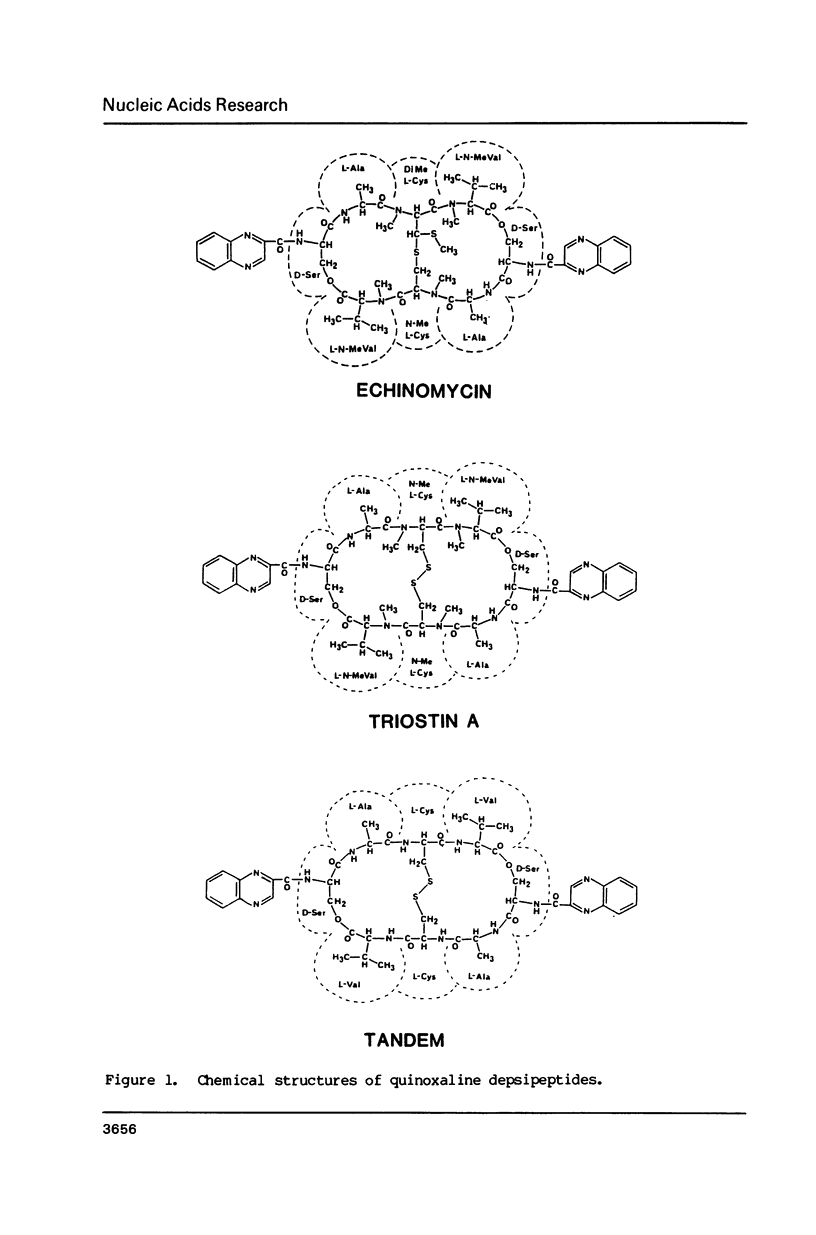
Abstract
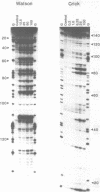
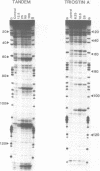
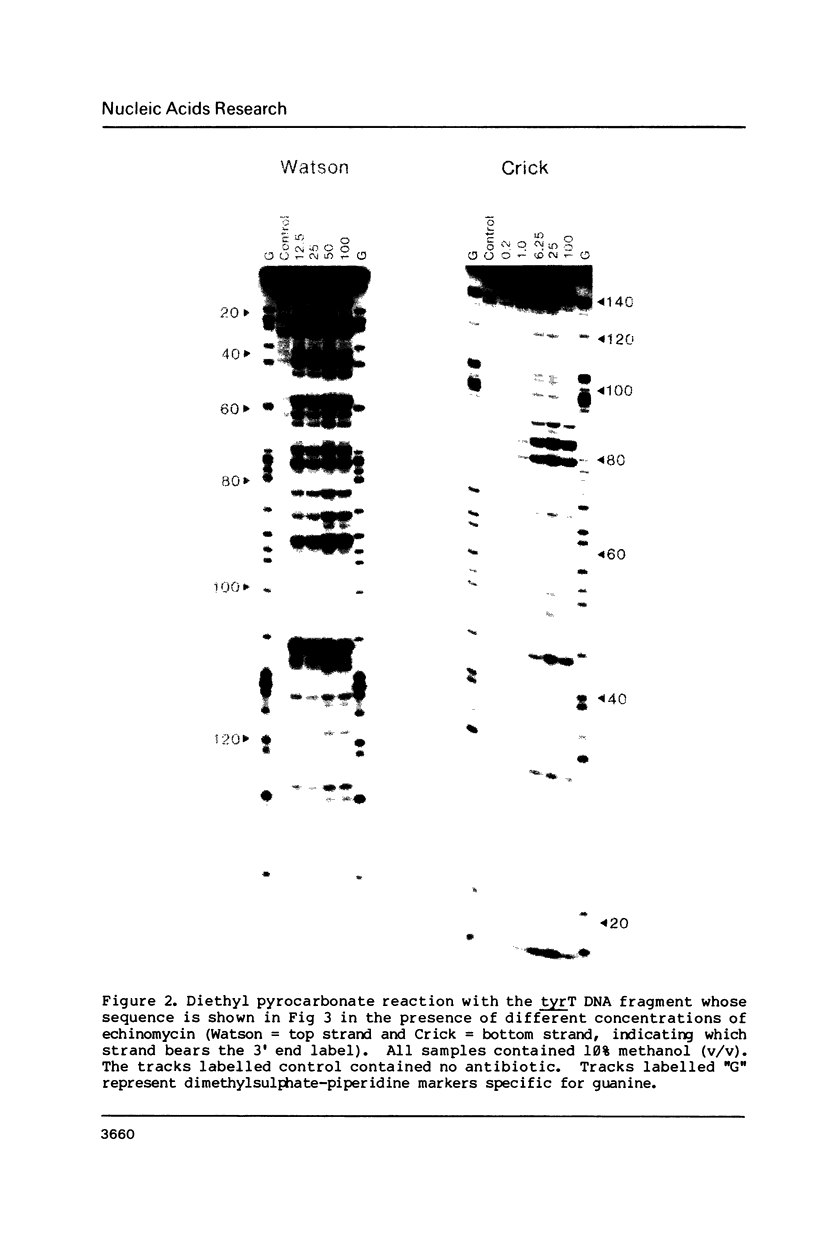
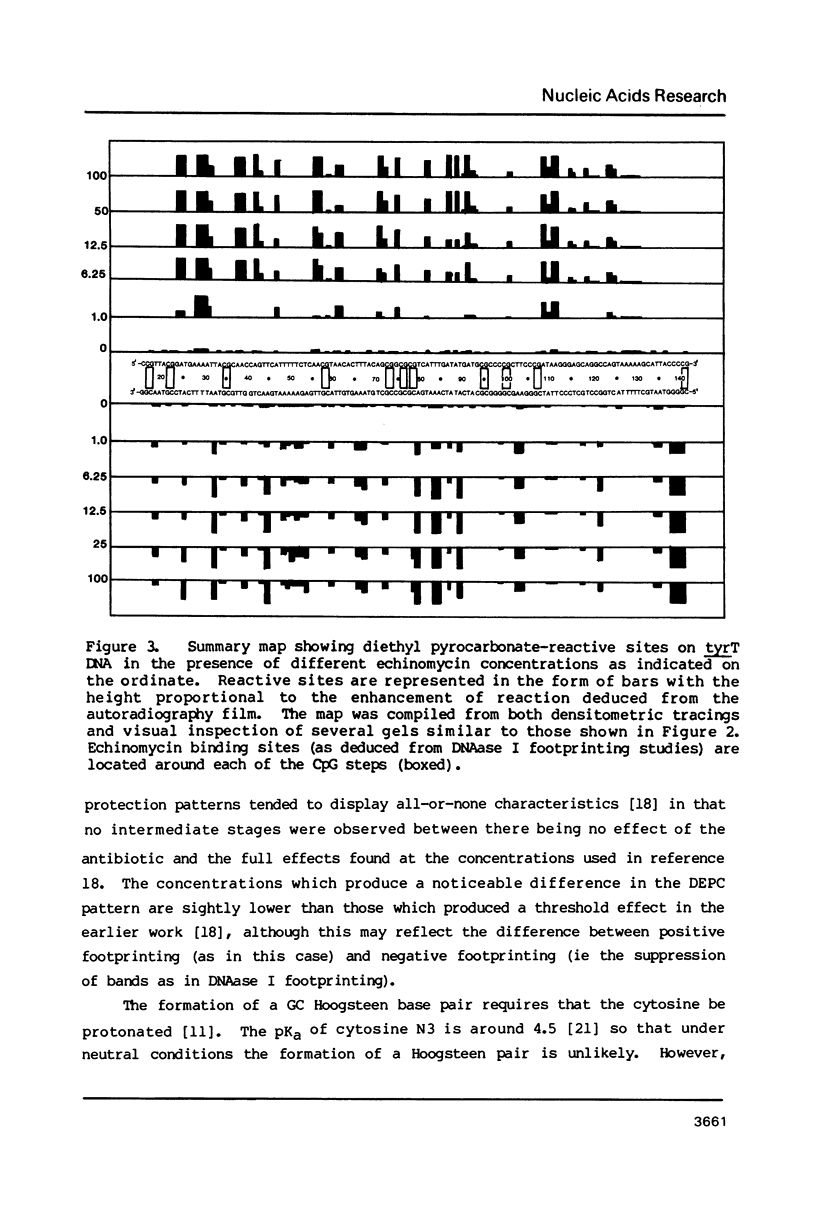
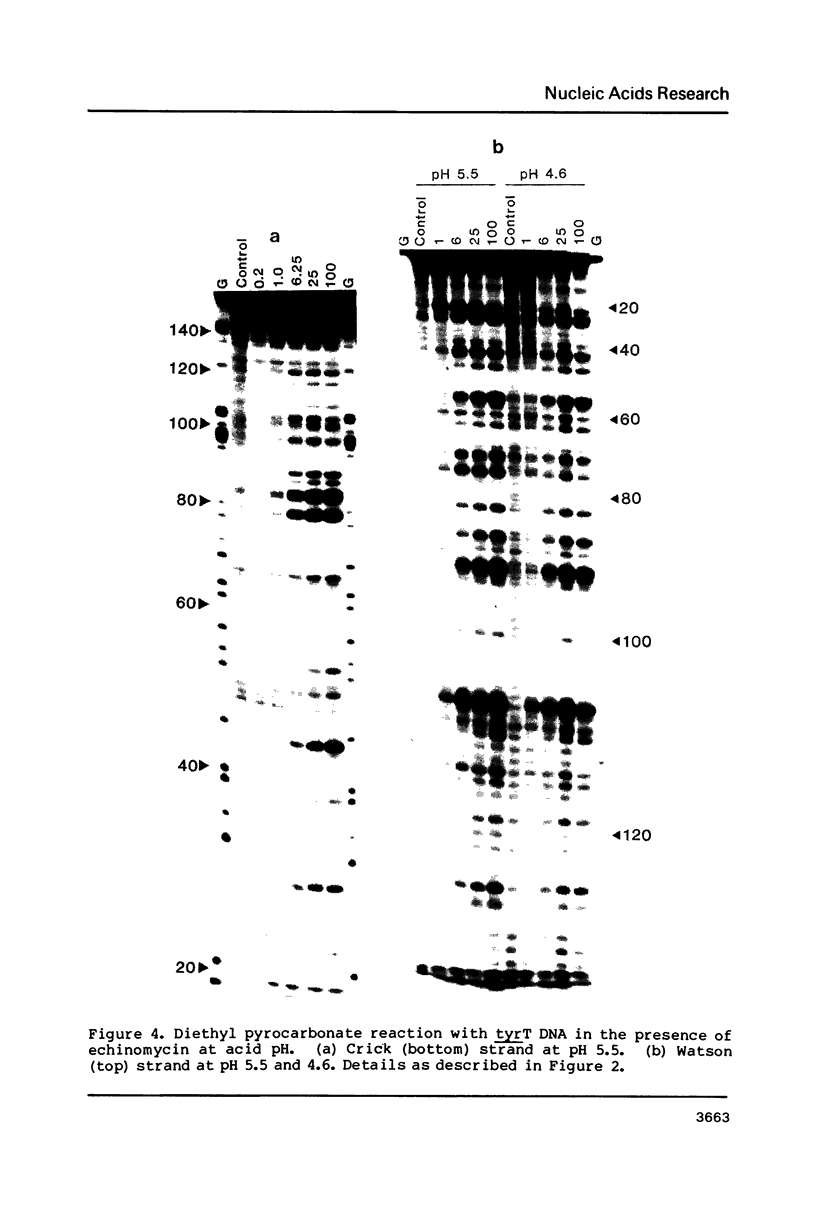
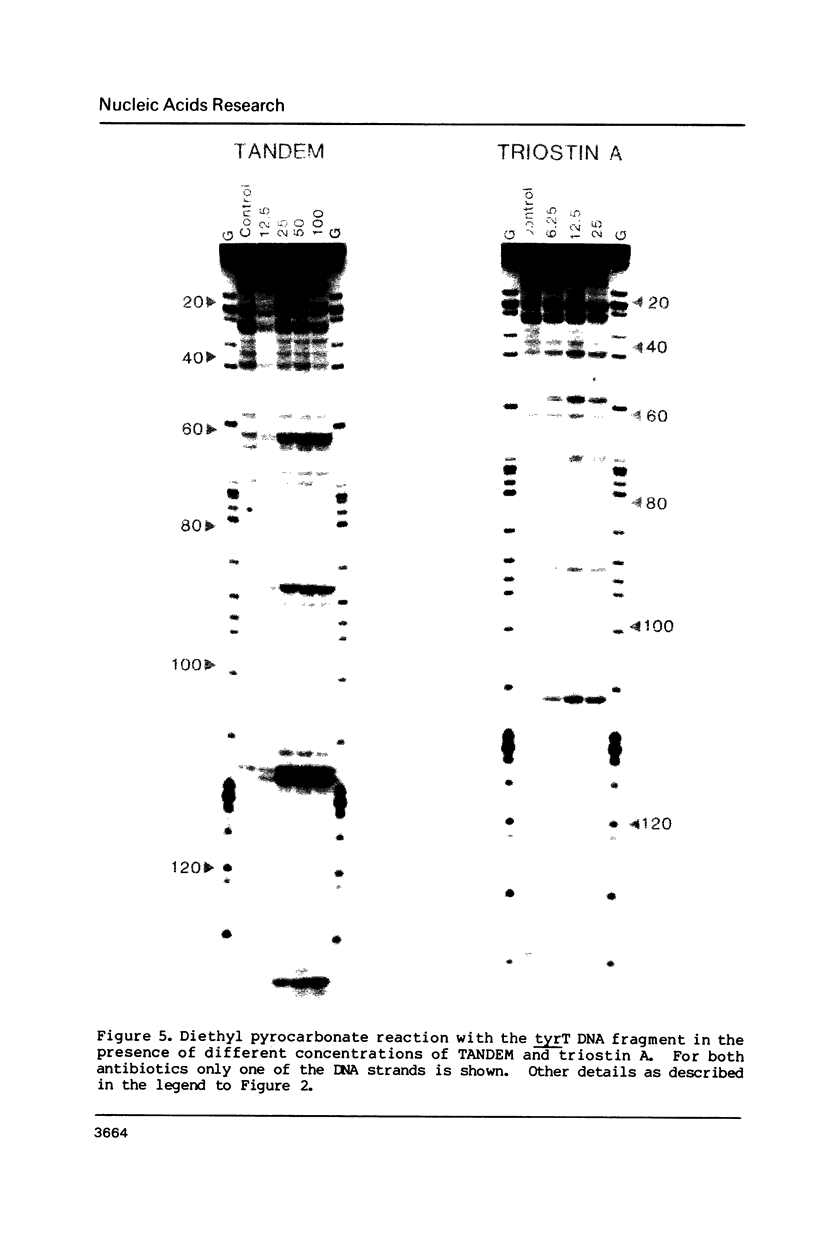
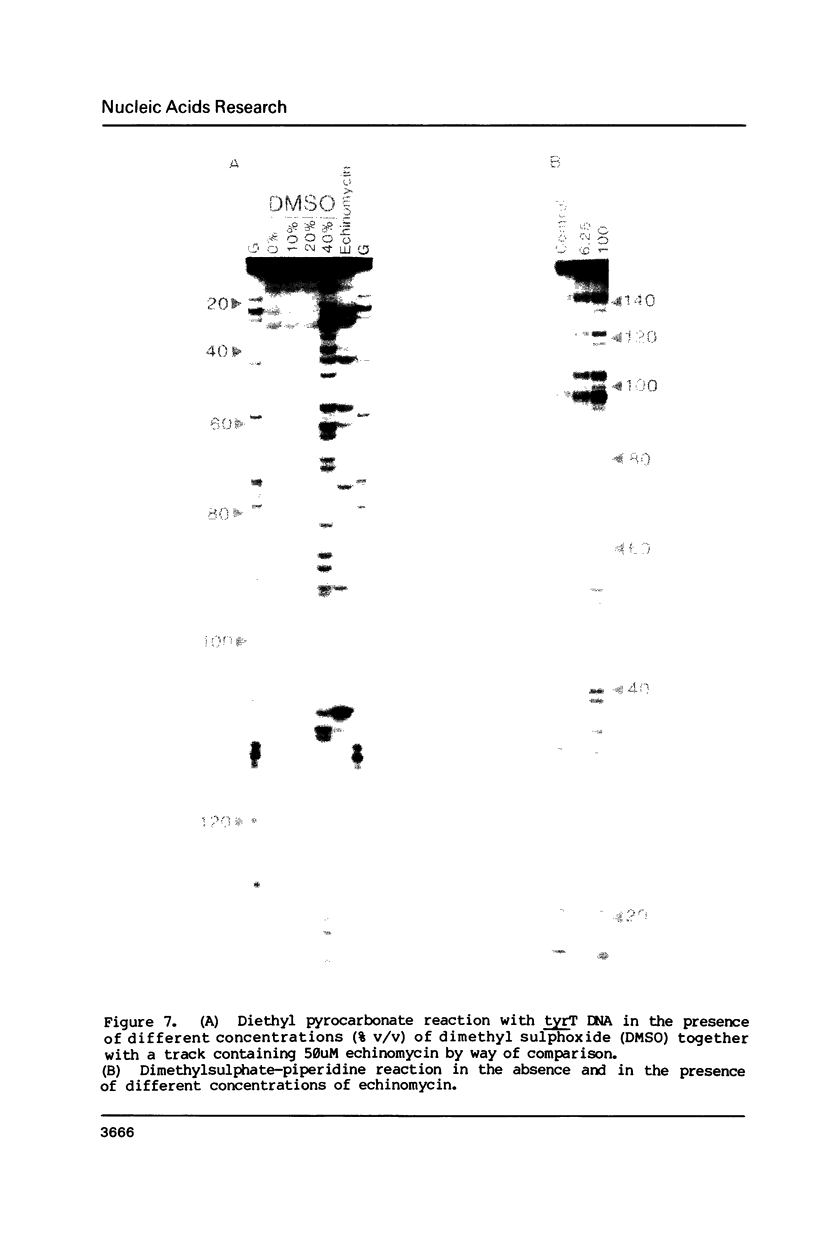
The reactivity of the 160 bp tyrT DNA fragment towards diethyl pyrocarbonate (DEPC) has been investigated in the presence of bis-intercalating quinoxaline antibiotics and the synthetic depsipeptide TANDEM. At moderate concentrations of each ligand, specific purine residues (mainly adenosines) exhibit enhanced reactivity towards the probe, and several sites of enhancement appear to be related to the sequence selectivity of drug binding. Further experiments were performed with echinomycin at pH 5.5 and 4.6 to facilitate the protonation of cytosine required for formation of Hoogsteen GC base pairs. No significant increase in reactivity was observed under these conditions. Additionally, no protection of deoxyguanosine residues from methylation