Abstract
Dietary fish oil containing ω3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), elicit cardioprotective and anti-inflammatory effects through unresolved mechanisms that may involve competition and inhibition at multiple levels. Here, we report the effects of arachidonic acid (AA), EPA, and DHA supplementation on membrane incorporation, phospholipase A2 catalyzed release, and eicosanoid production in RAW264.7 macrophages. Using a targeted lipidomics approach, we observed that Toll-like receptor 4 and purinergic receptor activation of supplemented cells leads to the release of 22-carbon fatty acids that potently inhibit cyclooxygenase pathways. This inhibition was able to shunt metabolism of AA to lipoxygenase pathways, augmenting leukotriene and other lipoxygenase mediator synthesis. In resident peritoneal macrophages, docosapentaenoic acid (DPA) was responsible for cyclooxygenase inhibition after EPA supplementation, offering fresh insights into how EPA exerts anti-inflammatory effects indirectly through elongation to 22-carbon DPA.
A mechanistic understanding of the therapeutic benefits associated with fish oil ω3 fatty acid supplementation has been sought since the 1950s, when cod liver oil was found to be beneficial in treating eczema, hypercholesterolemia, and arthritis. In the 1980s, epidemiological studies showed that Greenland Eskimos have lower mortality rates than mainland Danes, which correlated with an increased intake of polyunsaturated fatty acids (PUFA) vs. saturated fatty acids, as well as higher ω3 PUFAs vs. ω6 PUFAs (1). This result was attributed to the Eskimo diet, which largely consists of seal and fish from marine sources rather than food from mainland sources. Subsequent human trials showed that ω3 PUFA supplementation resulted in lower mortality rates and lower incidence of major coronary events in subjects with heart disease (2).
Further studies have established that ω3 PUFA supplementation can decrease the ratio of ω6 arachidonic acid (AA)/ω3 PUFAs in membrane phospholipids (3, 4), where AA is the most common highly unsaturated fatty acid in North Americans and Western Europeans. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are widely accepted as the key ω3 PUFAs that elicit therapeutic effects because they are found in fish oil and can additionally be formed in humans via elongation of α-linolenic acid, the essential ω3 fatty acid. It is now widely accepted that fish oil diets are cardioprotective, as well as anti-inflammatory and anticarcinogenic.
There is great current interest and focus on elucidating the metabolic changes that result from increased ω3 PUFAs. AA is a C20:4 fatty acid that is mostly esterified at the sn-2 position of membrane phospholipids in resting cells, but can be released by phospholipase A2 (PLA2) (5, 6) after various stimuli. As a free fatty acid, AA can be oxygenated by cyclooxygenases (COX), lipoxygenases (LOX), and cytrochrome P450 enzymes, and further by a host of downstream enzymes in each pathway to form a wide spectrum of unique lipid mediators (7), generally referred to as eicosanoids. Many of these metabolites are bioactive and can signal through their own natural receptors to evoke a wide variety of physiological changes. Eicosanoids play major roles in initiating the innate immune response and promoting the resolution of inflammation.
Biochemical characterizations of EPA and DHA have generally suggested that these fatty acids are less prone to metabolism by eicosanoid pathway enzymes, and that ω3 eicosanoids generally elicit lower activation responses of receptors than AA eicosanoids (8). In addition, their increased presence through supplementation presents the potential to compete for, and inhibit AA eicosanoid production and receptor stimulation, particularly in the COX pathway (8, 9). Although DHA is generally considered to be cardioprotective after membrane incorporation via mechanisms that improve receptor and ion channel function, EPA can also be metabolized to resolvin E1, which has recently been demonstrated to have potent cardioprotective action (10).
Herein, we report a complete lipidomic characterization of the effects of AA, EPA, and DHA supplementation on membrane phospholipid PUFA composition and subsequent PUFA release and eicosanoid production in RAW264.7 macrophages after short- (purinergic) and long-term (Toll-like receptor 4, TLR4) inflammatory stimulations. Using a combination of GC-MS and LC-MS/MS analysis (11), we have been able to accurately quantify PUFA phospholipid incorporation and release and relate this to the quantities and identities of nearly all AA-derived eicosanoids and key EPA- and DHA-derived eicosanoids produced.
Unexpectedly, we found that both supplementation and elongation dramatically affected the quantity and specificity of eicosanoid production. We were able to demonstrate that both ω3- and ω6-supplemented 22-carbon fatty acids have differential inhibitory properties on COX-1 and COX-2 metabolism that increased with additional double bonds, and that docosapentaenoic acid (DPA), which is elongated from EPA, is likely a major source of inhibition.
Results
Effects of PUFA Supplementation on Membrane Composition.
Fatty acid analysis of cell membranes after saponification was carried out to determine the total levels of AA, EPA, and DHA incorporated after 24 h of supplementation. We had previously observed the formation of adrenic acid (AdA) as well as dihomo-prostaglandins derived from AdA after supplementing RAW cells with AA (12); thus, we also monitored possible 2-carbon elongation products of AA, EPA, and DHA using GC/MS methodology.
AA supplementation increased AA in membranes nearly twofold, and increased AdA ∼fourfold relative to control (nonsupplemented) cells, as shown in Fig. 1A. EPA supplementation resulted in a roughly 10-fold increase in membrane EPA relative to control, and a roughly threefold increase in DPA. DHA supplementation increased membrane DHA levels nearly fourfold relative to control and elevated 24:6 (THA) levels (Fig. 1B) by more than fivefold. The overall profiles of AA, EPA, and DHA in phospholipids of control, EPA-, and DHA-supplemented cells closely resembled those of human serum phospholipids before and after EPA and DHA supplementation (4).
