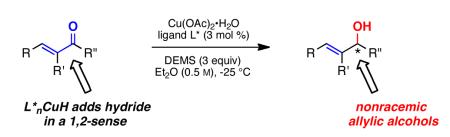
Abstract
The first study describing a general technology for arriving at valued nonracemic allylic alcohols using asymmetric ligand-accelerated catalysis by copper hydride is described.
Asymmetric copper hydride chemistry has become an especially powerful tool for controlling chirality in a variety of substrate types.1 Most notably, nonracemically ligated CuH can be used to direct remarkably selective hydride delivery to the β-site in a variety of Michael acceptors (Scheme 1, path A). In the absence of extended conjugation, asymmetric 1,2-additions of CuH are now known for aromatic ketones,2 diaryl3 and heteroaromatic ketones,4 and imines.5 Redirecting the natural tendency for copper complexes away from additions in a 1,4-sense can be challenging. The potential to alter, in the achiral manifold, such regioselectivity toward the 1,2-mode by a “subtle interplay of steric and electronic factors” of the phosphine ligand on copper was recognized years ago by Stryker.6 Overcoming the inherent mechanistic preference for initial d-π*-complexation associated, e.g., with Cu(I)-olefin soft-soft interactions in α,β-unsaturated ketones, remains an unsolved problem notwithstanding the synthetic potential of the resulting nonracemic allylic alcohols (Scheme 1, path B). While isolated examples of copper-catalyzed enantioselective 1,2-reductions of enones exist,7 any semblance of a general asymmetric protocol resulting from the correlation of substrate substitution pattern with ligand biases and/or tuning of reaction conditions for this important transformation is still lacking. Herein, we describe new methodology for the enantioselective CuH-catalyzed 1,2-reduction of α-substituted unsaturated ketones leading to secondary allylic alcohols (Scheme 1).
Scheme 1.
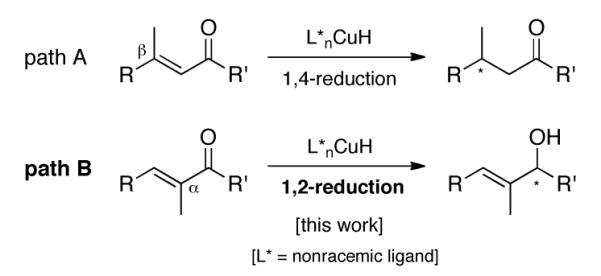
Pathways for addition of CuH to unsaturated ketones
As illustrated in Table 1, optimization studies using enone 1 revealed that (1) 1,2-addition to arrive at cinnamyl alcohol 2 is strongly favored over conjugate addition; (2) ee’s on the order of 90% could be achieved; (3) ligands in both the SEGPHOS8 and BIPHEP9 series give similar levels of induction; (4) diethoxymethylsilane (DEMS) as the stoichiometric source of hydride10 gives the best ee’s; (5) Et2O is the solvent of choice; (6) reactions should be run at −25 °C for optimal conversion and enantioselectivity; (7) the sense of induction is such that (L2)CuH11 produces the S-allylic alcohol, while (L3b)CuH leads to the enantiomeric product.
Table 1.
Selected optimization conditions for regio- and stereo-controlled 1,2-reductions (see SI for full details)a

| Entry | Ligand | Solv. | T (°C) | Yield of 2 (%)b | ee of 2 (%)c |
|---|---|---|---|---|---|
| 1 | L1 | THF | rt | 90 | 50 (S) |
| 2 | L2 | THF | rt | 78 | 75 (S) |
| 3 | L2 | THF | −25 | 87 | 86 (S) |
| 4 | L2 | Et2O | −25 | 83 (98)d | 91 (S) |
| 5e | L2 | Et2O | −35 | n.d. | n.d. |
| 6 | L3a | Et2O | −25 | 96 | 89 (R) |
| 7 | L3b | Et2O | −25 | 95 | 91 (R) |
| 8 | L3c | Et2O | −25 | 99 | 90 (S) |
| 9f | BDP | THF | rt | - | - |
Performed on a 0.1 mmol scale in 0.3 mL solvent.
