Abstract
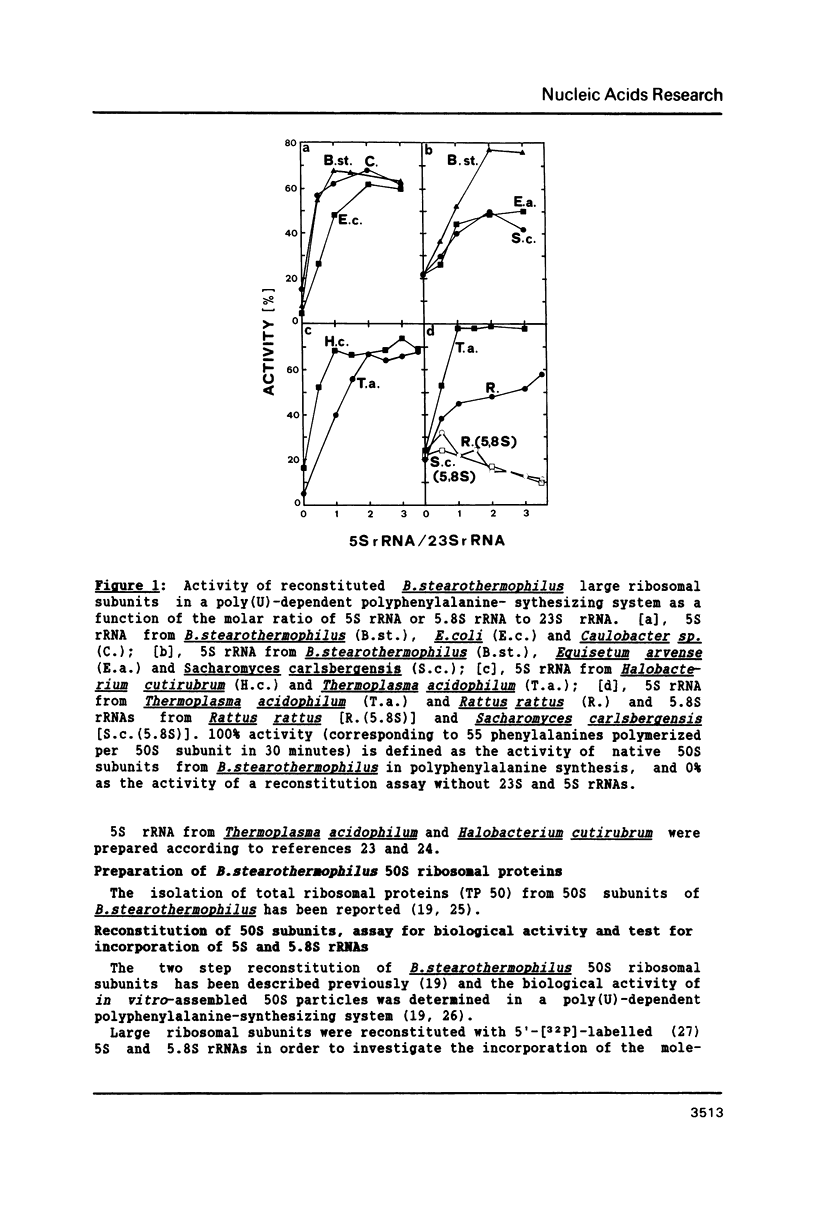
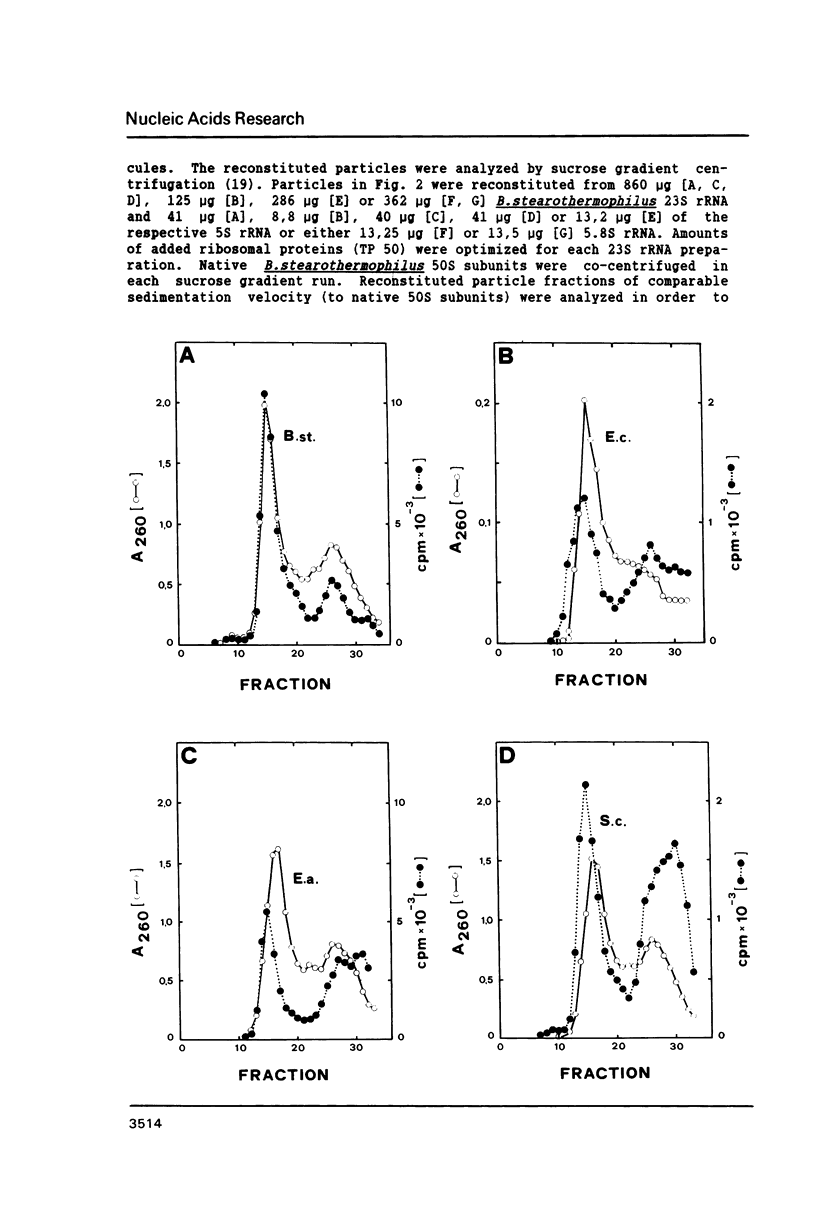
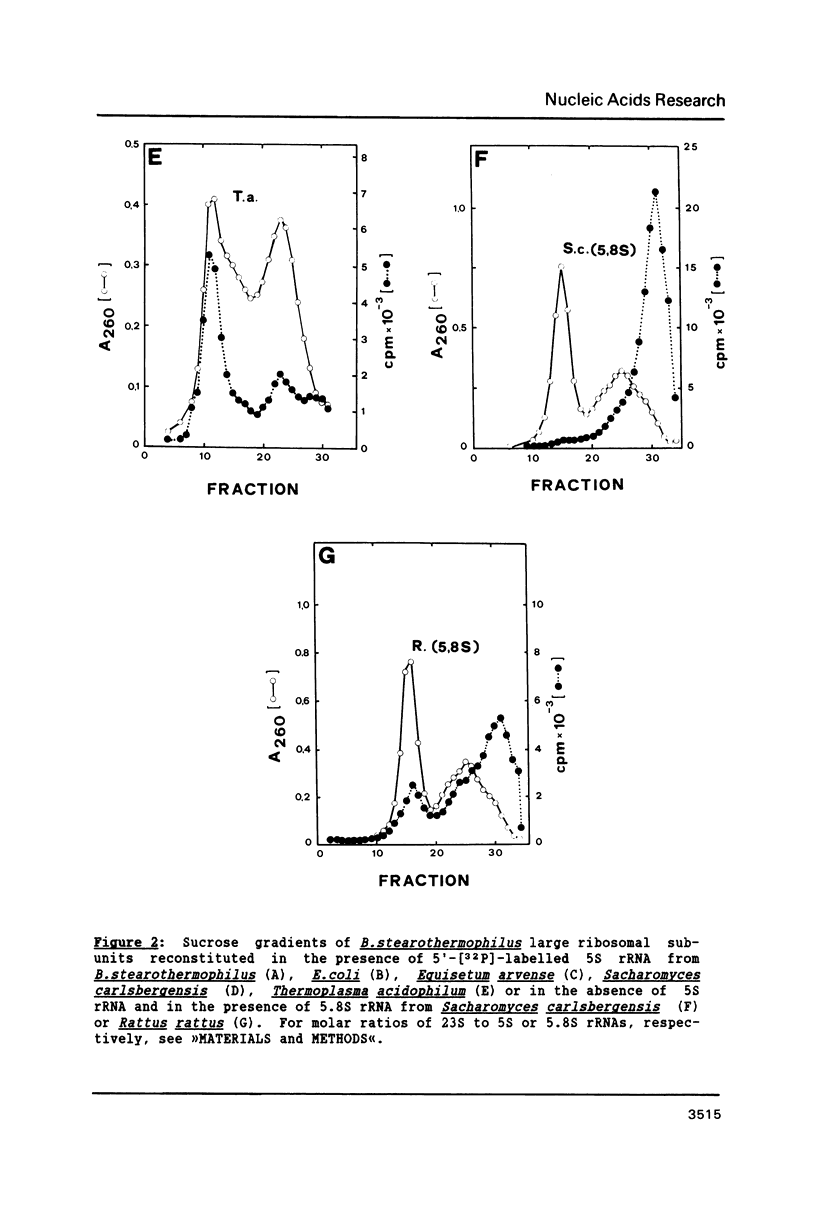
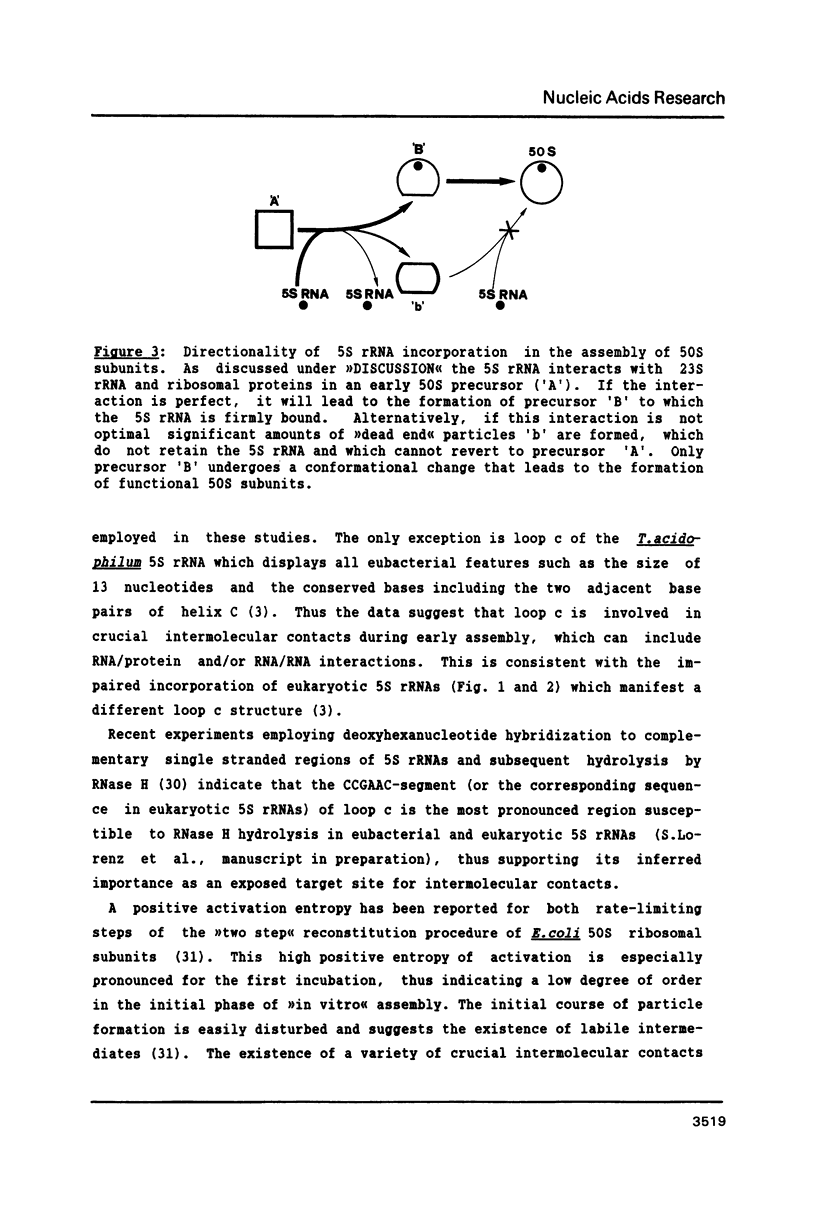
Bacillus stearothermophilus large ribosomal subunits were reconstituted in the presence of 5S rRNAs from different origins and tested for their biological activities. The results obtained have shown that eubacterial and archaebacterial 5S rRNAs can easily substitute for B. stearothermophilus 5S rRNA in the reconstitution, while eukaryotic 5S rRNAs yield ribosomal subunits with reduced biological activities. From our results we propose an interaction between nucleotides 42-47 of 5S rRNA and nucleotides 2603-2608 of 23S rRNA during the assembly of the 50S ribosomal subunit. Other experiments with eukaryotic 5.8S rRNAs reveal, if at all, a very low incorporation of these RNA species into the reconstituted ribosomes.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azad A. A., Deacon N. J. The 3'-terminal primary structure of five eukaryotic 18S rRNAs determined by the direct chemical method of sequencing. The highly conserved sequences include an invariant region complementary to eukaryotic 5S rRNA. Nucleic Acids Res. 1980 Oct 10;8(19):4365–4376. doi: 10.1093/nar/8.19.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. G., Tague B. W., Ware V. C., Gerbi S. A. Xenopus laevis 28S ribosomal RNA: a secondary structure model and its evolutionary and functional implications. Nucleic Acids Res. 1984 Aug 10;12(15):6197–6220. doi: 10.1093/nar/12.15.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wachter R., Chen M. W., Vandenberghe A. Conservation of secondary structure in 5 S ribosomal RNA: a uniform model for eukaryotic, eubacterial, archaebacterial and organelle sequences is energetically favourable. Biochimie. 1982 May;64(5):311–329. doi: 10.1016/s0300-9084(82)80436-7. [DOI] [PubMed] [Google Scholar]