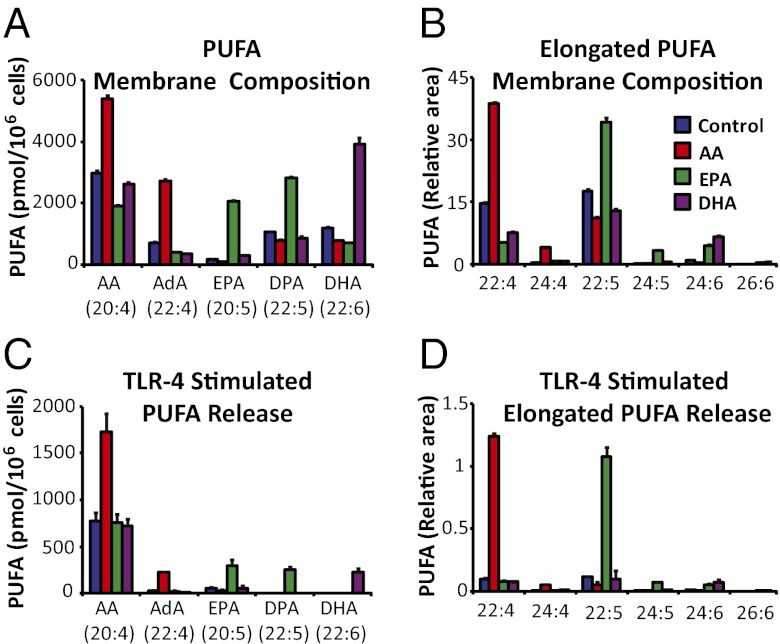
Fig. 1.
Supplemented PUFAs are rapidly elongated, robustly incorporated into membranes, and released by PLA2. RAW cells were incubated for 24 h in nonsupplemented media, or with 25 μM AA, EPA, or DHA. (A and B) The supplemented PUFAs were increased in membrane phospholipids in both their original forms and in their elongated forms. (C and D) After 2-h KLA (100 ng/mL) stimulation, supplemented and elongated PUFA species were increased in the media. Values in B and D represent area of the indicated fatty acid normalized to a deuterated DHA internal standard. PUFAs were analyzed with GC-MS and data are mean values of three biological replicates ± SEM.
In AA-supplemented cells, membrane-incorporated AA significantly increased and, although somewhat unexpectedly, AdA increased by about the same amount as AA, likely via rapid 2-carbon elongation of AA (Fig. 1A). EPA was similarly elongated to DPA in EPA-supplemented cells. DHA appears to be elongated after supplementation (Fig. 1B) but was present at much lower levels than AdA and DPA. AA and EPA were further elongated to 24-carbon PUFAs, but only to a small extent; thus, elongation to 22-carbons was essentially the limit.
Analysis of cell membrane composition established that supplemented ω3 PUFAs and their elongated analogs could be incorporated into membrane phospholipids, presumably at the sn-2 position, at a significant level consistent with traditional phospholipid remodeling (13). The compositional analysis above suggests that the increased quantity of AA in AA-enriched cells is roughly equal to half of the increased amount of EPA + DPA in EPA-enriched cells, and ∼equal to that of DHA in DHA-enriched cells. In control cells, AA is at a ∼10:1 excess over EPA and ∼3:1 excess over DHA. Interestingly, AA supplementation caused a reduction in the AA/AdA ratio from 5:1 in control cells to 2:1.
Analysis of PUFA Release After Supplementation.
AA-, EPA-, or DHA-supplemented RAW cells were stimulated for 2 h with the TLR4 agonist Kdo2-Lipid A (KLA) and the media were analyzed by GC-MS to determine if the dramatic elevations in membrane PUFAs would lead to significantly higher release by Group IVA cytosolic PLA2 (cPLA2), and if there is a competition for the fatty acids at the sn-2 position of the membrane phospholipids.
All of the supplemented and further elongated PUFAs increased in membrane phospholipids were also significantly increased in media with KLA stimulation (Fig. 1C). Further elongated PUFAs (24:4, 24:5, and 24:6) that increased in phospholipids also increased in media, but were quantitatively insignificant (Fig. 1D).
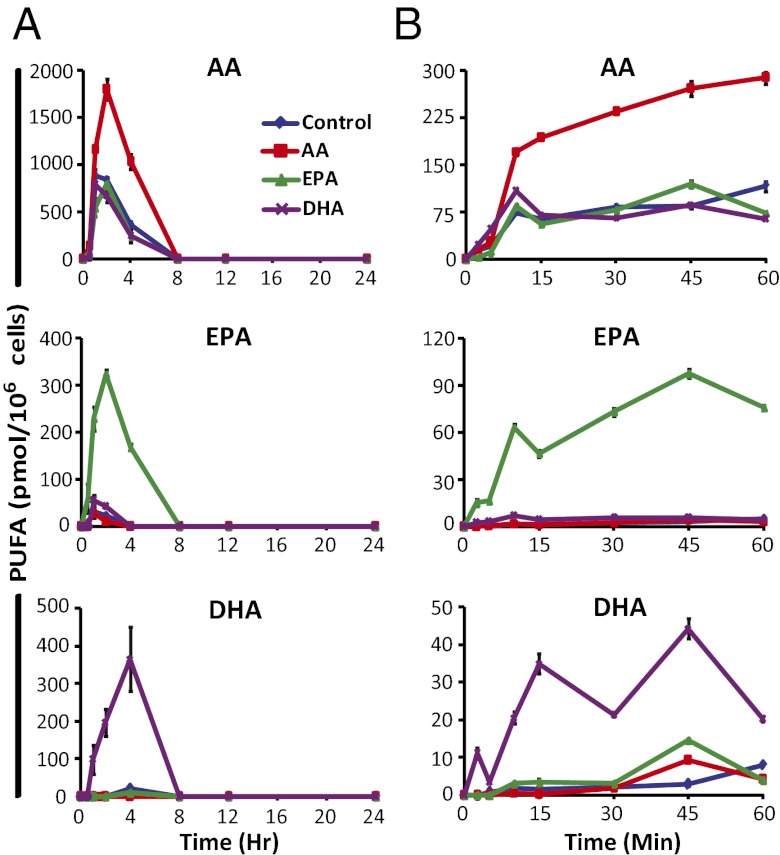
Supplemented cells were subjected to TLR4 stimulation with KLA over 24 h and to purinergic stimulation with 2 mM ATP over 1 h. PUFAs and eicosanoids in cell media were analyzed by LC-MS/MS. With TLR4 stimulation, supplemented PUFAs were increased significantly relative to control and peaked by 2 h with AA and EPA, and by 4 h with DHA supplementation (Fig. 2A). Without stimulation, only EPA and DHA were significantly released from supplemented cells, likely through turnover, indicating that cPLA2 mostly released AA with TLR4 stimulation (Fig. S1A). However, stimulation caused EPA and DHA to more rapidly diminish from the media than without stimulation, which likely resulted from more rapid reincorporation because of stimulation and dramatic release of AA. Most importantly, TLR4 stimulation resulted in peak PUFA release before the majority of eicosanoids are formed in the delayed phase; perhaps other PLA2s, such as sPLA2, play a role in this stimulation (see Discussion).
