development and meiosis. Dev Cell 2010; 19: 675 686 [DOI] [PubMed] [Google Scholar]
- Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science 2010; 330: 622 627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JR, Susiarjo M, Bartolomei MS. Imprinting and epigenetic changes in the early embryo. Mamm Genome 2009; 20: 532 543 [DOI] [PubMed] [Google Scholar]
- Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 2008; 452: 877 881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson T, Liu N, Arkov A, Lehmann R, Lasko P. Isolation of new polar granule components in Drosophila reveals P body and ER associated proteins. Mech Dev 2008; 125: 865 873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike DL, Hachey SJ, Kreher J, Strome S. P granules extend the nuclear pore complex environment in the C. elegans germ line. J Cell Biol 2011; 192: 939 948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma S, Hosokawa M, Tanaka T, Nakatsuji N. Ultrastructural characterization of spermatogenesis and its evolutionary conservation in the germline: germinal granules in mammals. Mol Cell Endocrinol 2009; 306: 17 23 [DOI] [PubMed] [Google Scholar]
- Russell L, Frank B. Ultrastructural characterization of nuage in spermatocytes of the rat testis. Anat Rec 1978; 190: 79 97 [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Eddy EM, Phillips DM. Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod 1970; 2: 129 153 [DOI] [PubMed] [Google Scholar]
- Targeting Anne J. and anchoring Tudor in the pole plasm of the Drosophila oocyte. PLoS One 2010; 5: e14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne J. Arginine methylation of SmB is required for Drosophila germ cell development. Development 2010; 137: 2819 2828 [DOI] [PubMed] [Google Scholar]
- de Sousa Lopes SM, Roelen BA. An overview on the diversity of cellular organelles during the germ cell cycle. Histol Histopathol 2010; 25: 267 276 [DOI] [PubMed] [Google Scholar]
- Kotaja N, Sassone-Corsi P. The chromatoid body: a germ-cell-specific RNA-processing centre. Nat Rev Mol Cell Biol 2007; 8: 85 90 [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Shoji M, Kitamura K, Tanaka T, Noce T, Chuma S, Nakatsuji N. Tudor-related proteins TDRD1/MTR-1, TDRD6 and TDRD7/TRAP: domain composition, intracellular localization, and function in male germ cells in mice. Dev Biol 2007; 301: 38 52 [DOI] [PubMed] [Google Scholar]
- Idler RK, Yan W. Control of messenger RNA fate by RNA binding proteins: an emphasis on mammalian spermatogenesis. J Androl 2012; 33: 309 337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev 2000; 14: 841 853 [PMC free article] [PubMed] [Google Scholar]
- Soper SF, van der Heijden GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, Bortvin A. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell 2008; 15: 285 297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodier JL, Zhang L, Vetter MR, Kazazian HH., Jr LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex. Mol Cell Biol 2007; 27: 6469 6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodier JL, Mandal PK, Zhang L, Kazazian HH., Jr Discrete subcellular partitioning of human retrotransposon RNAs despite a common mechanism of genome insertion. Hum Mol Genet 2010; 19: 1712 1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AK, Kai T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc Natl Acad Sci U S A 2007; 104: 6714 6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Buchold GM, Greenbaum MP, Roy A, Burns KH, Zhu H, Han DY, Harris RA, Coarfa C, Gunaratne PH, Yan W, Matzuk MM. GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet 2009; 5: e1000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E. et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 2008; 22: 908 917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development 2003; 130: 859 871 [DOI] [PubMed] [Google Scholar]
- Aravin AA, van der Heijden GW, Castaneda J, Vagin VV, Hannon GJ, Bortvin A. Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet 2009; 5: e1000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL. et al. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell 2009; 17: 775 787 [DOI] [PubMed] [Google Scholar]