- Delihas N., Andersen J. Generalized structures of the 5S ribosomal RNAs. Nucleic Acids Res. 1982 Nov 25;10(22):7323–7344. doi: 10.1093/nar/10.22.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digweed M., Erdmann V. A., Odom O. W., Hardesty B. Fluorescence modification of Escherichia coli 5S RNA. Nucleic Acids Res. 1981 Jul 10;9(13):3187–3198. doi: 10.1093/nar/9.13.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohme F., Nierhaus K. H. Total reconstitution and assembly of 50 S subunits from Escherichia coli Ribosomes in vitro. J Mol Biol. 1976 Nov 15;107(4):585–599. doi: 10.1016/s0022-2836(76)80085-x. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. E., Doolittle W. F. Complete nucleotide sequence of the 23S rRNA gene of the Cyanobacterium, Anacystis nidulans. Nucleic Acids Res. 1984 Apr 11;12(7):3373–3386. doi: 10.1093/nar/12.7.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Kössel H. The rRNA operon from Zea mays chloroplasts: nucleotide sequence of 23S rDNA and its homology with E.coli 23S rDNA. Nucleic Acids Res. 1981 Jun 25;9(12):2853–2869. doi: 10.1093/nar/9.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Doberer H. G. Structure and function of 5S RNA: the role of the 3' terminus in 5S RNA function. Mol Gen Genet. 1972;114(2):89–94. doi: 10.1007/BF00332779. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A. Structure and function of 5S and 5.8 S RNA. Prog Nucleic Acid Res Mol Biol. 1976;18:45–90. [PubMed] [Google Scholar]
