Abstract
Supplementation of standardized fermented papaya preparation (FPP) to adult diabetic mice improves dermal wound healing outcomes. Peripheral blood mononuclear cells (PBMC) from type II diabetes mellitus (T2DM) patients elicit a compromised respiratory burst activity resulting in increased risk of infections for the diabetic patients. Aims: The objectives of the current study were to determine the effect of FPP supplementation on human diabetic PBMC respiratory burst activity and to understand underlying mechanisms of such action of FPP. Results: When stimulated with phorbol 12-myristate 13-acetate, the production of reactive oxygen species by T2DM PBMC was markedly compromised compared to that of the PBMC from non-DM donors. FPP treated ex vivo improved respiratory burst outcomes in T2DM PBMC. FPP treatment significantly increased phosphorylation of the p47phox subunit of NADPH oxidase. In addition, the protein and mRNA expression of Rac2 was potently upregulated after FPP supplemention. The proximal human Rac2 gene promoter is G–C rich and contains consensus binding sites for Sp1 and AP-1. While FPP had no significant effect on the AP-1 DNA binding activity, the Sp1 DNA binding activity was significantly upregulated in PBMC after treatment of the cells with FPP. Innovation: This work provided first evidence that compromised respiratory burst performance of T2DM PBMC may be corrected by a nutritional supplement. Conclusion: FPP can correct respiratory burst performance of T2DM PBMC via an Sp-1-dependant pathway. Studies testing the outcome of FPP supplementation in diabetic patients are warranted. Antioxid. Redox Signal. 17, 485–491.
Introduction
The Centers for Disease Control and Prevention report that diabetes affects 21 million Americans. The incidence of infection is known to be increased in patients with diabetes mellitus (DM) (42). In a prospective study of 101,293 adult hospitalized patients, 1640 episodes of bacteremia were diagnosed. Of 1000 hospitalized patients studied, two-third of DM patients suffered from bacteremia, while only one-third of non-DM patients were affected (12). Compromised immune defenses, including leukocyte dysfunction, represent a key mechanism underlying the high infection rate noted in patients with DM (7, 8, 19). Leukocytes (neutrophil and monocytes), through their characteristic respiratory burst activity, produce superoxide anion (O2•−) and derivative reactive oxygen species (ROS), which fight infection (4). Leukocyte NADPH oxidase, found in professional phagocytes, catalyzes the production of O2•− by the one-electron reduction of oxygen, using NADPH as the electron donor (4, 5). It is widely reported that patients with type II DM (T2DM) suffer from systemic oxidative stress (16). However, the ability of peripheral blood monocytes of T2DM to mount respiratory burst response in response to appropriate pathogenic stimulus is known to be compromised, increasing the risk of infection-related complications in diabetics (14).
Innovation.
This work provides the first evidence demonstrating that respiratory burst dysfunction in monocytes from diabetic patients may be corrected by fermented papaya preparation (FPP). Induction of p47phox phosphorylation as well as upregulation of Rac2 transcription emerged as two potential underlying mechanisms. Because FPP has a long track-record of safe human consumption (1, 27–29, 44) and has proved to be beneficial in influencing wound healing outcomes (15), findings of this study lay a strong rationale for the design of phase-II clinical trial testing the effects of FPP supplementation on wound infection outcomes of type II diabetes mellitus patients suffering from chronic wounds.
Carica papaya Linn is widely recognized as a medicinal fruit (2). Fermented papaya preparation (FPP), a granular substance, is available over the counter (32). FPP possesses antioxidant properties (3, 11, 22, 35) that provide benefit against age-related complications (27). FPP is also known to protect red blood cells against oxidative damage (27, 28) and help against severe forms of thalassemia (1). Several independent observations convergently point toward the hypothesis that treatment with papaya preparations may facilitate wound healing responses (2, 17, 21, 30, 33, 34). Chronic wounds represent a major public health problem in diabetics. Our previous studies have demonstrated that wound-site macrophages of diabetics are compromised in their ability to support wound healing (23). Recently, our laboratory reported first evidence demonstrating that FPP may improve diabetic wound outcomes by specifically influencing the response of wound-site macrophages and the subsequent angiogenic response (15). FPP has a long track-record of safe human consumption (26, 28). The objectives of the current study were twofold: to determine whether FPP is able to improve inducible respiratory burst outcomes in peripheral blood mononuclear cells (PBMC) of diabetic patients, and to investigate the underlying mechanisms. The overall goal was to develop data toward future phase-II clinical studies.