- Thomson T, Lin H. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol 2009; 25: 355 376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkov AL, Ramos A. Building RNA-protein granules: insight from the germline. Trends Cell Biol 2010; 20: 482 490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma S, Pillai RS. Retrotransposon silencing by piRNAs: ping-pong players mark their sub-cellular boundaries. PLoS Genet 2009; 5: e1000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AJ, Stathaki E, Migliavacca E, Brahmachary M, Montgomery SB, Dupre Y, Antonarakis SE. DNA methylation profiles of human active and inactive X chromosomes. Genome Res 2011; 21: 1592 1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem 2005; 74: 481 514 [DOI] [PubMed] [Google Scholar]
- Schaefer CB, Ooi SK, Bestor TH, Bourc'his D. Epigenetic decisions in mammalian germ cells. Science 2007; 316: 398 399 [DOI] [PubMed] [Google Scholar]
- Marques CJ, Joao Pinho M, Carvalho F, Bieche I, Barros A, Sousa M. DNA methylation imprinting marks and DNA methyltransferase expression in human spermatogenic cell stages. Epigenetics 2011; 6: 1354 1361 [DOI] [PubMed] [Google Scholar]
- Guibert S, Forne T, Weber M. Dynamic regulation of DNA methylation during mammalian development. Epigenomics 2009; 1: 81 98 [DOI] [PubMed] [Google Scholar]
- Bestor TH. Cytosine methylation mediates sexual conflict. Trends Genet 2003; 19: 185 190 [DOI] [PubMed] [Google Scholar]
- Gaudet F, Rideout WM, III, Meissner A, Dausman J, Leonhardt H, Jaenisch R. Dnmt1 expression in pre- and postimplantation embryogenesis and the maintenance of IAP silencing. Mol Cell Biol 2004; 24: 1640 1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilkaitis G, Suetake I, Klimasauskas S, Tajima S. Processive methylation of hemimethylated CpG sites by mouse Dnmt1 DNA methyltransferase. J Biol Chem 2005; 280: 64 72 [DOI] [PubMed] [Google Scholar]
- Suetake I, Shinozaki F, Miyagawa J, Takeshima H, Tajima S. DNMT3L stimulates the DNA methylation activity of Dnmt3a and Dnmt3b through a direct interaction. J Biol Chem 2004; 279: 27816 27823 [DOI] [PubMed] [Google Scholar]
- La Salle S, Mertineit C, Taketo T, Moens PB, Bestor TH, Trasler JM. Windows for sex-specific methylation marked by DNA methyltransferase expression profiles in mouse germ cells. Dev Biol 2004; 268: 403 415 [DOI] [PubMed] [Google Scholar]
- Mertineit C, Yoder JA, Taketo T, Laird DW, Trasler JM, Bestor TH. Sex-specific exons control DNA methyltransferase in mammalian germ cells. Development 1998; 125: 889 897 [DOI] [PubMed] [Google Scholar]
- Maatouk DM, Kellam LD, Mann MR, Lei H, Li E, Bartolomei MS, Resnick JL. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 2006; 133: 3411 3418 [DOI] [PubMed] [Google Scholar]
- Takashima S, Takehashi M, Lee J, Chuma S, Okano M, Hata K, Suetake I, Nakatsuji N, Miyoshi H, Tajima S, Tanaka Y, Toyokuni S. et al. Abnormal DNA methyltransferase expression in mouse germline stem cells results in spermatogenic defects. Biol Reprod 2009; 81: 155 164 [DOI] [PubMed] [Google Scholar]
- Bourc'his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 2004; 431: 96 99 [DOI] [PubMed] [Google Scholar]
- Shovlin TC, Bourc'his D, La Salle S, O'Doherty A, Trasler JM, Bestor TH, Walsh CP. Sex-specific promoters regulate Dnmt3L expression in mouse germ cells. Hum Reprod 2007; 22: 457 467 [DOI] [PubMed] [Google Scholar]
- Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M, Sasaki H. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet 2007; 16: 2272 2280 [DOI] [PubMed] [Google Scholar]
- Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 2006; 311: 395 398 [DOI] [PubMed] [Google Scholar]
- Rai K, Chidester S, Zavala CV, Manos EJ, James SR, Karpf AR, Jones DA, Cairns BR. Dnmt2 functions in the cytoplasm to promote liver, brain, and retina development in zebrafish. Genes Dev 2007; 21: 261 266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksakova IA, Mager DL, Reiss D. Keeping active endogenous retroviral-like elements in check: the epigenetic perspective. Cell Mol Life Sci 2008; 65: 3329 3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, Depaux A, Hery P, Barral S, Thuret JY, Dimitrov S, Gerard M. Histone H3 trimethylation at lysine 36 is associated with constitutive and facultative heterochromatin. Genome Res 2011; 21: 1426 1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego A, Sinclair PB, Tao W, Kireev I, Belmont AS. The facultative heterochromatin of the inactive X chromosome has a distinctive condensed ultrastructure. J Cell Sci 2008; 121: 1119 1127 [DOI] [PubMed] [Google Scholar]