Fig. 2.
Supplementation increases release of specific PUFA species. RAW cells were incubated for 24 h in nonsupplemented media, or with 25 μM AA, EPA, or DHA before stimulation with (A) KLA (100 ng/mL) for the indicated times over 24 h, or (B) 2 mM ATP for the indicated times over 60 min. PUFAs were analyzed by LC-MS/MS; 0 h values were subtracted from each time point and data are mean values of three biological replicates ± SEM.
ATP activates P2X receptor cation channels, producing sustained increases in intracellular Ca2+ leading to translocation of cPLA2 to cell membranes. Of all purinergic receptors (P2X and P2Y), only P2X7 requires 2 mM ATP for activation (14, 15). This receptor is expressed and functional in RAW cells, and is reported to be the receptor responsible for the majority of AA release after millimolar ATP stimulation in murine macrophages (16). PUFA release and eicosanoid production in this setting is rapid and takes 10–30 min for product formation to peak, but TLR4 activation requires several hours for COX-2 induction and subsequent eicosanoid production.
After purinergic stimulation, supplemented PUFAs were again significantly increased relative to control (Fig. 2B). Without stimulation, minimal release of supplemented PUFAs was observed (Fig. S1B), indicating release of AA, EPA, and DHA was dependent on P2X7 stimulation. We then incubated DHA-enriched cells in serum-free media (to minimize turnover) and then subjected them to TLR4 or P2X7 stimulation, or both (Fig. S2), and found that cPLA2 hydrolysis of DHA was dependent on P2X7, but not TLR4 stimulation. Interestingly, TLR4 priming and subsequent stimulation of P2X7 led to synergistic release of DHA, which we have previously demonstrated with AA (17).
Effects of PUFA Supplementation on TLR4-Stimulated Eicosanoid Metabolism.
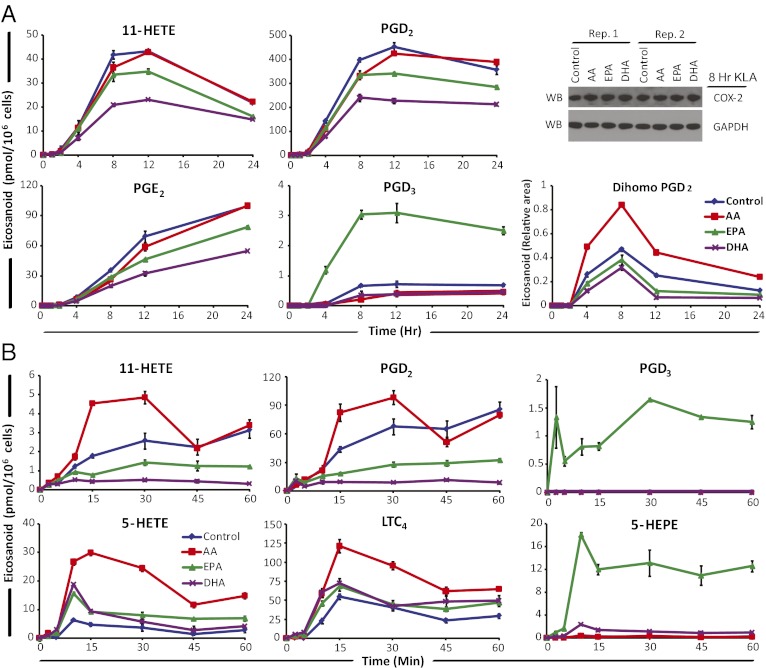
Using KLA stimulation, we previously observed up-regulation of COX-2 and microsomal prostaglandin E2 synthase-1 (mPGES-1) in RAW cells, as well as production of ∼10-fold higher levels of PGD2 vs. PGE2, which was proportional to the mRNA levels of mPGES-1 and hematopoietic prostaglandin D synthase (H-PGDS) (18). Because TLR4 stimulation does not lead to changes in intracellular Ca2+ levels, 5-LOX is not activated, thus 5-hydroxyeicosatetraenoic acid (HETE) and leukotriene production in RAW cells have not been observed (17, 18). We also have not detected prostaglandin I2 synthase (PGIS) mRNA or the breakdown product of PGI2 (6-keto-PGF1a), and virtually no thromboxane synthase (TBXAS) mRNA or the breakdown product of TxA2 (TxB2) (18). The effects of PUFA supplementation were also assessed by monitoring the COX side product 11-HETE as an indicator of COX activity (17, 18).