- Georgiev O. I., Nikolaev N., Hadjiolov A. A., Skryabin K. G., Zakharyev V. M., Bayev A. A. The structure of the yeast ribosomal RNA genes. 4. Complete sequence of the 25 S rRNA gene from Saccharomyces cerevisae. Nucleic Acids Res. 1981 Dec 21;9(24):6953–6958. doi: 10.1093/nar/9.24.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. L., Gonzalez I. L., Schmickel R. D. The secondary structure of human 28S rRNA: the structure and evolution of a mosaic rRNA gene. J Mol Evol. 1987;24(3):236–251. doi: 10.1007/BF02111237. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Georgiev O. I., Nosikov V. V., Yavachev L. P. Primary and secondary structure of rat 28 S ribosomal RNA. Nucleic Acids Res. 1984 Apr 25;12(8):3677–3693. doi: 10.1093/nar/12.8.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassouna N., Michot B., Bachellerie J. P. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 1984 Apr 25;12(8):3563–3583. doi: 10.1093/nar/12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Noller H. F. A fragment of 23S RNA containing a nucleotide sequence complementary to a region of 5S RNA. FEBS Lett. 1975 May 1;53(2):248–252. doi: 10.1016/0014-5793(75)80030-5. [DOI] [PubMed] [Google Scholar]
- Kop J., Wheaton V., Gupta R., Woese C. R., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Bacillus stearothermophilus. DNA. 1984 Oct;3(5):347–357. doi: 10.1089/dna.1984.3.347. [DOI] [PubMed] [Google Scholar]