- Trojer P, Reinberg D. Facultative heterochromatin: is there a distinctive molecular signature? Mol Cell 2007; 28: 1 13 [DOI] [PubMed] [Google Scholar]
- Munshi A, Shafi G, Aliya N, Jyothy A. Histone modifications dictate specific biological readouts. J Genet Genomics 2009; 36: 75 88 [DOI] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 2006; 75: 243 269 [DOI] [PubMed] [Google Scholar]
- Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol 2002; 14: 286 298 [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell 2008; 31: 449 461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey AM, Chaturvedi CP, Brand M. Crosstalk between histone modifications maintains the developmental pattern of gene expression on a tissue-specific locus. Epigenetics 2010; 5: 273 281 [DOI] [PubMed] [Google Scholar]
- Suganuma T, Workman JL. Crosstalk among histone modifications. Cell 2008; 135: 604 607 [DOI] [PubMed] [Google Scholar]
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 2007; 134: 2627 2638 [DOI] [PubMed] [Google Scholar]
- Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 2010; 463: 1101 1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K, Matsui Y. Epigenetic profiles in primordial germ cells: global modulation and fine tuning of the epigenome for acquisition of totipotency. Dev Growth Differ 2010; 52: 517 525 [DOI] [PubMed] [Google Scholar]
- Brunmeir R, Lagger S, Simboeck E, Sawicka A, Egger G, Hagelkruys A, Zhang Y, Matthias P, Miller WJ, Seiser C. Epigenetic regulation of a murine retrotransposon by a dual histone modification mark. PLoS Genet 2010; 6: e1000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Chaurasia P, Bhaumik SR. Histone methylation and ubiquitination with their cross-talk and roles in gene expression and stability. Cell Mol Life Sci 2009; 66: 1419 1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Elgin SC. Heterochromatin: new possibilities for the inheritance of structure. Curr Opin Genet Dev 2002; 12: 178 187 [DOI] [PubMed] [Google Scholar]
- Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 2002; 108: 489 500 [DOI] [PubMed] [Google Scholar]
- Godmann M, Auger V, Ferraroni-Aguiar V, Di Sauro A, Sette C, Behr R, Kimmins S. Dynamic regulation of histone H3 methylation at lysine 4 in mammalian spermatogenesis. Biol Reprod 2007; 77: 754 764 [DOI] [PubMed] [Google Scholar]
- Payne C, Braun RE. Histone lysine trimethylation exhibits a distinct perinuclear distribution in Plzf-expressing spermatogonia. Dev Biol 2006; 293: 461 472 [DOI] [PubMed] [Google Scholar]
- Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol 2005; 278: 440 458 [DOI] [PubMed] [Google Scholar]
- Carrell DT, Hammoud SS. The human sperm epigenome and its potential role in embryonic development. Mol Hum Reprod 2010; 16: 37 47 [DOI] [PubMed] [Google Scholar]
- Hall IM, Shankaranarayana GD, Noma K, Ayoub N, Cohen A, Grewal SI. Establishment and maintenance of a heterochromatin domain. Science 2002; 297: 2232 2237 [DOI] [PubMed] [Google Scholar]
- Girton JR, Johansen KM. Chromatin structure and the regulation of gene expression: the lessons of PEV in Drosophila. Adv Genet 2008; 61: 1 43 [DOI] [PubMed] [Google Scholar]
- Yasuhara JC, Wakimoto BT. Molecular landscape of modified histones in Drosophila heterochromatic genes and euchromatin-heterochromatin transition zones. PLoS Genet 2008; 4: e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer DR, Henikoff S. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 1994; 77: 993 1002 [DOI] [PubMed] [Google Scholar]
- Allen E, Horvath S, Tong F, Kraft P, Spiteri E, Riggs AD, Marahrens Y. High concentrations of long interspersed nuclear element sequence distinguish monoallelically expressed genes. Proc Natl Acad Sci U S A 2003; 100: 9940 9945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat Genet 2000; 25: 338 342 [DOI] [PubMed] [Google Scholar]
- Montoya-Durango DE, Liu Y, Teneng I, Kalbfleisch T, Lacy ME, Steffen MC, Ramos KS. Epigenetic control of mammalian LINE-1 retrotransposon by retinoblastoma proteins. Mutat Res 2009; 665: 20 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A, Lein S, Schotta G, Reuter G. Histone modification and the control of heterochromatic gene silencing in Drosophila. Chromosome Res 2006; 14: 377 392 [DOI] [PubMed] [Google Scholar]
- Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat Rev Genet 2005; 6: 24 35 [DOI] [PubMed] [Google Scholar]
- Farazi TA, Juranek SA, Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development 2008; 135: 1201 1214 [DOI] [PubMed] [Google Scholar]
- Hock J, Meister G. The Argonaute protein family. Genome Biol 2008; 9: 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, Perrimon N, Kellis M, Wohlschlegel JA, Sachidanandam R, Hannon GJ, Brennecke J. An endogenous small interfering RNA pathway in Drosophila. Nature 2008; 453: 798 802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, Surani MA, Sakaki Y. et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 2008; 453: 539 543 [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, Xu J, Kittler EL, Zapp ML, Weng Z, Zamore PD. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 2008; 320: 1077 1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WJ, Okamura K, Martin R, Lai EC. Endogenous RNA interference provides a somatic defense against Drosophila transposons. Curr Biol 2008; 18: 795 802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other microprocessor-independent, Dicer-dependent small RNAs. Genes Dev 2008; 22: 2773 2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Hennig GW, Wu Q, Jose C, Zheng H, Yan W. Male germ cells express abundant endogenous siRNAs. Proc Natl Acad Sci U S A 2011; 108: 13159 13164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissier T, Wassenegger MA. DNA target of 30 bp is sufficient for RNA-directed DNA methylation. RNA 2000; 6: 55 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JI, Lamm AT, Pavelec DM, Maniar JM, Parameswaran P, Tao L, Kennedy S, Fire AZ. Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol Cell 2010; 37: 679 689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JI, Schvarzstein M, Villeneuve AM, Gu SG, Jantsch V, Fire AZ, Baudrimont AA. Caenorhabditis elegans RNA-directed RNA polymerase in sperm development and endogenous RNA interference. Genetics 2009; 183: 1297 1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Z, May B, Yordan C, Singer T, Martienssen R. Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol 2003; 1: e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramke V, Sheedy DM, Denli AM, Bonila C, Ekwall K, Hannon GJ, Allshire RC. RNA-interference-directed chromatin modification coupled to RNA polymerase II transcription. Nature 2005; 435: 1275 1279 [DOI] [PubMed] [Google Scholar]
- Djupedal I, Portoso M, Spahr H, Bonilla C, Gustafsson CM, Allshire RC, Ekwall K. RNA. Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev 2005; 19: 2301 2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi C, Paterson BM. Identification of an RNA-dependent RNA polymerase in Drosophila involved in RNAi and transposon suppression. Proc Natl Acad Sci U S A 2009; 106: 15645 15650 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lipardi C, Paterson BM. Identification of an RNA-dependent RNA polymerase in Drosophila establishes a common theme in RNA silencing. Fly (Austin) 2010; 4: 30 35 [DOI] [PubMed] [Google Scholar]
- Meyer P. DNA methylation systems and targets in plants. FEBS Lett 2011; 585: 2008 2015 [DOI] [PubMed] [Google Scholar]
- Kim DH, Villeneuve LM, Morris KV, Rossi JJ. Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat Struct Mol Biol 2006; 13: 793 797 [DOI] [PubMed] [Google Scholar]
- Fagegaltier D, Bouge AL, Berry B, Poisot E, Sismeiro O, Coppee JY, Theodore L, Voinnet O, Antoniewski C. The endogenous siRNA pathway is involved in heterochromatin formation in Drosophila. Proc Natl Acad Sci U S A 2009; 106: 21258 21263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M, Bhadra U, Birchler JA. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol Cell 2002; 9: 315 327 [DOI] [PubMed] [Google Scholar]