AA supplemented vs. control cells yielded little difference in eicosanoid levels throughout the 24-h time course (Fig. S3A). Interestingly, this was true for AA COX-derived PGE2, PGD2, and 11-HETE, which were actually ∼10% lower in AA-supplemented cells vs. control cells (Fig. 3A). We reasoned that the slightly lower level of AA-derived prostanoids was a result of the increased release of AdA in AA-supplemented cells. AdA-derived dihomo-prostaglandins were in fact the only eicosanoids increased in the COX pathway. The level of dihomo-PGs based on dihomo-PGF2a (the only available quantitative standard) was less than 1% of total AA-derived prostanoids (Fig. S4A).
Fig. 3.
Effects of PUFA supplementation on TLR4 and purinergic stimulated eicosanoid production. RAW cells were incubated for 24 h in nonsupplemented media, or with 25 μM AA, EPA, or DHA before stimulation with (A) KLA (100 ng/mL) for the indicated times over 24 h, or (B) 2 mM ATP for the indicated times over 60 min; 0 h values were subtracted from each time point. Eicosanoids were analyzed by LC-MS/MS from the same experiment shown in Fig. 2, and data are mean values of three biological replicates ± SEM. Western blot of COX-2 protein after 8-h KLA stimulation in biological duplicate (A, Upper Right); dihomo-PGD2 is expressed as area relative to deuterated PGD2 internal standard (A, Lower Right).
EPA-supplemented cells produced lower levels of AA-derived COX metabolites and higher levels of EPA-derived COX metabolites PGD3 and PGE3 (Fig. S3A). AA COX metabolites were reduced ∼20% (Fig. 3A). PGD3 was the predominant EPA-derived metabolite produced in EPA-supplemented cells and was about threefold higher than in control cells; however, EPA COX metabolites were 100-fold lower than AA prostanoids. H-PGDS and mPGES-1 have about 17% and 30% activity with PGH3 vs. PGH2 (8, 19–22), respectively.
DHA supplementation overall reduced AA-derived COX metabolites to a greater extent than EPA-supplemented cells (Fig. S3A) and were ∼40% lower than in control cells (Fig. 3A). The DHA-derived COX side product, 13-hydroxy DHA (HDoHE), was ∼40% higher than in control cells (Fig. S4B); however, the absolute level was ∼100-fold lower than 11-HETE; thus, PGH4 is likely produced at a very low level compared with PGH2. Furthermore, COX-2 expression was not reduced in any supplemented cells relative to control (Fig. 3A, Upper Right).
Effects of PUFA Supplementation on Purinergic-Stimulated Eicosanoid Metabolism.
We have previously observed eicosanoid production in RAW cells after P2X7 stimulation and have identified increases in COX and 5-LOX pathway eicosanoids (17). In this intracellular Ca2+ mobilizing condition, 5-LOX is translocated to the membrane and can act on released PUFAs with the assistance of 5-LOX activating protein (23), concomitantly with constitutive COX-1. In this scenario, the effects of PUFA supplementation on multiple, simultaneously active eicosanoid pathways can be observed.
Eicosanoid production in AA supplemented cells differed substantially from that of control cells after purinergic stimulation (Fig. S3B). AA prostanoids slightly increased, AdA dihomo-PGs increased to a greater extent, and 5-LOX metabolites also increased relative to control. Prostanoid production was nearly twofold higher than in control cells from 15 to 30 min (Fig. 3B). This result shows that COX-1 is not rate-limiting with purinergic stimulation, unlike COX-2 in long-term TLR4 stimulation. Dihomo-PGD2 was the only AdA prostanoid detected in all four cell conditions and was at least threefold higher than in control (Fig. S5A). Relative to PGD2, dihomo-PGD2 was 100-fold lower in abundance (Fig. S5 A and B), thus COX-1 AdA metabolism was minimal.
In the 5-LOX pathway, 5-HETE and leukotriene A4 (LTA4) are formed before further metabolism by downstream enzymes. Although LTA4 was not monitored because of rapid nonenzymatic breakdown or enzymatic conversion, LTC4 and 5-HETE were robustly increased in AA supplemented cells to ∼fivefold higher levels than in control cells (Fig. 3B). Overall, 5-LOX AA metabolites increased more than COX metabolites with purinergic stimulation.
EPA supplementation decreased AA-derived COX metabolite production vs. control cells with purinergic stimulation (Fig. S3B), but EPA metabolites, PGD3 and PGE3, were increased over fourfold vs. control cells. COX metabolites were ∼50% lower than in control cells (Fig. 3B), and PGD3 was ∼30-fold lower than PGD2. 5-LOX AA metabolites were increased at a level proportional to the decreased COX-1 metabolites, suggesting a shunting effect caused by selective inhibition by EPA. The EPA version of 5-HETE, 5-HEPE, was dramatically increased in EPA-supplemented cells and was produced at a comparable level to 5-HETE.
DHA supplementation decreased AA prostanoid levels vs. control to a greater extent than EPA supplementation and also increased 5-LOX metabolite production (Fig. S3B). COX-dependent AA metabolites were reduced by ∼90% relative to control cells (Fig. 3B) and 13-HDoHE was not detected, suggesting that DHA was not significantly metabolized by COX-1 but greatly inhibited AA metabolism. 5-HETE and LTC4 were increased to similar levels as in EPA-supplemented cells. DHA can be converted by 5-LOX to 4-HDoHE and 7-HDoHE. These metabolites were only detected in DHA-supplemented cells (Fig. S5 C and D), and at a combined level ∼fourfold lower than 5-HETE in control cells (Fig. 3B). This finding suggests DHA can be metabolized by 5-LOX to a much greater extent than by COX-1 or COX-2 because DHA release was ∼twofold lower than AA release with DHA supplementation (Fig. 2B). Importantly, standards for the cysteinyl leukotrienes that would be formed from EPA, DHA, DPA, and AdA are not presently available, so they cannot be quantified; thus, the full extent of metabolism of these substrates by the 5-LOX pathway cannot be ascertained.