Results
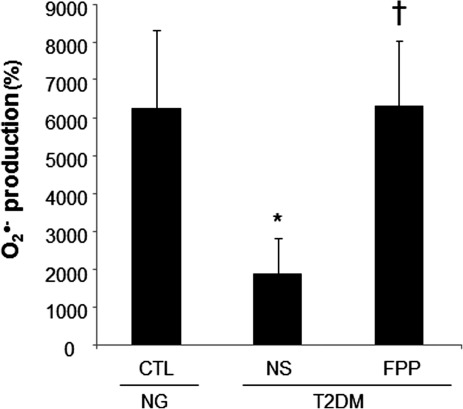
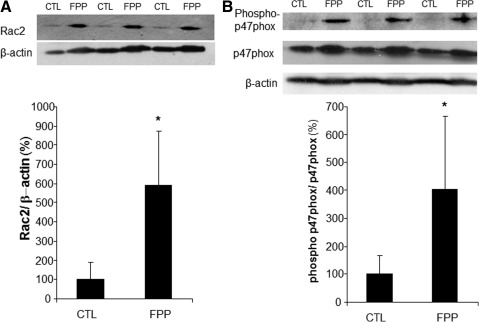
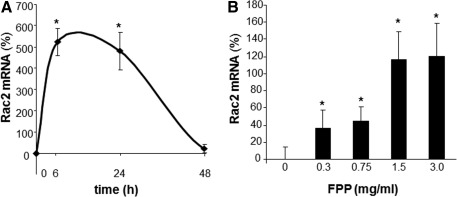
Phorbol 12-myristate 13-acetate (PMA) stimulated a potent induction of superoxide anion production from PBMCs. This response was severely blunted in PBMC isolated from T2DM patients compared to results from normoglycemic control human subjects. Intriguingly, the compromise in inducible respiratory burst activity in T2DM PBMC was corrected in response to exposure to FPP (Fig. 1). FPP induces ROS expression in cells isolated from normoglycemic subjects; the extent of induction, however, is less (20% vs. 230% in T2DM) compared to cells from T2DM (data not shown). NADPH oxidase represents the primary enzyme that is responsible for PMA-inducible respiratory burst production in PBMC. To investigate the underlying mechanisms of FPP action on respiratory burst, isolated PBMCs were cultured in presence of FPP for 24 h. Expression of the major subunits of NADPH oxidase complex was determined using Western blot (Fig. 2). Interestingly, the expression of Rac2 was significantly upregulated after FPP supplementation (Fig. 3A). FPP contains large amounts (∼90%) of carbohydrates (29). We have previously shown that matching amount of glucose supplementation did not result in ROS production as observed after FPP supplementation (15). To confirm these observation in ROS production by human PBMC, glucose (matching to that present in FPP) treatment to the PBMCs was performed (data not shown). Glucose treatment alone did not further stimulate PMA-induced ROS production in PBMCs as observed after FPP treatment, suggesting that FPP is specifically effective in inducing ROS production, which is independent of the glucose present in this nutritional supplement. In addition to upregulation of Rac2 expression, FPP exposure also resulted in increased phosphorylation of p47phox (Fig. 3B). With the goal to understand how FPP induces Rac2 expression, the effect on gene transcription was investigated. Expression of Rac2 mRNA was significantly upregulated after FPP exposure, suggesting that the Rac2 transcriptional machinery was sensitive to FPP. Maximal induction of Rac2 mRNA was noted after 6 h of treatment, indicating that the regulation by FPP was rapid (Fig. 4A). Next, to determine the minimum dose of FPP required eliciting induction of Rac2 mRNA expression, a dose–response study was performed. A dose of 0.3 mg/ml was noted to be sufficient to significantly induce Rac2 transcription, whereas the response was at a peak with 1.5 mg/ml of FPP (Fig. 4B).
FIG. 1.
Ex vivo supplementation of fermented papaya preparation (FPP) corrects type II diabetes mellitus (T2DM)-mediated compromised respiratory burst production by human peripheral blood mononuclear cells (PBMC). Peripheral blood was collected from T2DM donors with good glycemic control (hemoglobin A1c [HbA1c]<7) or healthy normoglycemic (NG) subjects (CTL). Freshly isolated mononuclear leukocytes (PBMC) were cultured in presence or absence of FPP (3 mg/ml) for 24 h. Superoxide anion production was measured after phorbol 12-myristate 13-acetate (PMA; 1 μg/ml) activation for 30 min. Data are expressed as percent change compared to corresponding PMA-unstimulated cells. Data are mean±standard deviation (SD; n=5). *p<0.05 compared to CTL. †p<0.005 compared to nonsupplemented (NS) T2DM group.