By 1H NMR using Ph3CH as internal standard.
By chiral HPLC. Absolute stereochemistry was determined by comparing optical rotation to that of the known compound.
Isolated yield (0.25 mmol scale).
Low conversion after prolonged reaction time.
1,2-/1,4-ratio = 1:7, 60% isolated yield of 1,4-reduced enone.
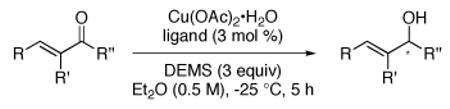
Several additional examples of acyclic and cyclic enones can be found in Table 2. α’-Substitution with an alkyl group 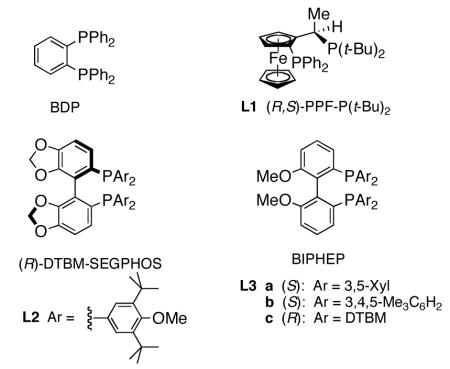 other than methyl in 1 leads to the desired product 3 in high ee using L3b, while α-substitution with residues including ethyl and n-pentyl (4 and 5) gives consistently high yields and ee’s of 1,2-addition products with one or both ligand systems.12 Modified educts with either α-phenyl (6) or α-bromo (7), likewise, lead to 1,2-adducts, albeit in somewhat lower ee’s. Replacing the β-phenyl group in 1 with an alkyl moiety (as in 8) did not alter the outcome of the reaction.
other than methyl in 1 leads to the desired product 3 in high ee using L3b, while α-substitution with residues including ethyl and n-pentyl (4 and 5) gives consistently high yields and ee’s of 1,2-addition products with one or both ligand systems.12 Modified educts with either α-phenyl (6) or α-bromo (7), likewise, lead to 1,2-adducts, albeit in somewhat lower ee’s. Replacing the β-phenyl group in 1 with an alkyl moiety (as in 8) did not alter the outcome of the reaction.
Table 2.
CuH cat. asymmetric 1,2-reductions of α-substituted enonesa
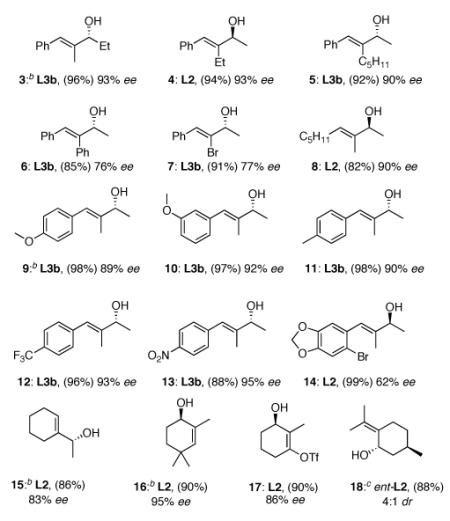 |
Reactions were carried out on 0.25 mmol scale in 0.5 mL Et2O. Isolated yields after column chromatography are given in parentheses. Ee’s were determined by chiral HPLC or GC analyses. Stereochemistry shown was determined by analogy to 2 (see Table 1).
Absolute stereochemistry determined by comparing optical rotations with known compounds.
See text.
See SI.