- Moazed D, Buhler M, Buker SM, Colmenares SU, Gerace EL, Gerber SA, Hong EJ, Motamedi MR, Verdel A, Villen J, Gygi SP. Studies on the mechanism of RNAi-dependent heterochromatin assembly. Cold Spring Harb Symp Quant Biol 2006; 71: 461 471 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Watanabe S, Ichimura T, Kawasuji M, Koseki H, Baba H, Nakao M. Overlapping roles of the methylated DNA-binding protein MBD1 and polycomb group proteins in transcriptional repression of HOXA genes and heterochromatin foci formation. J Biol Chem 2007; 282: 16391 16400 [DOI] [PubMed] [Google Scholar]
- Cerutti H. RNA interference: traveling in the cell and gaining functions? Trends Genet 2003; 19: 39 46 [DOI] [PubMed] [Google Scholar]
- Schramke V, Allshire R. Hairpin RNAs and retrotransposon LTRs effect RNAi and chromatin-based gene silencing. Science 2003; 301: 1069 1074 [DOI] [PubMed] [Google Scholar]
- Grewal SI. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev 2010; 20: 134 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature 1999; 399: 166 169 [DOI] [PubMed] [Google Scholar]
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011; 12: 99 110 [DOI] [PubMed] [Google Scholar]
- Piriyapongsa J, Marino-Ramirez L, Jordan IK. Origin and evolution of human microRNAs from transposable elements. Genetics 2007; 176: 1323 1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker A, Hohlfeld R, Meinl E. The emerging role of microRNAs in multiple sclerosis. Nat Rev Neurol 2011; 7: 56 59 [DOI] [PubMed] [Google Scholar]
- Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011; 469: 336 342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, Lao K, O'Carroll D, Das PP, Tarakhovsky A, Miska EA, Surani MA. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One 2008; 3: e1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL, Surani MA, Daley GQ. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 2009; 460: 909 913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev 2007; 21: 682 693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 2005; 19: 489 501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Torvik VI. Mammalian microRNAs derived from genomic repeats. Trends Genet 2005; 21: 322 326 [DOI] [PubMed] [Google Scholar]
- Gu TJ, Yi X, Zhao XW, Zhao Y, Yin JQ. Alu-directed transcriptional regulation of some novel miRNAs. BMC Genomics 2009; 10: 563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, Hannon GJ. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 2008; 453: 534 538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig JV, Tomari Y. Forstemann K. piRNAs—the ancient hunters of genome invaders. Genes Dev 2007; 21: 1707 1713 [DOI] [PubMed] [Google Scholar]
- Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development 2008; 135: 3 9 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Chuma S, Yamamoto Y, Kuramochi-Miyagawa S, Totoki Y, Toyoda A, Hoki Y, Fujiyama A, Shibata T, Sado T, Noce T, Nakano T. et al. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev Cell 2011; 20: 364 375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ. Frohman MA. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell 2011; 20: 376 387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Bourc'his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell 2008; 31: 785 799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 2007; 12: 503 514 [DOI] [PubMed] [Google Scholar]
- Wang J, Saxe JP, Tanaka T, Chuma S, Lin H. Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis. Curr Biol 2009; 19: 640 644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development 2002; 129: 1983 1993 [DOI] [PubMed] [Google Scholar]
- Simonelig M. Developmental functions of piRNAs and transposable elements: a Drosophila point-of-view. RNA Biol 2011; 8: 754 759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang JY, Huang Y, Li Z, Gong W, Lehmann R, Xu RM. Structural basis for methylarginine-dependent recognition of Aubergine by Tudor. Genes Dev 2010; 24: 1876 1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Chen C, Guo Y, Lam R, Bian C, Xu C, Zhao DY, Jin J, MacKenzie F, Pawson T, Min J. Structural basis for recognition of arginine methylated Piwi proteins by the extended Tudor domain. Proc Natl Acad Sci U S A 2010; 107: 18398 18403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev 2009; 23: 1749 1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, Pawson T. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci U S A 2009; 106: 20336 20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai RS. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat Struct Mol Biol 2009; 16: 639 646 [DOI] [PubMed] [Google Scholar]