Effects of 22-Carbon Fatty Acid Supplementation on Eicosanoid Metabolism.
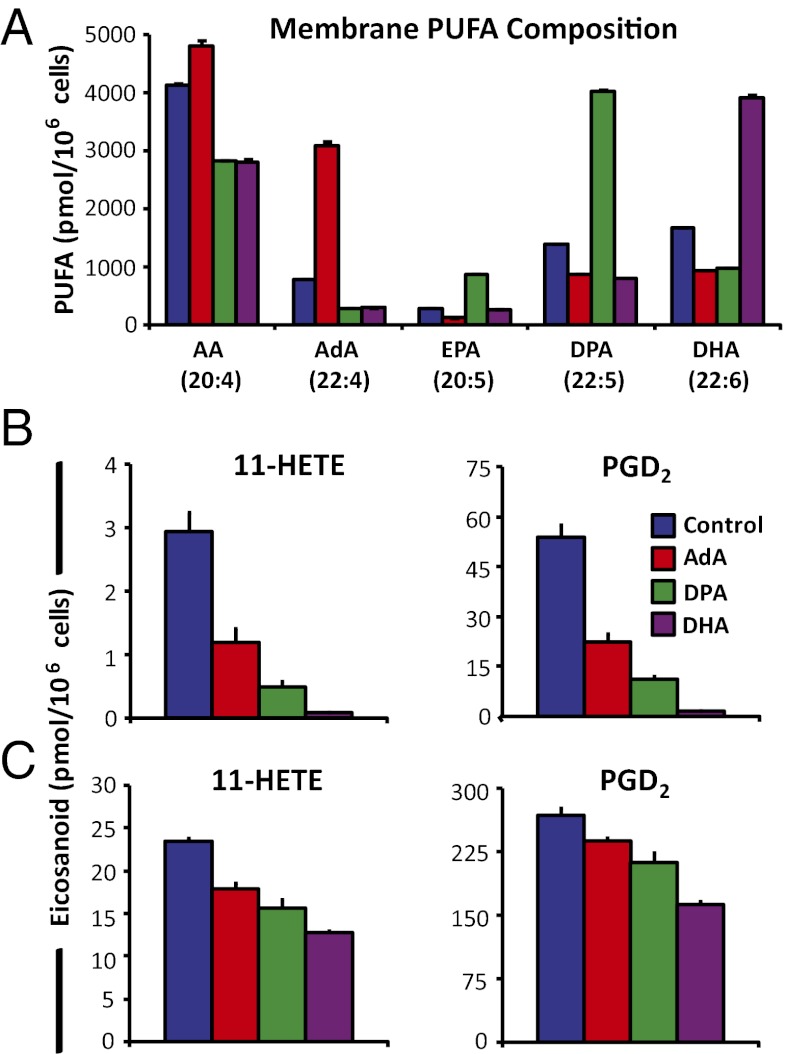
RAW cells were supplemented with 25 μM AdA, DPA, or DHA to compare the degree of inhibition of COX AA metabolism after purinergic and TLR4 stimulation. Similar increases in each PUFA were observed in phospholipids with only minor conversion to 20-carbon PUFAs (Fig. 4A). This process allowed us to more clearly compare the inhibitory effects of 22-carbon PUFAs that were obscured in the previous experiments.
Fig. 4.
22-carbon PUFAs decrease COX-1 and COX-2 eicosanoid production to varying degrees. RAW cells were incubated for 24 h in nonsupplemented media, or with 25 μM AdA, DPA, or DHA and (A) were increased in membrane phospholipids. 11-HETE and PGD2 were decreased relative to control after (B) 15 min stimulation with 2 mM ATP or (C) 100 ng/mL KLA for 6 h. Data are mean values of three biological replicates ± SEM.
After 15 min of ATP stimulation, 11-HETE and PGD2 levels measured from the media were decreased by ∼60%, 80%, and 90% with AdA, DPA, and DHA supplementation vs. control cells, respectively (Fig. 4B). After 6-h KLA stimulation, levels of 11-HETE and PGD2 were reduced by ∼20%, 30%, and 40% with AdA, DPA, and DHA supplementation, respectively (Fig. 4C). Thus, AA COX inhibition increased as the number of double bonds of the supplemented 22-carbon PUFA increased.
We then determined that supplementation with 10 vs. 25 μM EPA led to similar increases in EPA and DPA in phospholipids, inhibition of P2X7-stimulated AA prostanoids, and increased AA 5-LOX metabolites (Fig. S6). Nonesterified levels of ω3 PUFAs as high as 12.5 μM have been observed in human serum after 1.5 g/d supplementation (24). Finally, when murine resident peritoneal macrophages (RPM) were supplemented with 10 μM EPA, ∼75% of the increased EPA in phospholipids was in the elongated form, DPA. Ultimately, DPA was the only PUFA released at a comparable level to AA for competition after P2X7 stimulation, and COX AA eicosanoids were reduced after P2X7 and TLR4 stimulation similar to RAW cells (Fig. 3 and Fig. S7). The overall summary of our findings on PUFA supplementation effects is illustrated in Fig. 5.
Fig. 5.