by dimethyl sulphate was observed in the presence of echinomycin. We conclude that the structural anomaly giving rise to drug-dependent enhanced DEPC reaction is not simply the formation of Hoogsteen base pairs adjacent to antibiotic binding sites. Nor is it due to a general unwinding of the double helix, since we show that conditions which are supposed to unwind the helix lead to a uniform increase in purine reactivity, regardless of the surrounding nucleotide sequence.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clauwaert J., Stockx J. Interactions of polynucleotides and thier components. I. Dissociation constants of the bases and their derivatives. Z Naturforsch B. 1968 Jan;23(1):25–30. [PubMed] [Google Scholar]
- Dattagupta N., Hogan M., Crothers D. M. Does irehdiamine kink DNA? Proc Natl Acad Sci U S A. 1978 Sep;75(9):4286–4290. doi: 10.1073/pnas.75.9.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. DNA structural variations produced by actinomycin and distamycin as revealed by DNAase I footprinting. Nucleic Acids Res. 1984 Dec 21;12(24):9271–9285. doi: 10.1093/nar/12.24.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. The use of micrococcal nuclease as a probe for drug-binding sites on DNA. Biochim Biophys Acta. 1987 Jul 14;909(2):145–155. doi: 10.1016/0167-4781(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Furlong J. C., Lilley D. M. Highly selective chemical modification of cruciform loops by diethyl pyrocarbonate. Nucleic Acids Res. 1986 May 27;14(10):3995–4007. doi: 10.1093/nar/14.10.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Diethyl pyrocarbonate: a chemical probe for secondary structure in negatively supercoiled DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8009–8013. doi: 10.1073/pnas.82.23.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. Binding of an antitumor drug to DNA, Netropsin and C-G-C-G-A-A-T-T-BrC-G-C-G. J Mol Biol. 1985 Jun 25;183(4):553–563. doi: 10.1016/0022-2836(85)90171-8. [DOI] [PubMed] [Google Scholar]
