Background: The tomato leucine aminopeptidase (LAP) regulates wound signaling, and its mode of action is unknown.
Results: Plant LAPs are molecular chaperones, and the chaperone activity of the tomato LAP-A is independent of its peptidase and enhanced upon hexamer disruption.
Conclusion: Plant LAPs are bifunctional, with both aminopeptidase and chaperone activities.
Significance: Plant LAPs are a new class of molecular chaperone with roles in plant defense.
Keywords: Aminopeptidase, Molecular Chaperone, Multifunctional Enzymes, Plant Defense, Site-directed Mutagenesis, Herbivory, Plant-Insect Interactions
Abstract
Leucine aminopeptidases (LAPs) are present in animals, plants, and microbes. In plants, there are two classes of LAPs. The neutral LAPs (LAP-N and its orthologs) are constitutively expressed and detected in all plants, whereas the stress-induced acidic LAPs (LAP-A) are expressed only in a subset of the Solanaceae. LAPs have a role in insect defense and act as a regulator of the late branch of wound signaling in Solanum lycopersicum (tomato). Although the mechanism of LAP-A action is unknown, it has been presumed that LAP peptidase activity is essential for regulating wound signaling. Here we show that plant LAPs are bifunctional. Using three assays to monitor protein protection from heat-induced damage, it was shown that the tomato LAP-A and LAP-N and the Arabidopsis thaliana LAP1 and LAP2 are molecular chaperones. Assays using LAP-A catalytic site mutants demonstrated that LAP-A chaperone activity was independent of its peptidase activity. Furthermore, disruption of the LAP-A hexameric structure increased chaperone activity. Together, these data identify a new class of molecular chaperones and a new function for the plant LAPs as well as suggesting new mechanisms for LAP action in the defense of solanaceous plants against stress.
Introduction
All organisms contain a complex array of aminopeptidases that cleave N-terminal residues from proteins and peptides (1). Aminopeptidases have important roles in N-terminal Met removal, protein turnover, protein maturation, and generation or catabolism of bioactive peptides that are important in a variety of physiological processes (2, 3). Aminopeptidases may be important in exposing penultimate amino acid residues that may profoundly affect a protein's half-life as predicted by the N-end rule (4–6). The plant aminopeptidase complement is distinctive, with a paucity of vacuolar-localized and membrane-bound aminopeptidases relative to animals (6, 7). In plants, aminopeptidases modulate wound signaling (8), meiotic recombination (9), cell cycle progression (10), and embryonic and seedling development (10, 11).
Leucyl aminopeptidases (LAPs3; EC.3.4.11.1) belong to the M17 family of peptidases. LAPs are highly conserved, di-zinc metallopeptidases found in plants, animals, and microbes (1). Animal LAPs may have a role in the turnover of oxidatively damaged proteins in the lens of the eye (12). Human LAP was proposed to process peptides released from the 26S proteasome for use in MHC I presentation; however, its role in this process is not essential (3). In contrast, a Escherichia coli LAP (PepA) is multifunctional. It is an aminopeptidase and a DNA-binding protein that mediates site-specific recombination in ColE1 plasmids and acts as a transcription factor to modulate the carAB operon (13). The complement of LAPs in plants is more complex, and their roles are being elucidated.
In plants, there are two classes of LAPs, which are 70–77% identical (14). The LAPs with neutral pIs (LAP-N) are detected in all plants and are constitutively expressed (14, 15). The LAPs with acidic pIs (LAP-A) are found only in a subset of the Solanaceae and are induced in response to both biotic and abiotic stresses (14–16). Recently, LAPs were shown to be important in insect deterrence in Solanum lycopersicum (tomato) and Solanum nigrum (nightshade) (8, 17). Silencing of LAPs in transgenic tomatoes or transiently in nightshade plants made plants more susceptible to Manduca sexta feeding, and insect masses were larger than insects grown on wild-type plants (8, 17). Reciprocally, transgenic tomatoes that ectopically express the tomato LAP-A were more resistant to M. sexta feeding, and delays in insect growth and development were displayed (8). The tomato LAP-A modulates the late branch of wound signaling downstream of the synthesis of the defense hormone jasmonic acid. By controlling the late branch of wounding, LAP regulates the levels of critical defense proteins that deter herbivore growth and development. To date, the in vivo substrates of LAP-A have yet to be discovered.