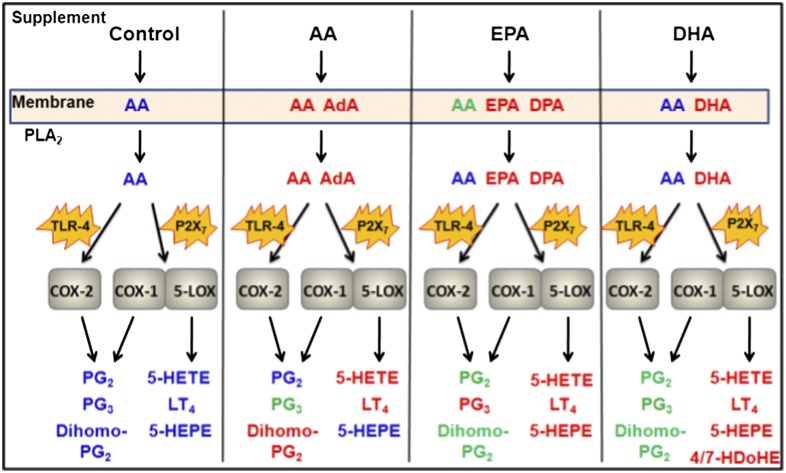
Global effects of PUFA supplementation on cellular eicosanoid metabolism. RAW cells were incubated without supplemental PUFAs (Control), or with 25 μM AA, EPA, or DHA. Subsequent increases (relative to control) of PUFAs in membrane phospholipids, released fatty acids, and eicosanoids after TLR4 (100 ng/mL KLA) or P2X7 (2 mM ATP) receptor stimulation are indicated in red; unchanged PUFA/eicosanoid levels relative to control in blue; decreases in green. Presence of PUFA species in addition to AA in phospholipids indicate levels higher than 50% of control AA levels; additional released PUFA species by PLA2 indicate levels were significantly higher than in controls and were within an order of magnitude of the control level of AA released; eicosanoid species in red and green indicate sustained increases or decreases of at least 20% relative to control (in the majority of time points). Abbreviations: LT4, 4-series leukotrienes; 4/7-HDoHE = 4-hydroxy DHA and 7-hydroxy DHA; PG2, 2-series prostaglandins; PG3, 3-series prostaglandins.
Discussion
Fatty Acid Elongation and Membrane Incorporation.
Free fatty acids are toxic to cells at high levels and are normally esterified within phospholipids. Turnover and incorporation of PUFAs in resting macrophages has been shown to be highly dependent on the constitutively expressed Ca2+-independent iPLA2 by hydrolyzing fatty acids at the sn-2 position of phospholipids to generate lysophospholipids, allowing PUFA esterification by a CoA-dependent acyltransferase (25). Supplementation with AA, EPA, and DHA led to dramatic elevations in the levels of the respective PUFAs incorporated in phospholipids, and the levels of other PUFAs in each condition were nonspecifically lowered as much as ∼50% relative to control nonsupplemented cells (Fig. 1 A and B).
We anticipated that PUFA supplementation would increase levels of elongated PUFAs in phospholipids based on the observation by Rouzer et al. (26), along with our previous finding that AA supplementation results in de novo elongation of AA to AdA and subsequent dihomo-prostaglandin formation (12), but were surprised that about half of the AA and EPA were elongated. At least part of the elongation of AA and EPA in RAW cells is presumably attributed to elongase of very long-chain fatty acids (ELOVL)2 because this enzyme elongates saturated and unsaturated fatty acids between 18 and 22 carbons, and because ELOVL5 does not appear to elongate the 22-carbon fatty acids (27, 28) that we observed in Fig. 1B. Elongation in other cell types, including other macrophages, as we observed with RPM, likely varies and is important to consider during supplementation studies.
PLA2 Activity on Different Membrane PUFA Compositions.
With P2X7 stimulation, cPLA2 is translocated to the membrane in a Ca2+-dependent manor, where AA and EPA appear to be equally good substrates, and DHA is essentially not released after activation (29, 30). Our results with purinergic stimulation were consistent with these observations, although DHA release was significant when dramatically increased in phospholipids, as was DPA.
With long-term TLR4 stimulation, we observed overall higher levels of PUFA release (Fig. 2A) than with P2X7 stimulation (Fig. 2B). In this setting, cPLA2 is translocated to the membrane in a Ca2+-independent fashion by increased membrane affinity and enhanced specific activity that has been linked to generation of PIP2 and conformational changes (5, 31–33), as well as generation of C1P (34), ceramide (35), and diacylglycerol (36). Additionally, sPLA2 is reported to significantly contribute to prostaglandin production during late-phase activation, but not during 10-min calcium ionophore-induced metabolism (37). Our results suggest that non-AA PUFAs are not significantly released by cPLA2 activated via TLR4, but can affect delayed-phase eicosanoid production possibly through hydrolysis by sPLA2, which has much less PUFA specificity.
Inhibition of COX-1 and COX-2.
In numerous cell types, COX-2 is up-regulated after stimulation by IL-1, TNF-α, LPS, and other inflammatory stimuli. We have previously observed that various macrophage phenotypes induce COX-2 to different levels, dictating the amount of total COX metabolites produced in late-phase TLR4 stimulation (18). Here, we found that increased AA release after AA supplementation does not increase COX metabolite levels during TLR4 stimulation as further evidence that COX-2 is rate-limiting in macrophages (Figs. 2A and 3A).
EPA has been shown in vitro to inhibit COX-1 more than COX-2 (8). However, DPA was the predominant PUFA increased in RPM cells (Fig. S7 A and B) after EPA supplementation and appears to be the primary inhibiting PUFA. In fact, DPA is reported to be a more potent COX-1 inhibitor than EPA (38). DPA-derived, resolvin-like anti-inflammatory molecules have also been recently identified (39).