FIG. 2.
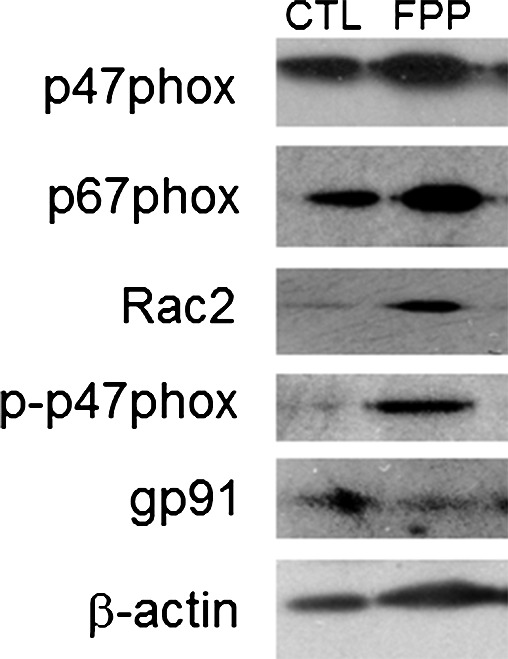
Effect of FPP supplementation on expression of NADPH oxidase subunits. PBMC were isolated from human peripheral blood buffy coat fraction followed by culturing with FPP (3 mg/ml) for 24 h. The control cells (CTL) were cultured under identical conditions without FPP. Total protein was extracted followed by Western blot analysis of the major proteins of the NADPH oxidase complex. Representative data set out of three independent experiments has been presented.
FIG. 3.
Rac2 and p-p47phox expression after FPP supplementation. PBMC were isolated from human peripheral blood buffy coat fraction followed by culturing with FPP (3 mg/ml) for 24 h. The control cells (CTL) were cultured under identical conditions without FPP. Total protein was extracted followed by Western blot analysis of Rac2 (A) and phospho-p47phox (B). Densitometry was done for quantification (bar graphs). Data are expressed as mean±SD (n=3). *p<0.05 compared to non FPP CTL.
FIG. 4.
FPP potently upregulates Rac2 transcription in human PBMC. PBMC were isolated from human blood buffy coats and cultured with FPP. (A) To determine the effect on Rac2 transcription, PBMCs were treated with FPP (3 mg/ml) for 6–72 h. Rac2 mRNA was quantified via real-time polymerase chain reaction (PCR). Data are expressed as mean±SD (n=3). *p<0.05 compared to non-FPP CTL. (B) FPP induces Rac2 mRNA in a dose- dependent (0.3–3 mg/ml) manner. PBMCs were supplemented with FPP for 6 h. Rac2 mRNA levels were measured. Data are expressed as mean±SD (n=3). *p<0.05 compared to non-FPP CTL.
To test whether compromised inducible respiratory burst function in PBMC from T2DM patients was associated with lower Rac2 expression, PBMC were obtained from patients and subjected to RTPCR. Indeed, the expression of Rac2 was noted to be significantly lower in PBMC of T2DM compared to that in PBMC from normoglycemic control donors. The lower expression of Rac2 in PBMC of T2DM was significantly corrected in response to FPP treatment ex vivo (Fig. 5).
FIG. 5.
FPP supplementation corrects T2DM-mediated compromise in Rac2 mRNA in human PBMC. Peripheral blood was collected from T2DM donors with good glycemic control (HbA1c<7) or healthy normoglycemic (NG). Freshly isolated mononuclear leukocytes (PBMC) were cultured in presence or absence of FPP (3 mg/ml) for 24 h. Levels of mRNA were measured via quantitative real-time PCR. Data are expressed as mean±SD (n=5) *p<0.05 compared to CTL. †p<0.005 compared to nonsupplemented (NS) T2DM group.