- Lane M. J., Laplante S., Rehfuss R. P., Borer P. N., Cantor C. R. Actinomycin D facilitates transition of AT domains in molecules of sequence (AT)nAGCT(AT)n to a DNAse I detectable alternating structure. Nucleic Acids Res. 1987 Jan 26;15(2):839–852. doi: 10.1093/nar/15.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H., Mizusawa H., Kakefuda T. Unwinding of double-stranded DNA helix by dehydration. Proc Natl Acad Sci U S A. 1981 May;78(5):2838–2842. doi: 10.1073/pnas.78.5.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Waring M. J. Interaction between synthetic analogues of quinoxaline antibiotics and nucleic acids. Changes in mechanism and specificity related to structural alterations. Biochem J. 1978 Jul 1;173(1):129–144. doi: 10.1042/bj1730129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Echinomycin and distamycin induce rotation of nucleosome core DNA. Nucleic Acids Res. 1986 Sep 11;14(17):6785–6801. doi: 10.1093/nar/14.17.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Sequence-specific binding of echinomycin to DNA: evidence for conformational changes affecting flanking sequences. Nucleic Acids Res. 1984 Jun 25;12(12):4865–4879. doi: 10.1093/nar/12.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Fox K. R., Olsen R. K., Waring M. J. DNA sequence recognition by under-methylated analogues of triostin A. Nucleic Acids Res. 1986 Mar 11;14(5):2015–2033. doi: 10.1093/nar/14.5.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Olsen R. K., Waring M. J. Sequence preferences in the binding to DNA of triostin A and TANDEM as reported by DNase I footprinting. FEBS Lett. 1984 Oct 29;176(2):414–420. doi: 10.1016/0014-5793(84)81209-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle S., Pearl L. H., Skelly J. V. DNA structure and perturbation by drug binding. Biochem J. 1987 Apr 1;243(1):1–13. doi: 10.1042/bj2430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Antibiotics which can alter the rotational orientation of nucleosome core DNA. Nucleic Acids Res. 1986 Nov 25;14(22):8735–8754. doi: 10.1093/nar/14.22.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Ughetto G., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science. 1986 Jun 6;232(4755):1255–1258. doi: 10.1126/science.3704650. [DOI] [PubMed] [Google Scholar]
- Scholten P. M., Nordheim A. Diethyl pyrocarbonate: a chemical probe for DNA cruciforms. Nucleic Acids Res. 1986 May 27;14(10):3981–3993. doi: 10.1093/nar/14.10.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U. C., Pattabiraman N., Langridge R., Kollman P. A. Molecular mechanical studies of d(CGTACG)2: complex of triostin A with the middle A - T base pairs in either Hoogsteen or Watson-Crick pairing. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6402–6406. doi: 10.1073/pnas.83.17.6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ughetto G., Wang A. H., Quigley G. J., van der Marel G. A., van Boom J. H., Rich A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985 Apr 11;13(7):2305–2323. doi: 10.1093/nar/13.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincze A., Henderson R. E., McDonald J. J., Leonard N. J. Reaction of diethyl pyrocarbonate with nucleic acid components. Bases and nucleosides derived from guanine, cytosine, and uracil. J Am Chem Soc. 1973 Apr 18;95(8):2677–2682. doi: 10.1021/ja00789a045. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]