The impact of variation in substituents on a β-aryl ring in an educt was also investigated. Electron-donating as well as electron-withdrawing groups were tolerated and gave secondary allylic alcohols 9-14 in high yields and good ee’s. Surprisingly, a strong electron-withdrawing group (e.g. a nitro group) led to significant amounts of the corresponding 1,4-reduced product when L2 was used (see SI), whereas L3b gave the desired alcohol 13 with excellent regio- and stereocontrol.12
Various cyclic arrays (15-17) fit into the anticipated pattern of regio- and enantio-control using (DTBM-SEGPHOS)CuH. The mild conditions involved allow for isolation of a nonracemic cyclohexenol 17 bearing a cross-coupling partner vinyl triflate without losses due to ring fragmentation observed with harsher reducing agents.13 While treatment of (R)-pulegone with catalytic [(R)-L2]CuH gave the highly favored anticipated cis-product (93%; 99:1 dr), CuH complexed by ent-L2 led predominantly to the less common trans isomer 18 (88%; 4:1 dr).14
The influence exerted by an α-substituent is further highlighted by the case of exocyclic olefin-containing enone 19. Notwithstanding full accessibility of CuH to the β-site, delivery of hydride takes place in a 1,2-fashion, giving allylic alcohol 20 in 78% ee (Scheme 2).
Scheme 2.
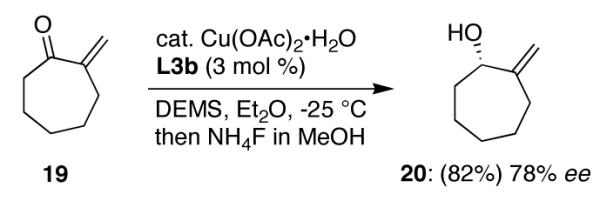
(L3b)CuH catalyzed 1,2-addition to a β,β-unsubstituted enone
The potential for a ligated CuH complex to induce asymmetry in two distinct functional groups within the same pot is illustrated in Scheme 3. Simultaneous exposure of enone 1 and enoate 21 (1:1 ratio) to conditions first favoring enone 1,2-reduction gave 2, with <5% conjugate reduction of 1 being observed. Without isolation, addition of t-BuOH (1.1 equiv), as originally reported by Stryker,6,15 was used to enhance the rate of catalyst regeneration. The presence of this additive, along with added silane (1.1 equiv), led to asymmetric 1,4-reduction of 21 to ester 22, both processes taking place in high isolated yields and excellent ee’s.
Scheme 3.
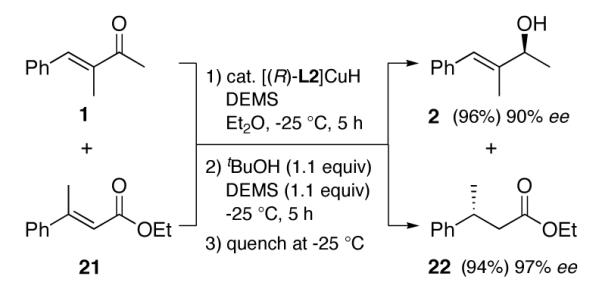
One reagent, two reactions: 1-pot asymmetric 1,2-reduction of an enone and 1,4-reduction of an enoate
In summary, regioselectivity in reactions of non-racemicaly ligated, in situ-generated CuH can be dramatically shifted to favor asymmetric 1,2-over normally observed 1,4-reductions of α,β-unsaturated ketones. This powerful methodology affords high yields and ee’s of resulting allylic alcohols of defined olefin geometries and central chirality.
Supplementary Material
Acknowledgement
Support for this work by the NIH is gratefully acknowledged. We are indebted to Takasago and Roche for supplying the SEGPHOS and BIPHEP ligands, respectively.