- O'Donnell KA, Burns KH, Boeke JD. A descent into the nuage: the maelstrom of transposon control. Dev Cell 2008; 15: 179 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Hosokawa M, Vagin VV, Reuter M, Hayashi E, Mochizuki AL, Kitamura K, Yamanaka H, Kondoh G, Okawa K, Kuramochi-Miyagawa S, Nakano T. et al. Tudor domain containing 7 (Tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis. Proc Natl Acad Sci U S A 2011; 108: 10579 10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci U S A 2010; 107: 11841 11846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, Olson EN. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci U S A 2010; 107: 11847 11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Tomizawa S, Mitsuya K, Totoki Y, Yamamoto Y, Kuramochi-Miyagawa S, Iida N, Hoki Y, Murphy PJ, Toyoda A, Gotoh K, Hiura H. et al. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science 2011; 332: 848 852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhkov NV, Aravin AA, Zelentsova ES, Schostak NG, Sachidanandam R, McCombie WR, Hannon GJ, Evgen'ev MB. Small RNA-based silencing strategies for transposons in the process of invading Drosophila species. RNA 2010; 16: 1634 1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden GW, Bortvin A. Transient relaxation of transposon silencing at the onset of mammalian meiosis. Epigenetics 2009; 4: 76 79 [DOI] [PubMed] [Google Scholar]
- Branciforte D, Martin SL. Developmental and cell type specificity of LINE-1 expression in mouse testis: implications for transposition. Mol Cell Biol 1994; 14: 2584 2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004; 429: 900 903 [DOI] [PubMed] [Google Scholar]
- Liang G, Chan MF, Tomigahara Y, Tsai YC, Gonzales FA, Li E, Laird PW, Jones PA. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol Cell Biol 2002; 22: 480 491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999; 99: 247 257 [DOI] [PubMed] [Google Scholar]
- Webster KE, O'Bryan MK, Fletcher S, Crewther PE, Aapola U, Craig J, Harrison DK, Aung H, Phutikanit N, Lyle R, Meachem SJ, Antonarakis SE. et al. Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis. Proc Natl Acad Sci U S A 2005; 102: 4068 4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev 2008; 22: 1607 1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Robertson KD. DNMT1 knockout delivers a strong blow to genome stability and cell viability. Nat Genet 2007; 39: 289 290 [DOI] [PubMed] [Google Scholar]
- Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 2001; 107: 323 337 [DOI] [PubMed] [Google Scholar]
- O'Carroll D, Scherthan H, Peters AH, Opravil S, Haynes AR, Laible G, Rea S, Schmid M, Lebersorger A, Jerratsch M, Sattler L, Mattei MG. et al. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Mol Cell Biol 2000; 20: 9423 9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fazio S, Bartonicek N, Di Giacomo M, Abreu-Goodger C, Sankar A, Funaya C, Antony C, Moreira PN, Enright AJ, O'Carroll D. The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature 2011; 480: 259 263 [DOI] [PubMed] [Google Scholar]
- Reuter M, Berninger P, Chuma S, Shah H, Hosokawa M, Funaya C, Antony C, Sachidanandam R, Pillai RS. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 2011; 480: 264 267 [DOI] [PubMed] [Google Scholar]
- Deng W. Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2002; 2: 819 830 [DOI] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006; 442: 199 202 [DOI] [PubMed] [Google Scholar]
- Grivna ST, Pyhtila B, Lin H. MIWI associates with translational machinery and PIWI interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci U S A 2006; 103: 13415 13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Kuramochi-Miyagawa S, Chuma S, Tanaka T, Nakatsuji N, Kimura T, Nakano T. Associations between PIWI proteins and TDRD1/MTR-1 are critical for integrated subcellular localization in murine male germ cells. Genes Cells 2009; 14: 1155 1165 [DOI] [PubMed] [Google Scholar]
- Yan W, Ma L, Zilinski CA, Matzuk MM. Identification and characterization of evolutionarily conserved pufferfish, zebrafish, and frog orthologs of GASZ. Biol Reprod 2004; 70: 1619 1625 [DOI] [PubMed] [Google Scholar]