- Londei P., Teixidò J., Acca M., Cammarano P., Amils R. Total reconstitution of active large ribosomal subunits of the thermoacidophilic archaebacterium Sulfolobus solfataricus. Nucleic Acids Res. 1986 Mar 11;14(5):2269–2285. doi: 10.1093/nar/14.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S., Hartmann R. K., Piel N., Ulbrich N., Erdmann V. A. Structural analysis of 5S rRNA, 5S rRNA-protein complexes and ribosomes employing RNase H and d(GTTCGG). Eur J Biochem. 1987 Mar 2;163(2):239–246. doi: 10.1111/j.1432-1033.1987.tb10793.x. [DOI] [PubMed] [Google Scholar]
- Luehrsen K. R., Fox G. E., Kilpatrick M. W., Walker R. T., Domdey H., Krupp G., Gross H. J. The nucleotide sequence of the 5S rRNA from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981 Feb 25;9(4):965–970. doi: 10.1093/nar/9.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus K. H., Dohme F. Total reconstitution of functionally active 50S ribosomal subunits from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4713–4717. doi: 10.1073/pnas.71.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Erdmann V. A. Reconstitution of 50S ribosomal subunits from dissociated molecular components. Nature. 1970 Nov 21;228(5273):744–748. doi: 10.1038/228744a0. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Nomiyama H., Yoshida H., Kukita T., Kuhara S., Sakaki Y. Complete nucleotide sequence of the 26S rRNA gene of Physarum polycephalum: its significance in gene evolution. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3163–3167. doi: 10.1073/pnas.80.11.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace B., Matthews E. A., Johnson K. D., Cantor C. R., Pace N. R. Conserved 5S rRNA complement to tRNA is not required for protein synthesis. Proc Natl Acad Sci U S A. 1982 Jan;79(1):36–40. doi: 10.1073/pnas.79.