The term asthma was coined by Hippocrates to refer to the attacks of breathlessness and wheezing experienced by sufferers. Contributors to our understanding of bronchial asthma read like a “Who’s Who” of medical history including, among the ancients, Hippocrates and Galen, in the 12th century, Moses Maimonides, and in modern times, European, especially British, physicians (1). For centuries, the attacks of breathlessness characteristic of asthma were thought to be due to abnormalities of bronchial smooth muscle. Over the past two decades, the underlying inflammation associated with asthma, characterized by marked infiltration of eosinophils, has received increasing attention (2). With this recognition has come a renewed appreciation of the value of therapies directed at this inflammation, particularly the effective use of glucocorticoids, and especially inhaled steroids (3). Nonetheless, in spite of new insights into the nature of asthma, it continues to exert a significant toll on patients; evidence shows that it is increasing in prevalence and severity, especially in westernized societies. Indeed, current information suggests that the prevalence of asthma has risen steadily and has doubled over the past 20 years (4). Moreover, asthma continues to be a source of significant mortality in spite of the improved pharmacopoeia available to physicians (5).
Asthma is a member of the family of atopic diseases. In 1923, Coca and Cooke (6) proposed the term atopy to refer to the familial occurrence of asthma, allergic rhinitis (hay fever), and dermatitis (atopic dermatitis) associated with positive immediate skin test reactions to environmental antigens, such as ragweed pollen extracts. Prausnitz and Küstner (7) then showed that the sera of allergic patients contain a specific active substance, which in the 1960s was identified by K. Ishizaka and coworkers (8) as the fifth immunoglobulin class, namely IgE. Subsequently, the discovery of an IgE myeloma protein by Johansson and coworkers (9) made reagents for measurement of total IgE protein and IgE antibodies readily available. These new reagents permitted precise characterization of IgE protein levels and revealed that the atopic individual differs from the normal individual by an increased concentration of IgE protein and by a propensity to produce IgE antibodies to a variety of commonly encountered environmental antigens (9, 10). In the 1980s, interleukin (IL)-4 and IL-5 were discovered as critical cytokines regulating the commitment of B cells to IgE and the production of eosinophils by the bone marrow, respectively (11, 12). Immune responses by T lymphocytes were also divided into two classes (13): TH1 responses associated with the production of IL-2 and interferon (IFN)-γ and TH2 responses associated with the production of IL-4 and IL-5. Analyses of lymphocytes from patients showed that IL-4 and IL-5 expression is associated with atopic disease (14). The increased prevalence of atopic diseases in westernized societies is a subject of great interest especially because it may be due to decreased infectious diseases exposure, especially to tuberculosis, and to a shift of the immune response to the TH2 type (15). Knowledge that bronchial asthma is associated with IgE elevations, especially in younger people, has pointed to an important role for IgE in the pathophysiology of bronchial asthma. Yet, clinicians experienced in the care of patients with asthma recognize that a subset of patients, especially those developing the disease later in life, around the age of 40, experience a form of asthma that is not associated with IgE (and usually not familial), referred to by Rackemann (16) as intrinsic asthma. These patients are similar to their younger sufferers, except that they often display a more marked blood eosinophilia, frequently develop nasal polyps, and occasionally experience worsening of asthma after ingestion of aspirin and other nonsteroidal analgesics. Few investigations of these patients have been conducted, but certain studies have concluded that IL-5 and not IL-4 is expressed in these patients (17), and many clinicians believe that intrinsic asthma is initiated by viral infections. Thus, the observations from clinical medicine point to the possibility of two kinds of asthma, one associated with IgE and allergy to environmental antigens and another not associated with IgE elevation or with allergy to antigens.
Recognition that asthma is an inflammatory disease has focused attention on the mechanisms of this inflammation. Over the past decade, animal models, including guinea pigs, monkeys, and mice, have been employed to dissect the inflammation associated with asthma (18). These studies have examined the importance of IL-5 and eosinophils, and the roles of adhesion molecules, T cells, and immune reactants, including immunoglobulins. The models have also been useful in tests of new drugs for the treatment of asthma. Because of the ability to manipulate the genome of mice by production of transgenic lines and by ablation of specific genes through homologous recombination, increasingly, the mouse has served as the model for analyses of the inflammation associated with asthma.