LAP-A resides within the plastid (18), which is a dynamic compartment subject to many cellular stresses during development and biotic/abiotic stresses that can result in protein damage and aggregation. In order to prevent the accumulation of misfolded proteins, cells express a wide range of molecular chaperones (19). Molecular chaperones can act as “holdases” by binding to misfolded proteins to prevent aggregation or as “foldases” by actively refolding misfolded proteins (19, 20). There are five major classes of chaperones in plants (21, 22). The most abundant and diverse class of chaperones is the “holdase” class of small heat-shock proteins (sHSPs).
Molecular chaperone activity has also been revealed in proteins with other primary biological functions, including thioredoxin, peroxidase, elongation factors (eEF1a and EF-TU), and several protein-degrading enzymes (23–27). For example, several plastid ATP-dependent proteases are molecular chaperones (28, 29). Currently, no plant aminopeptidase is known to function as a molecular chaperone. However, the tomato LAP-A has several compelling biochemical characteristics that are shared with molecular chaperones, including its stability, high temperature optimum (60–70 °C), high pH optimum (9.0), and induction by a wide range of stresses (16, 30). Finally, two microbial proteins are known to possess both chaperone and aminopeptidase activity in vitro: the E. coli Hsp31 chaperone and a Shizosaccharomyes pombe aspartyl aminopeptidase (25, 31).
Although these Hsp31 and aspartyl aminopeptidases do not share conserved protein domains with the plant M17 peptidases, the discovery of the aminopeptidase-chaperone bifunctionality in these microbial enzymes prompted investigations into the plant LAPs. Here, the in vitro chaperone activities of plant LAPs were studied by assaying the ability of the tomato and Arabidopsis LAPs to prevent protein unfolding, prevent protein aggregation, and promote protein refolding. These assays indicated that the tomato LAP-A and LAP-N and Arabidopsis LAP1 and LAP2 form a new class of molecular chaperones in plants. Assays performed on LAP-A active site mutants also indicated that the chaperone activity of LAP-A was independent of its peptidase activity and that disruption of the LAP-A hexameric structure, which is essential for its peptidase activity, increased chaperone activity. In contrast, a catalytically inactive LAP-N was impaired in chaperone activity. These data shed new light on the complexity of plant LAPs and suggest new potential roles for LAPs in defending tomato against stress.
EXPERIMENTAL PROCEDURES
Isolation of AtLAP1 and AtLAP2 cDNAs
RNA was isolated from 1-week-old seedlings of Arabidopsis thaliana ecotype Columbia by the hot phenol method (32). Total RNA (5 μg) was used to synthesize first-strand cDNA using the Smart PCR cDNA synthesis kit (Clontech, Palo Alto, CA) and an oligo(dT) primer. LAP1 (At2g24200) and LAP2 (At4g30920) coding regions were cloned by RT-PCR using gene-specific primers (supplemental Table 1). The resulting PCR-amplified cDNA fragments included the entire coding region of LAP1 and the mature protein of LAP2 (excluding the plastid transit peptide) (6, 33).
PCR was performed with two cycles of 30 s at 94 °C, 30 s at 52 °C, and 2 min at 72 °C. This was followed with 30 cycles of 2 min at 95 °C, 30 s at 65 °C, and 2 min at 72 °C. In healthy leaves, the AtLAP2 RNA is present at low levels, and the LAP2 RNA was not detected after 30 PCR cycles. Therefore, 1 μl of the primary PCR product was used as a template for a second round of 30 PCR cycles. The PCR products amplified using Ex-Taq (Dakara, Madison, WI) and were cloned into pGEM T-easy (Promega, Madison, WI) to generate pGEM-LAP1 and pGEM-LAP2. Fidelity of the cDNA sequences was determined by DNA sequencing at the Genomics Core Facility at the Institute of Integrative Genome Biology (University of California, Riverside). pGEM-LAP clones were digested with corresponding restriction enzymes and cloned into the pET28 expression vector (Novagen, Darmstadt, Germany). The resulting clones, pET-LAP1 and pET-LAP2, expressed LAP proteins with N-terminal His6 fusions (His6-LAP1 and His6-LAP2, respectively).
Isolation of LAP-N Lys-357 Substitution Mutants
LAP-N Lys-357 mutants were generated using the QuikChange Lightning Multi Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The template was the pQLapN plasmid that contains a His6-LAP-N coding region (14). The primer used for introducing mutations in the Lys-357 codon (in boldface type) was 5′-GGTGGCTACAACATCNNKACTGGACCTGGTTG-3′. Plasmids containing putative Lys-357 mutations were sequenced to identify the residue substitution and confirm that no polymerase errors occurred at other locations in the His6-LAP-N coding region. Twelve mutants that had a 357-residue substitution were confirmed, and eight mutants (K357E, K357R, K357L, K357C, K357M, K357G, K357T, and K357P) were further characterized.