Footnotes
Supporting Information Available: Experimental details and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1)(a).Deutsch C, Krause N, Lipshutz BH. Chem. Rev. 2008;108:2916–2927. doi: 10.1021/cr0684321. Review: [DOI] [PubMed] [Google Scholar]; (b) Yun J, Kim D, Lee D. Angew. Chem., Int. Ed. 2006;45:2785–2787. doi: 10.1002/anie.200600184. [DOI] [PubMed] [Google Scholar]; (c) Lipshutz BH, Servesko JM, Taft BR. J. Am. Chem. Soc. 2004;126:8352–8353. doi: 10.1021/ja049135l. [DOI] [PubMed] [Google Scholar]; (d) Buchwald SL, Aye Y, Rainka MP. Proc. Nat. Acad. Sci. U.S.A. 2004;101:5821–5823. doi: 10.1073/pnas.0307764101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Czekelius C, Carreira EM. Angew. Chem., Int. Ed. 2003;42:4793–4795. doi: 10.1002/anie.200352175. [DOI] [PubMed] [Google Scholar]
- (2).Lipshutz BH, Noson K, Chrisman W. J. Am. Chem. Soc. 2001;123:12917–12918. doi: 10.1021/ja011529e. [DOI] [PubMed] [Google Scholar]
- (3).Lee C-T, Lipshutz BH. Org. Lett. 2008;10:4187–4190. doi: 10.1021/ol801590j. [DOI] [PubMed] [Google Scholar]
- (4).Lipshutz BH, Lower A, Noson K. Org. Lett. 2002;4:4045–4048. doi: 10.1021/ol026755n. [DOI] [PubMed] [Google Scholar]
- (5).Lipshutz BH, Shimizu H. Angew. Chem., Int. Ed. 2004;43:2228–2230. doi: 10.1002/anie.200353294. [DOI] [PubMed] [Google Scholar]
- (6).Chen J-X, Daeuble JF, Brestensky DM, Stryker JM. Tetrahedron. 2000;56:2153–2166. [Google Scholar]
- (7).For chemoselective Cu-catalyzed hydrogenation of enals: Shimizu H, Sayo N, Saito T. Synlett. 2009:1295–1298. for chemoselective Cu-cataylzed asymmetric hydrogenation of cyclic and acyclic enones: Shimizu H, Nagano T, Sayo N, Saito T, Ohshima T, Mashima K. Synlett. 2009:3143–3146. for chemoselective Cu-catalyzed reduction of α,β-unsaturated amino ketones: Pelss A, Kumpulainen ETT, Koskinen AMP. J. Org. Chem. 2009;74:7598–7601. doi: 10.1021/jo9017588. for chemo- and enantioselective hydrosilylation of enones using monodentate binaphthophosphepine ligands: Junge K, Wendt B, Addis D, Zhou S, Das S, Beller M. Chem. Eur. J. 2009;16:68–73. doi: 10.1002/chem.200902442.
- (8).Saito T, Yokozawa T, Moroi T, Sayo N, Miura T, Kumobayashi H. Adv. Synth. Catal. 2001;343:264–267. [Google Scholar]
- (9)(a).Schmid R, Broger EA, Cereghetti M, Crameri Y, Foricher J, Lalonde M, Mueller RK, Scalone M, Schoettel G, Zutter U. Pure & Appl. Chem. 1996;68:131–138. [Google Scholar]; (b) Schmid R, Foricher J, Cereghetti M, Schonholzer P. Helv. Chim. Acta. 1991;74:370–389. [Google Scholar]
- (10).Nishiyama H, Shiomi T, Tsuchiya Y, Matsuda I. J. Am. Chem. Soc. 2005;127:6972–6973. doi: 10.1021/ja050698m. [DOI] [PubMed] [Google Scholar]
- (11).Shimizu H, Nagasaki I, Saito T. Tetrahedron. 2005;61:5405–5432. [Google Scholar]
- (12).For results obtained using ligands other than the ones shown in Table 2 see Supporting Information.
- (13)(a).Stork G, Danheiser RL. J. Org. Chem. 1973;38:1775–1776. [Google Scholar]; (b) Kamijo S, Dudley GB. J. Am. Chem. Soc. 2006;128:6499–6507. doi: 10.1021/ja0608085. [DOI] [PubMed] [Google Scholar]
- (14).Ohkuma T, Ikehira H, Ikariya T, Noyori R. Synlett. 1997:467–468. [Google Scholar]
- (15)(a).Stryker JM, Mahoney WS, Daeuble JF, Brestensky DM. Catalysis of Organic Reactions. In: Pascoe WE, editor. Chem. Ind. Vol. 47. Marcel Dekker; New York: 1992. pp. 29–44. [Google Scholar]; (b) Hughes G, Kimura M, Buchwald SL. J. Am. Chem. Soc. 2003;125:11253–11258. doi: 10.1021/ja0351692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.