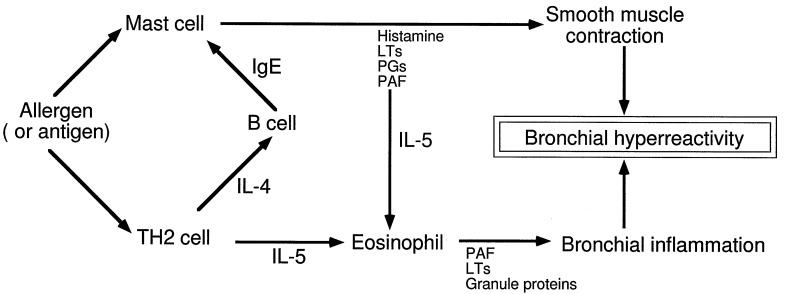
In a recent report, Mehlhop and coworkers (19) used IgE-deficient mice to explore the role of IgE in the inflammation associated with bronchial asthma. Surprisingly, they found that bronchial inflammation is undiminished as judged by the occurrence of eosinophils in the lung, infiltration of cells into the bronchi, and the development of bronchial hyperresponsiveness, namely the exaggerated bronchoconstrictor response of the airways to a variety of specific and nonspecific stimuli. This result is surprising because of the presumed primacy of IgE in allergic sensitization and because of prior results showing the importance of IgE in murine asthma (20, 21). By inference, the results of Mehlhop and coworkers (19) also cast doubt on the role of the mast cell in murine asthma. Yet in the mouse, two classes of immunoglobulins can sensitize mast cells and trigger anaphylaxis, namely the IgE and IgG isotypes (22, 23), and it is likely that the animals in the report (19) made an IgG response. Thus, mast cells sensitized by IgG antibodies could play a role in the IgE-deficient mice by secreting mediators and cytokines; if so, then the underlying pathophysiology, requiring production of IL-5 and recruitment of eosinophils, might not differ appreciably from the wild-type animals. Analyses of bronchoalveolar fluids from the IgE-deficient mice failed to show evidence of histamine, but, as the authors note, this could be an assay sensitivity problem (19). Perhaps measurement of another marker of mast cell degranulation, such as a mast cell protease, would have been useful to determine whether mast cell activation and degranulation occurred. Still, prior analyses of mast cell-deficient mice have shown that these animals demonstrate degrees of bronchial eosinophilia and inflammation comparable to those of normal mice (21, 24). Therefore, the observations by Mehlhop and coworkers (19) along with the prior findings in the mast cell-deficient mice make it unlikely that TH2 cytokines were produced by the mast cell. Rather T cells become the most likely suspects (Fig. 1), and abundant evidence exists that they are able to produce the TH2 cytokines needed in the murine model of asthma (25). Furthermore, the importance of IL-5 is shown by the inability of IL-5-deficient mice to develop eosinophilia, lung damage, and bronchial hyperreactivity after aeroallergen challenge (26).
Figure 1.
Mechanisms of inflammation in murine asthma. The observations that IgE-deficient and mast cell-deficient mice are able to develop bronchial eosinophilia and hyperreactivity suggest that the TH2 pathway is the critical pathway. PAF, platelet-activating factor; PGs, prostaglandins; LTs, leukotrienes.
So what does the IgE-deficient mouse teach us about asthma? First, the mere presence of IgE is not critical. Either another immunoglobulin, such as IgG, permits sensitization and activation of mast cells with production of IL-5 and/or IL-5 is produced by TH2 lymphocytes. Investigation of the cells producing cytokines in the model, with particular attention to IL-5, will be of interest. Second, we recognize intrinsic asthma as a form of the disease occurring in older individuals and related neither to antigen sensitization and exposure nor to elevations of IgE. Is the model of murine asthma here analogous to intrinsic asthma? One presumes that the IgE-deficient mice have produced IgG antibodies, and this raises the question of whether clinicians should be concerned with the occurrence of IgG antibodies in patients with intrinsic asthma. Of interest, IgG antibodies bind to FcγRII (CD32) and activate eosinophils, resulting in eosinophil degranulation with release of its biologically active cationic proteins (27). Third, one must keep in mind the caveat that all of the animal models of asthma are antigen-driven and, thus, are useful only to the degree that they permit sharp dissection of the mechanisms of inflammation. The IgE-deficient mouse may be a useful model for intrinsic asthma, and the knowledge that eosinophil-associated inflammation occurs in this model should stimulate investigations of the mechanisms of inflammation in the mouse, as well as parallel investigations of patients with intrinsic asthma. Finally, the animal models of asthma faithfully reproduce some but not all of the manifestations of the human disease. For example, in patients, the pathology of asthma usually involves only the respiratory tract, whereas the pathology in the models often extends into the lung parenchyma and even to the visceral pleura. Also the degree of bronchial hyperreactivity is more marked in patients and often strikingly so. Indeed, one cannot perform a complete concentration response analysis in patients because of the danger this procedure would entail. Thus, one must be cognizant not only of the power of the murine model but also of its weaknesses.