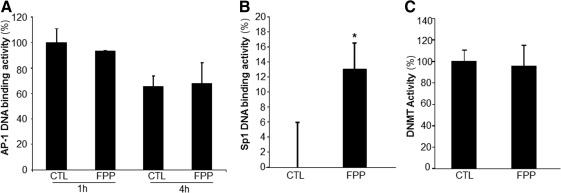
The human Rac2 gene promoter contains binding sites for the transcription factors Sp1 and AP-1. DNA binding activity of AP-1 and Sp1 were therefore tested in response to FPP treatment of PBMC. While FPP had no significant effect on the AP-1 DNA binding activity (Fig. 6A), the Sp1 DNA binding activity was significantly upregulated in PBMC after treatment of the cells with FPP (Fig. 6B). The Rac2 genomic locus exhibits distinct patterns of DNA methylation in expressing versus nonexpressing cells. A role of DNA methylation in Rac2 gene transcription has been shown (25). To determine if FPP influences Rac2 gene expression by modifying DNA methyltransferase (DNMT) activity, DNMT activity was measured from nuclear protein extracts. No effect of FPP supplementation on the DNMT activity was observed (Fig. 6C).
FIG. 6.
Effect of FPP on AP-1/Sp1 DNA binding and DNA methyltransferase activities. PBMC were isolated from human peripheral blood buffy coat fraction followed by culturing with FPP (3 mg/ml). Nuclear proteins were extracted. AP-1 (A) and Sp1 (B) DNA binding activities were quantified using the TransAM DNA binding assay. (C) DNA methyltransferase activity was measured from nuclear protein extracts. Data are expressed as mean±SD (n=3) *p<0.05 compared to non-FPP CTL.
Discussion
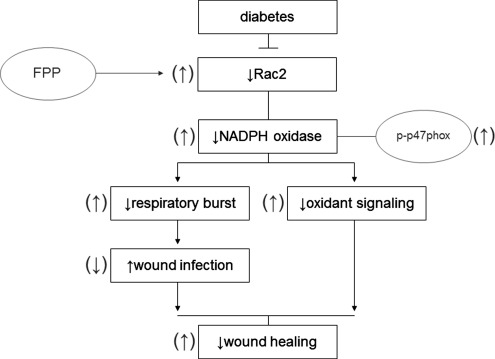
Respiratory burst, generating superoxide (O2•−) and derivative oxidant species, represents a major defense mechanism that fights infection (4). Immune dysfunction, including defective leukocyte respiratory burst activity, has been well documented in T2DM patients (14). Such dysfunction represents a key mechanism underlying the high infection rate in patients with DM (7, 8, 19). Findings of this study demonstrate for the first time that FPP may correct deficiencies in respiratory burst function by inducing Rac2 expression of PBMC obtained from T2DM patients (Fig. 7). Function of the NADPH oxidase complex is a key driver of respiratory burst (6). In response to appropriate stimuli, cytosolic components of this enzyme system, for example, p47phox and p67phox, associate with the membrane-bound gp91phox (NOX2) and p22phox (6). Under appropriate conditions, p47phox is rapidly phosphorylated at several serine residues and undergoes a conformational change. Phosphorylated p47phox then interacts with p22phox and gp91phox to promote the oxidase assembly and subsequent activation (20). Increased phosphorylation of p47phox is likely one of the mechanisms of action of FPP in correcting inducible respiratory burst function of T2DM PBMC.
FIG. 7.
Summary figure. A schematic illustration of mechanisms of action of FPP to induce respiratory burst capability in human PBMCs.
The small GTPase Rac is a member of Rho–GTPase superfamily and acts as a molecular switch to regulate various cellular functions, including NADPH oxidase activation (18). Rac has three isoforms: the ubiquitously expressed Rac1, a hematopoietic cell-specific isoform Rac2, and Rac3, which appears to be expressed in a variety of tissues (43). Rac1 is about fourfold more abundant than Rac2 in monocytes/macrophages and was suggested to be primarily involved in NADPH oxidase activation in monocytes (13). Macrophages derived from Rac2-null mice show compromised PMA-induced NADPH oxidase activation pointing toward a key role of Rac2 in enabling macrophage respiratory burst (43). Specifically, Rac2 was shown to be essential for oxidant production elicited by PMA and FcγR stimulation (43). Observations of the current study unveil the potential for FPP to bolster macrophage respiratory burst function via a Rac2-dependent mechanism.