DHA is known to be a potent inhibitor of AA prostaglandin production (9). Here, we were able to confirm this in immune cells during acute inflammatory activations, and show that DHA is a more effective COX inhibitor than other 22-carbon PUFAs when supplemented.
Greater inhibition of COX-1 vs. COX-2 with ω3 PUFA supplementation is consistent with decreased platelet aggregation and longer bleeding times in Eskimos and other groups on high marine-fish diets (9, 40). Our data suggest that these effects may be because of highly decreased COX-1–derived TxA2 and PGH2 by platelets, but endothelial PGI2 coupled with COX-2 would be inhibited to a significantly lesser extent to shift the vascular eicosanoid balance toward antiplatelet aggregation. The levels of ω3 prostanoids generated in our study paled in comparison with even the inhibited levels of AA after supplementation, meaning prostanoid receptor competition with ω3 metabolites would be minimal.
AA, EPA, and DHA Supplementation Shift AA Metabolism to Higher 5-LOX vs. COX-1.
We have previously observed a preferential increase in 5-LOX vs. COX-1 AA metabolites when RAW cells were primed with TLR4 stimulation and subsequent P2X7 stimulation that was independent of protein synthesis (17). In this same study, AA was synergistically increased along with AA 5-LOX metabolites but not COX-1 metabolites. Here, we further probed these pathways using supplementation and confirmed that increased AA release (Fig. 2B) more significantly enhances 5-LOX metabolism (Fig. 3B), even without priming. In addition, DHA can be synergistically activated (Fig. S2), thus the dual activation of different binding modes of cPLA2 appears to increase activity without great specificity.
In contrast to the inhibitory nature of DHA toward COX AA activity, 5-LOX AA metabolism was previously found to be minimally inhibited by DHA using enzyme derived from RBL-1 cells (9). This presents a potential shunting scenario which we were able to observe. Interestingly AA, EPA, and DHA supplementations can boost 5-LOX/COX-1 AA metabolite ratios using different mechanisms. In activated macrophages, there is a significant capacity for metabolism of EPA and DHA through 5-LOX, which helps to explain how resolvins can form in vivo (41). In theory, these effects by ω3 PUFAs would not reduce LTB4, allowing sufficient neutrophil recruitment and potential generation of proresolving lipoxins and resolvins through LOX pathways. However, fish oil supplementation should be carefully considered with additional COX inhibitor therapies because this shunting effect could augment production of the cysteinyl leukotrienes. Ultimately, fish oil could exacerbate aspirin-induced asthma.
Importance of Considering DHA and DPA in Understanding ω3 Metabolism.
AA and EPA are indeed hydrolyzed by cPLA2 preferentially vs. DHA, although DHA can still be significantly released. Other 22-carbon PUFAs can also be significantly released and can dramatically affect eicosanoid signaling. This finding is important because DHA was previously assumed to be minimally released, downplaying its significance, so that EPA has been more thoroughly examined in vitro (8). Finally, we demonstrated that even without supplementation of AdA and DPA, these 22-carbon PUFAs can be significantly increased in membrane phospholipids with AA and EPA supplementation via rapid elongation, and can lead to COX inhibition. EPA and DHA are the two common PUFAs available as therapeutic supplements, although DPA may be partly responsible for anti-inflammatory effects that have likely been overlooked. Considering that 5-LOX is synergistically activated via priming and can metabolize ω3 and ω6 PUFAs to similar extents, discovery of DPA-derived metabolites formed via 5-LOX pathways constitutes an important additional consideration in understanding ω3 PUFA metabolism and fish oil supplementation.
Materials and Methods
RAW264.7 cells were cultured in DMEM media supplemented with 10% FBS and 100 units/mL penicillin/streptomycin at 37 °C in a humidified 5% CO2 atmosphere. Cells were plated in six-well culture plates with 2.0 mL of media with 10% (vol/vol) FBS (5 × 105 cells per well) and allowed to adhere for 24 h in the presence of 25 μM AA, EPA, DHA, AdA, DPA, or no supplement (control). Media were aspirated and cells were washed twice with media, and 1.0 mL of media was added to each well and incubated for 1 h. Then, 1.0 mL of 2× KLA (200 ng/mL) or ATP (4 mM) medium was added, bringing the total volume to 2.0 mL and 1× concentration of stimulant. Media during stimulation contained 10% FBS for the 24-h KLA time course and were serum-free for the 1 h ATP time course. Detailed protocols for eicosanoid, PUFA, and COX-2 protein analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Darren Dumlao for advice on mass spectrometry and eicosanoid analysis, and Drs. Christopher Glass and Nathanael Spann for supplying mice for animal studies. This work was supported by the Lipid Metabolites and Pathways Strategy (LIPID MAPS) Large Scale Collaborative Grant U54 GM069338 and Grant R01 GM64611 from the National Institutes of Health; and University of California at San Diego Graduate Training Program in Cellular and Molecular Pharmacology National Institutes of Health Grant T32 GM007752 (to P.C.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200189109/-/DCSupplemental.