Acknowledgments
This work was supported by National Institutes of Health Grants AI 09728, AI 34577, AI 34486, and AI 07047, and by the Mayo Foundation.
References
- 1.Bradding P, Freezer N J, Scheffer A L, Holgate S T. In: Samter’s Immunologic Diseases. 5th Ed. Frank M M, Austen K F, Clamen H N, Unanue E R, editors. Brown, Boston: Little; 1995. pp. 1293–1327. [Google Scholar]
- 2.Hamann K J, Gleich G J, Gundel R H, White S R. In: The Airway Epithelium: Physiology, Pathophysiology, and Pharmacology. Farmer S G, Hay D W P, editors. New York: Dekker; 1991. pp. 255–300. [Google Scholar]
- 3.Schleimer R P, Busse W W, O’Byrne P M. Inhaled Glucocorticoids in Asthma: Mechanisms and Clinical Actions. New York: Dekker; 1997. [Google Scholar]
- 4.Seaton A, Godden D J, Brown K. Thorax. 1994;49:171–174. doi: 10.1136/thx.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Morbid Mortal Wkly Rep. 1996;45:350–353. [Google Scholar]
- 6.Coca A F, Cooke R A. J Immunol. 1923;8:163–182. [Google Scholar]
- 7.Prausnitz C, Küstner H. Zentralbl Bakteriol Parasitenkd Infektionskrankh Hyg Abt. 1921;86:160–169. [Google Scholar]
- 8.Ishizaka K, Ishizaka T, Hornbrook M M. J Immunol. 1966;97:75–85. [PubMed] [Google Scholar]
- 9.Wide L, Bennich H, Johansson S G O. Lancet. 1967;ii:1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]
- 10.Johansson S G O. Lancet. 1967;ii:951–953. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- 11.Snapper C M, Finkelman F D, Paul W E. Immunol Rev. 1988;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson C J, Campbell H D, Young I G. Immunol Rev. 1988;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann T R, Coffman R L. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 14.Corrigan C J, Kay A B. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 15.Shirakawa T, Enomoto T, Shimazu S, Hopkin J M. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 16.Rackemann F M. J Lab Clin Med. 1927;12:1185–1197. [Google Scholar]
- 17.Walker C, Bode E, Boer L, Hansel T T, Blaser K, Virchow J C., Jr Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 18.Kung T T, Jones H, Adams G K, III, Umland S P, Kreutner W, Egan R W, Chapman R W, Watnick A S. Int Arch Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 19.Mehlhop P D, van de Rijn M, Goldberg A B, Brewer J P, Kurup V P, Martin T R, Oettgen H C. Proc Natl Acad Sci USA. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lack G, Oshiba A, Bradley K L, Loader J E, Amran D, Larsen G L, Gelfand E W. Am J Respir Crit Care Med. 1995;152:1765–1773. doi: 10.1164/ajrccm.152.6.8520735. [DOI] [PubMed] [Google Scholar]
- 21.Coyle A J, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. J Exp Med. 1996;183:1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussenzweig R S, Merryman C, Benacerraf B. J Exp Med. 1964;120:315–328. doi: 10.1084/jem.120.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oettgen H C, Martin T R, Wynshaw-Boris A, Deng C, Drazen J M, Leder P. Nature (London) 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 24.Brusselle G G, Kips J C, Tavernier J H, van der Heyden J G, Cuvelier C A, Pauwels R A, Bluethmann H. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 25.Gavett S H, Chen Z, Finkelman F, Wills-Karp M. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 26.Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko M, Swanson M C, Gleich G J, Kita H. J Clin Invest. 1995;95:2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]