In addition to the improper bactericidal action of the respiratory burst in T2DM patients, oxidant signaling necessary to the wound healing process is likely to be compromised in patients exhibiting improper respiratory burst in response to stimuli (41). NADPH-oxidase-produced superoxide anion is quickly converted to hydrogen peroxide, which continues down a signaling cascade creating more stable oxidants, such as hypochlorous acid, chloramines, and aldehydes, required in the wound healing process (41). FPP-induced correction of respiratory burst function may have implications in improving wound healing outcomes via correction of oxidant signaling pathways.
Human DNA is methylated at the cytosine-5 position within cytosine–guanine (CpG) dinucleotides. This epigenetic modification plays an important role in determining genomic stability and transcriptional regulation (9). The Rac2 genomic locus exhibits distinct patterns of DNA methylation in expressing versus nonexpressing cells (25). Cells that lack Rac2 expression exhibit increased cytosine methylation in the sequences flanking the gene, whereas cells that express Rac2 exhibit increased cytosine methylation within the body of the Rac2 gene (25). Treatment of nonexpressing cells with the DNMT inhibitor 5-aza-2′-deoxycytidine has been shown to be sufficient to induce Rac2 gene expression (25). (−)−Epigallocatechin-3-gallate (EGCG), the main polyphenol antioxidant in green tea, exhibits demethylating activity (10). Therefore, we tested whether FPP modifies DNMT activity. No effect of FPP on DNMT activity excludes this regulatory pathway of FPP-mediated Rac2 gene transcription.
The Rac2 gene is located on chromosome 22q12 and consists of seven exons spanning 18 kb of DNA (31). The human Rac2 gene promoter lacks TATA and CCAAT boxes, utilizing multiple transcription initiation sites, including AP-1 and several putative Sp1 binding sites (25, 31). Overexpression of AP-1 has been shown to be sufficient to induce expression of a transiently transfected Rac2 promoter/luciferase plasmid, but not of the endogenous Rac2 gene (31). In the current study, FPP did not modify AP-1 transactivation in PBMC ruling out AP-1 as a potential mechanism underlying Rac2 induction by FPP. Sp1 binding sites are common in promoters that lack TATA boxes. In mice, Sp1 binding sites in the promoter region play a key role in driving Rac2 gene expression (25). Indeed, we noted that FPP increased Sp1 DNA binding activity in PBMC. This observation suggests that FPP-induced Rac2 gene transcription may be Sp1 dependent. Antioxidants such as N-acetylcysteine are known to induce Sp1-dependent transcription of the CDKN1A gene by increasing the phosphorylation of the C terminus of Sp1 (24). FPP possesses potent antioxidant functions (22). Whether the antioxidant activity of FPP is involved in the regulation of Sp1 transactivation remains unresolved.
Materials and Methods
Human subjects and sample collection
All human studies were approved by The Ohio State University Institutional Review Board. Subjects (adults 40–60 years) participating in the study were either healthy normoglycemic or clinically diagnosed as T2DM with good glycemic control, that is, hemoglobin A1c (HbA1c)≤7%. T2DM subjects were recruited from the Diabetes Clinic at The Ohio State University Medical Center. Subject demographics are presented in Table 1. T2DM subjects, either immunosupressive or taking peroxisome proliferator-activated receptor gamma medications, were excluded from the study. Peripheral blood (60 cc) was drawn by venipuncture. For isolation of PBMC, blood samples were collected in sodium–heparin-coated blood-chemistry tubes (BD Medical, Franklin Lakes, NJ). For studies investigating the underlying mechanisms of FPP action, PBMC were also isolated from banked source leukocytes packets obtained from the American Red Cross of Greater Columbus (Columbus, OH).
Table 1.
Human Subject Demographics
| Normoglycemic (n=5) | T2DM (n=5) | |
|---|---|---|
| Sex (M/F) | 2/3 | 2/3 |
| Age (years) (mean±SD) | 50.0±7.8 | 54.0±5.5 |
| HbA1c (%) (mean±SD) | N/A | 6.3±0.4 |
| BMI (mean±SD) | 27.5±8.4 | 32.7±7.6 |
BMI, body mass index; HbA1c, hemoglobin A1c; SD, standard deviation.