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieler T., Digweed M., Bartsch M., Erdmann V. A. Comparative structural analysis of cytoplasmic and chloroplastic 5S rRNA from spinach. Nucleic Acids Res. 1983 Feb 11;11(3):591–604. doi: 10.1093/nar/11.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raué H. A., Lorenz S., Erdmann V. A., Planta R. J. Reconstitution of biologically active 50S ribosomal subunits with artificial 5S RNA molecules carrying disturbances in the base pairing within the molecular stalk. Nucleic Acids Res. 1981 Mar 11;9(5):1263–1269. doi: 10.1093/nar/9.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber G., Nierhaus K. H. Kinetic and thermodynamic parameters of the assembly in vitro of the large subunit from Escherichia coli ribosomes. Biochemistry. 1978 Aug 22;17(17):3505–3511. doi: 10.1021/bi00610a013. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Silberklang M., RajBhandary U. L., Lück A., Erdmann V. A. Chemical reactivity of E. coli 5S RNA in situ in the 50S ribosomal subunit. Nucleic Acids Res. 1983 Feb 11;11(3):605–617. doi: 10.1093/nar/11.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studnicka G. M., Eiserling F. A., Lake J. A. A unique secondary folding pattern for 5S RNA corresponds to the lowest energy homologous secondary structure in 17 different prokaryotes. Nucleic Acids Res. 1981 Apr 24;9(8):1885–1904. doi: 10.1093/nar/9.8.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. The complete nucleotide sequence of a 23-S rRNA gene from tobacco chloroplasts. Eur J Biochem. 1982 May;124(1):13–19. doi: 10.1111/j.1432-1033.1982.tb05901.x. [DOI] [PubMed] [Google Scholar]