References
- 1.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 2.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 3.Anti M, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- 4.Huang YC, et al. n-3 fatty acids decrease colonic epithelial cell proliferation in high-risk bowel mucosa. Lipids. 1996;31(Suppl):S313–S317. doi: 10.1007/BF02637099. [DOI] [PubMed] [Google Scholar]
- 5.Six DA, Dennis EA. Essential Ca(2+)-independent role of the group IVA cytosolic phospholipase A(2) C2 domain for interfacial activity. J Biol Chem. 2003;278:23842–23850. doi: 10.1074/jbc.M301386200. [DOI] [PubMed] [Google Scholar]
- 6.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada M, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 9.Corey EJ, Shih C, Cashman JR. Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc Natl Acad Sci USA. 1983;80:3581–3584. doi: 10.1073/pnas.80.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keyes KT, et al. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 11.Harkewicz R, Dennis EA. Applications of mass spectrometry to lipids and membranes. Annu Rev Biochem. 2011;80:301–325. doi: 10.1146/annurev-biochem-060409-092612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harkewicz R, Fahy E, Andreyev A, Dennis EA. Arachidonate-derived dihomoprostaglandin production observed in endotoxin-stimulated macrophage-like cells. J Biol Chem. 2007;282:2899–2910. doi: 10.1074/jbc.M610067200. [DOI] [PubMed] [Google Scholar]
- 13.Lands WE. Stories about acyl chains. Biochim Biophys Acta. 2000;1483:1–14. doi: 10.1016/s1388-1981(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.Di Virgilio F, et al. Nucleotide receptors: An emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 15.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 16.Balboa MA, Balsinde J, Johnson CA, Dennis EA. Regulation of arachidonic acid mobilization in lipopolysaccharide-activated P388D(1) macrophages by adenosine triphosphate. J Biol Chem. 1999;274:36764–36768. doi: 10.1074/jbc.274.51.36764. [DOI] [PubMed] [Google Scholar]
- 17.Buczynski MW, et al. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–22847. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 18.Norris PC, Reichart D, Dumlao DS, Glass CK, Dennis EA. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. J Leukoc Biol. 2011;90:563–574. doi: 10.1189/jlb.0311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinzar E, Miyano M, Kanaoka Y, Urade Y, Hayaishi O. Structural basis of hematopoietic prostaglandin D synthase activity elucidated by site-directed mutagenesis. J Biol Chem. 2000;275:31239–31244. doi: 10.1074/jbc.M000750200. [DOI] [PubMed] [Google Scholar]
- 20.Kanaoka Y, Urade Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fatty Acids. 2003;69:163–167. doi: 10.1016/s0952-3278(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 21.Thorén S, et al. Human microsomal prostaglandin E synthase-1: Purification, functional characterization, and projection structure determination. J Biol Chem. 2003;278:22199–22209. doi: 10.1074/jbc.M303227200. [DOI] [PubMed] [Google Scholar]
- 22.Ouellet M, et al. Purification and characterization of recombinant microsomal prostaglandin E synthase-1. Protein Expr Purif. 2002;26:489–495. doi: 10.1016/s1046-5928(02)00566-1. [DOI] [PubMed] [Google Scholar]
- 23.Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 24.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 25.Balsinde J, Bianco ID, Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc Natl Acad Sci USA. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouzer CA, et al. Lipid profiling reveals arachidonate deficiency in RAW264.7 cells: Structural and functional implications. Biochemistry. 2006;45:14795–14808. doi: 10.1021/bi061723j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J Lipid Res. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard AE, et al. Identification and expression of mammalian long-chain PUFA elongation enzymes. Lipids. 2002;37:733–740. doi: 10.1007/s11745-002-0955-6. [DOI] [PubMed] [Google Scholar]
- 29.Shikano M, et al. Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim Biophys Acta. 1994;1212:211–216. doi: 10.1016/0005-2760(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 30.Kramer RM, Sharp JD. Structure, function and regulation of Ca2+-sensitive cytosolic phospholipase A2 (cPLA2) FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- 31.Mosior M, Six DA, Dennis EA. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J Biol Chem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- 32.Grkovich A, Johnson CA, Buczynski MW, Dennis EA. Lipopolysaccharide-induced cyclooxygenase-2 expression in human U937 macrophages is phosphatidic acid phosphohydrolase-1-dependent. J Biol Chem. 2006;281:32978–32987. doi: 10.1074/jbc.M605935200. [DOI] [PubMed] [Google Scholar]
- 33.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H, Hirabayashi T, Shimizu M, Murayama T. Ceramide-1-phosphate activates cytosolic phospholipase A2alpha directly and by PKC pathway. Biochem Pharmacol. 2006;71:850–857. doi: 10.1016/j.bcp.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Huwiler A, Johansen B, Skarstad A, Pfeilschifter J. Ceramide binds to the CaLB domain of cytosolic phospholipase A2 and facilitates its membrane docking and arachidonic acid release. FASEB J. 2001;15:7–9. doi: 10.1096/fj.00-0370fje. [DOI] [PubMed] [Google Scholar]
- 36.Seeds MC, Jones DF, Chilton FH, Bass DA. Secretory and cytosolic phospholipases A2 are activated during TNF priming of human neutrophils. Biochim Biophys Acta. 1998;1389:273–284. doi: 10.1016/s0005-2760(97)00151-3. [DOI] [PubMed] [Google Scholar]
- 37.Kuwata H, et al. A novel role of group VIB calcium-independent phospholipase A2 (iPLA2gamma) in the inducible expression of group IIA secretory PLA2 in rat fibroblastic cells. J Biol Chem. 2007;282:20124–20132. doi: 10.1074/jbc.M611883200. [DOI] [PubMed] [Google Scholar]
- 38.Akiba S, Murata T, Kitatani K, Sato T. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull. 2000;23:1293–1297. doi: 10.1248/bpb.23.1293. [DOI] [PubMed] [Google Scholar]
- 39.Dangi B, et al. Biogenic synthesis, purification, and chemical characterization of anti-inflammatory resolvins derived from docosapentaenoic acid (DPAn-6) J Biol Chem. 2009;284:14744–14759. doi: 10.1074/jbc.M809014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bang HO, Dyerberg J. The bleeding tendency in Greenland Eskimos. Dan Med Bull. 1980;27:202–205. [PubMed] [Google Scholar]
- 41.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.