Human PBMC isolation and culture
Either fresh blood or source leukocytes were diluted 1:1 using sterile Dulbecco's phosphate buffered saline (Mediatech, Inc., Manasses, VA). PBMC were isolated using Ficoll density centrifugation followed by sorting with anti-CD14 coated magnetic microbeads (Miltenyi Biotech, Auburn, CA) as previously described (36). Purified CD14+ PBMCs were then cultured under standard conditions (5% CO2, 37°C, humidified incubator). RPMI 1640 (Mediatech, Inc.) supplemented with 5% heat-inactivated fetal bovine serum (Atlanta Biologicals, Inc., Lawrenceville, GA) and 1% antibiotic–antimycotic (Invitrogen Corp., Carlsbad, CA) was used as culture media.
FPP supplementation
FPP (Immun'Age; Osato International, Gifu, Japan) was dissolved in culture media by adding the desired FPP quantity in culture media, followed by sterile filtration using a 0.22-μm vacuum-driven filtration system (Millipore, Billerica, MA). The dose (3 mg/ml) of FPP was chosen based on prior studies (35).
Superoxide anion measurement
Induced superoxide anion generation was measured using a LumiMax® superoxide anion detection kit (Stratagene, La Jolla, CA). Briefly, freshly isolated PBMC were treated with PMA (1 μg/ml). Superoxide was measured at 430 nm using a luminometer (model Lumat LB9507; Berthold Technologies, Bad Wildbad, Germany) (36).
Western blot
Primary antibodies against Rac2 (Millipore, Temecula, CA), Nox2/gp91phox, phospho-p47phox (Abcam, Cambridge, MA), p47phox, and p67phox (Cell Signaling Technology, Beverly, MA) were used to detect the corresponding antigens. β-Actin was considered as housekeeping protein for normalization of sample loading.
Isolation of RNA, reverse transcription, and quantitative real-time polymerase chain reaction
Total RNA was extracted from PBMCs using a mirVana RNA isolation kit (Ambion, Austin, TX) according to the manufacturer's suggested protocol. mRNAs were quantified by real-time polymerase chain reaction assay using SYBR green-I (Applied Biosystems, Carlsbad, CA) as described previously (36, 38–40). β-Actin was used as the housekeeping gene. ΔΔCt comparative analysis was used to normalize gene expression data against housekeeping gene. The primer-set used for individual genes is listed below:
Rac2 5′ GCC TGG CAC TGG CCA AGG AG 3′
5′ CTA GGT GGG AGC GCT GGG GT 3′
β-Actin 5′ GTA CCA CTG GCA TCC TGA TGG ACT 3′
5′ CCG CTC ATT GCC AAT GGT GAT 3′
DNA binding activity of Sp1/Sp3
A TransAM Sp1/Sp3 family transcription assay ELISA-based kit (Active Motif, Carlsbad, CA) was used as described (37).
DNMT activity
DNMT activity was measured from nuclear protein extracts using DNMT activation/inhibition assay (Active Motif) according to the manufacturer's protocol.
Statistics
Data are reported as mean±standard deviation of at least three experiments. Comparisons among multiple groups were made using analysis of variance. p<0.05 was considered statistically significant.
Abbreviations Used
- DNMT
DNA methyltransferase
- FPP
fermented papaya preparation
- HbA1c
hemoglobin A1c
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- T2DM
type II diabetes mellitus
Acknowledgment
Supported by DK 076566 to S.R. and in part by a funding from Osato Research Institute, Japan.
Author Disclosure Statement
The authors declare that FPP and partial research funding was provided by Osato Research Institute of Japan.