- Ulbrich N., Wool I. G. Identification by affinity chromatography of the eukaryotic ribosomal proteins that bind to 5 S ribosomal ribonucleic acid. J Biol Chem. 1978 Dec 25;253(24):9049–9052. [PubMed] [Google Scholar]
- Veldman G. M., Klootwijk J., de Regt V. C., Planta R. J., Branlant C., Krol A., Ebel J. P. The primary and secondary structure of yeast 26S rRNA. Nucleic Acids Res. 1981 Dec 21;9(24):6935–6952. doi: 10.1093/nar/9.24.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel D. W., Hartmann R. K., Bartsch M., Subramanian A. R., Kleinow W., O'Brien T. W., Pieler T., Erdmann V. A. Reconstitution of 50 S ribosomal subunits from Bacillus stearothermophilus with 5 S RNA from spinach chloroplasts and low-Mr RNA from mitochondria of Locusta migratoria and bovine liver. FEBS Lett. 1984 Apr 9;169(1):67–72. doi: 10.1016/0014-5793(84)80291-4. [DOI] [PubMed] [Google Scholar]
- Willick G. E., Nazar R. N., Matheson A. T. 5S RNA-protein complex from an extreme halophile, Halobacterium cutirubrum. Comparative studies on reconstituted complexes. Biochemistry. 1979 Jun 26;18(13):2855–2859. doi: 10.1021/bi00580a028. [DOI] [PubMed] [Google Scholar]
- Wrede P., Erdmann V. A. Escherichia coli 5S RNA binding proteins L18 and L25 interact with 5.8S RNA but not with 5S RNA from yeast ribosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2706–2709. doi: 10.1073/pnas.74.7.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska L., Van Duin J., Noller H. F., Pace B., Johnson K. D., Pace N. R. The conserved 5 S rRNA complement to tRNA is not required for translation of natural mRNA. J Biol Chem. 1984 Mar 10;259(5):2798–2802. [PubMed] [Google Scholar]