References
- 1.Amer J. Goldfarb A. Rachmilewitz EA. Fibach E. Fermented papaya preparation as redox regulator in blood cells of beta-thalassemic mice and patients. Phytother Res. 2008;22:820–828. doi: 10.1002/ptr.2379. [DOI] [PubMed] [Google Scholar]
- 2.Anuar NS. Zahari SS. Taib IA. Rahman MT. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food Chem Toxicol. 2008;46:2384–2389. doi: 10.1016/j.fct.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Aruoma OI. Colognato R. Fontana I. Gartlon J. Migliore L. Koike K. Coecke S. Lamy E. Mersch-Sundermann V. Laurenza I. Benzi L. Yoshino F. Kobayashi K. Lee MC. Molecular effects of fermented papaya preparation on oxidative damage, MAP Kinase activation and modulation of the benzo[a]pyrene mediated genotoxicity. Biofactors. 2006;26:147–159. doi: 10.1002/biof.5520260205. [DOI] [PubMed] [Google Scholar]
- 4.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 5.Babior BM. The enzymatic basis for O-.2 production by human neutrophils. Can J Physiol Pharmacol. 1982;60:1353–1358. doi: 10.1139/y82-202. [DOI] [PubMed] [Google Scholar]
- 6.Babior BM. Lambeth JD. Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 7.Bagdade JD. Nielson KL. Bulger RJ. Reversible abnormalities in phagocytic function in poorly controlled diabetic patients. Am J Med Sci. 1972;263:451–456. doi: 10.1097/00000441-197206000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bagdade JD. Root RK. Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23:9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 10.Brueckner B. Lyko F. DNA methyltransferase inhibitors: old and new drugs for an epigenetic cancer therapy. Trends Pharmacol Sci. 2004;25:551–554. doi: 10.1016/j.tips.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Calzuola I. Gianfranceschi GL. Marsili V. Comparative activity of antioxidants from wheat sprouts, Morinda citrifolia, fermented papaya and white tea. Int J Food Sci Nutr. 2006;57:168–177. doi: 10.1080/09637480600658328. [DOI] [PubMed] [Google Scholar]
- 12.Carton JA. Maradona JA. Nuno FJ. Fernandez-Alvarez R. Perez-Gonzalez F. Asensi V. Diabetes mellitus and bacteraemia: a comparative study between diabetic and non-diabetic patients. Eur J Med. 1992;1:281–287. [PubMed] [Google Scholar]
- 13.Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 14.Chang FY. Shaio MF. Respiratory burst activity of monocytes from patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;29:121–127. doi: 10.1016/0168-8227(95)01123-4. [DOI] [PubMed] [Google Scholar]
- 15.Collard E. Roy S. Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid Redox Signal. 2010;13:599–606. doi: 10.1089/ars.2009.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandona P. Thusu K. Cook S. Snyder B. Makowski J. Armstrong D. Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 17.Dawkins G. Hewitt H. Wint Y. Obiefuna PC. Wint B. Antibacterial effects of Carica papaya fruit on common wound organisms. West Indian Med J. 2003;52:290–292. [PubMed] [Google Scholar]
- 18.Etienne-Manneville S. Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 19.Geerlings SE. Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 20.Groemping Y. Lapouge K. Smerdon SJ. Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 21.Gurung S. Skalko-Basnet N. Wound healing properties of Carica papaya latex: in vivo evaluation in mice burn model. J Ethnopharmacol. 2009;121:338–341. doi: 10.1016/j.jep.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Imao K. Wang H. Komatsu M. Hiramatsu M. Free radical scavenging activity of fermented papaya preparation and its effect on lipid peroxide level and superoxide dismutase activity in iron-induced epileptic foci of rats. Biochem Mol Biol Int. 1998;45:11–23. doi: 10.1080/15216549800202392. [DOI] [PubMed] [Google Scholar]
- 23.Khanna S. Biswas S. Shang Y. Collard E. Azad A. Kauh C. Bhasker V. Gordillo GM. Sen CK. Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KY. Rhim T. Choi I. Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- 25.Ladd PD. Butler JS. Skalnik DG. Identification of a genomic fragment that directs hematopoietic-specific expression of Rac2 and analysis of the DNA methylation profile of the gene locus. Gene. 2004;341:323–333. doi: 10.1016/j.gene.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Marotta F. Koike K. Lorenzetti A. Naito Y. Fayet F. Shimizu H. Marandola P. Nutraceutical strategy in aging: targeting heat shock protein and inflammatory profile through understanding interleukin-6 polymorphism. Ann N Y Acad Sci. 2007;1119:196–202. doi: 10.1196/annals.1404.011. [DOI] [PubMed] [Google Scholar]
- 27.Marotta F. Pavasuthipaisit K. Yoshida C. Albergati F. Marandola P. Relationship between aging and susceptibility of erythrocytes to oxidative damage: in view of nutraceutical interventions. Rejuvenation Res. 2006;9:227–230. doi: 10.1089/rej.2006.9.227. [DOI] [PubMed] [Google Scholar]
- 28.Marotta F. Weksler M. Naito Y. Yoshida C. Yoshioka M. Marandola P. Nutraceutical supplementation: effect of a fermented papaya preparation on redox status and DNA damage in healthy elderly individuals and relationship with GSTM1 genotype: a randomized, placebo-controlled, cross-over study. Ann N Y Acad Sci. 2006;1067:400–407. doi: 10.1196/annals.1354.057. [DOI] [PubMed] [Google Scholar]
- 29.Marotta F. Yoshida C. Barreto R. Naito Y. Packer L. Oxidative-inflammatory damage in cirrhosis: effect of vitamin E and a fermented papaya preparation. J Gastroenterol Hepatol. 2007;22:697–703. doi: 10.1111/j.1440-1746.2007.04937.x. [DOI] [PubMed] [Google Scholar]
- 30.Mikhal'chik EV. Ivanova AV. Anurov MV. Titkova SM. Pen'kov LY. Kharaeva ZF. Korkina LG. Wound-healing effect of papaya-based preparation in experimental thermal trauma. Bull Exp Biol Med. 2004;137:560–562. doi: 10.1023/b:bebm.0000042711.31775.f7. [DOI] [PubMed] [Google Scholar]
- 31.Muthukrishnan R. Skalnik DG. Identification of a minimal cis-element and cognate trans-factor(s) required for induction of Rac2 gene expression during K562 cell differentiation. Gene. 2009;440:63–72. doi: 10.1016/j.gene.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nau JY. [The Pope and the enlightenment of fermented papaya] Rev Med Suisse. 2005;1:459. [PubMed] [Google Scholar]
- 33.Nayak SB. Pinto Pereira L. Maharaj D. Wound healing activity of Carica papaya L. in experimentally induced diabetic rats. Indian J Exp Biol. 2007;45:739–743. [PubMed] [Google Scholar]
- 34.Pieper B. Caliri MH. Nontraditional wound care: A review of the evidence for the use of sugar, papaya/papain, and fatty acids. J Wound Ostomy Continence Nurs. 2003;30:175–183. doi: 10.1067/mjw.2003.131. [DOI] [PubMed] [Google Scholar]
- 35.Rimbach G. Park YC. Guo Q. Moini H. Qureshi N. Saliou C. Takayama K. Virgili F. Packer L. Nitric oxide synthesis and TNF-alpha secretion in RAW 264.7 macrophages: mode of action of a fermented papaya preparation. Life Sci. 2000;67:679–694. doi: 10.1016/s0024-3205(00)00664-0. [DOI] [PubMed] [Google Scholar]
- 36.Roy S. Dickerson R. Khanna S. Collard E. Gnyawali U. Gordillo GM. Sen CK. Particulate beta-glucan induces TNF-alpha production in wound macrophages via a redox-sensitive NF-kappabeta-dependent pathway. Wound Repair Regen. 2011;19:411–419. doi: 10.1111/j.1524-475X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy S. Khanna S. Azad A. Schnitt R. He G. Weigert C. Ichijo H. Sen CK. Fra-2 mediates oxygen-sensitive induction of transforming growth factor beta in cardiac fibroblasts. Cardiovasc Res. 2010;87:647–655. doi: 10.1093/cvr/cvq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy S. Khanna S. Kuhn DE. Rink C. Williams WT. Zweier JL. Sen CK. Transcriptome analysis of the ischemia-reperfused remodeling myocardium: temporal changes in inflammation and extracellular matrix. Physiol Genomics. 2006;25:364–374. doi: 10.1152/physiolgenomics.00013.2006. [DOI] [PubMed] [Google Scholar]
- 39.Roy S. Khanna S. Rink C. Biswas S. Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008;34:162–184. doi: 10.1152/physiolgenomics.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy S. Khanna S. Rink T. Radtke J. Williams WT. Biswas S. Schnitt R. Strauch AR. Sen CK. P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Mol Biol Cell. 2007;18:4837–4846. doi: 10.1091/mbc.E07-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen CK. Khanna S. Gordillo G. Bagchi D. Bagchi M. Roy S. Oxygen, oxidants, and antioxidants in wound healing: an emerging paradigm. Ann N Y Acad Sci. 2002;957:239–249. doi: 10.1111/j.1749-6632.2002.tb02920.x. [DOI] [PubMed] [Google Scholar]
- 42.Wheat LJ. Infection and diabetes mellitus. Diabetes Care. 1980;3:187–197. doi: 10.2337/diacare.3.1.187. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi A. Kim C. Li S. Marchal CC. Towe J. Atkinson SJ. Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J. Mori A. Chen Q. Zhao B. Fermented papaya preparation attenuates beta-amyloid precursor protein: beta-amyloid-mediated copper neurotoxicity in beta-amyloid precursor protein and beta-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Neuroscience. 2006;143:63–72. doi: 10.1016/j.neuroscience.2006.07.023. [DOI] [PubMed] [Google Scholar]