Overexpression and Purification of LAP Proteins
The E. coli vectors that express the His6-LAP-A, His6-LAP-N, and the His6-LAP-A mutants (R431A, K354R, K354E, D347N, D347R, E429R, and E429V) were previously described (14, 34). His6-LAP fusion proteins were expressed in and purified from E. coli according to Gu and Walling (35) with minor modifications. Cultures were grown at 37 °C overnight. Overnight cultures were diluted 1:20, and the cultures (0.1–1 liter) were grown at 37 °C (wild-type and mutant His6-LAP-As) or 30 °C (His6-LAP1, His6-LAP2, and wild-type and mutant His6-LAP-Ns) to A600 = 0.6. At this time, cultures were induced with 0.4 mm isopropyl 1-thio-β-d-galactopyranoside and allowed to grow for an additional 6–18 h at 37 °C (wild-type and mutant His6-LAP-As), 30 °C (His6-LAP1 and His6-LAP2), or 22 °C (wild-type and mutant His6-LAP-Ns). Cells were resuspended in 5 volumes of prechilled Buffer A (50 mm NaPO4, pH 8.0, 300 mm NaCl) with 75 mm lysozyme. After a 0.5-h incubation on ice, cells were lysed using six 10-s sonicator pulses followed by 10 s on ice. The lysate was cleared at 10,000 × g for 30 min at 4 °C.
With the exception of the His6-LAP-N mutants, His6-LAP proteins were purified using nickel-nitrilotriacetic acid resin columns (Qiagen, Valencia, CA) as described previously (35). For the His6-LAP-N mutant analyses, His6-LAP-N wild type and mutants (K357E, K357R, K357L, K357C, K357M, K357G, K357T, and K357P) were expressed (100 ml), and cleared lysates were prepared as described above. Cleared lysates were loaded onto 0.2-ml nickel-nitrilotriacetic acid spin resin columns (Thermo Fisher Scientific, Rockford, IL) equilibrated with Buffer A. The column was washed twice with 0.5 ml of Buffer A with 20 mm imidazole and twice with 0.5 ml of Buffer A with 40 mm imidazole. His6-LAP-N proteins were eluted with Buffer A with 250 mm imidazole and collected in 0.2-ml fractions. LAP-A wild-type and mutant proteins were stored in 25 mm sodium phosphate (pH 8.0), 250 mm NaCl, 125 mm imidazole, and 50% glycerol at −20 °C until use; LAP-A is stable for 1 year under these conditions. LAP-N wild-type and mutant proteins were used on the day they were purified, due to their limited stability (5 days) under these storage conditions (14).
Protein concentrations were determined with the Bradford method using IgG as a standard (Bio-Rad Protein Assay Kit I). To determine the molecular masses of the WT and mutant His6-LAP-A and His6-LAP-N complexes, purified His6-LAP-As and His6-LAP-Ns were fractionated on a set of four native polyacrylamide gels (7.5–12%, w/v) based on the methods of Bryan (36, 37). The proteins used as molecular mass standards included chicken egg albumin (45 kDa), bovine serum albumin monomer (66 kDa) and dimer (132 kDa), and tomato His6-LAP-A hexamer (357 kDa). Protein purity was determined by SDS-PAGE and LAP masses were determined by native PAGE by staining with Coomassie Brilliant Blue R-250 (38). For all assays, molar amounts of the mature WT and non-disruption mutant proteins were calculated using hexameric values (LAP-A, 357 kDa; LAP-N, 365 kDa; LAP1, 327 kDa; LAP2, 330 kDa), whereas molar amounts for mature proteins of disruption mutants were calculated using the mass of the monomer (55 kDa).
LAP Activity Assay
The peptidase activities of all LAPs were determined prior to use in chaperone assays. LAP activity was determined using the fluorescent substrate, leucine-amino methyl coumarin (Leu-AMC; Bachem, Bunderdorf, Switzerland). Purified His6-LAPs (2160 ng) were preincubated in assay buffer (50 mm Tris-HCl, pH 8, 0.5 mm MnCl2) in a total volume of 162 μl in a 96-well microtiter plate. Activity assays were initiated with the addition of Leu-AMC (1.58 μm) and proceeded for 30 min at 37 °C. Reactions were performed in duplicate. Leu-AMC hydrolysis was quantified by measuring the fluorescence emission of AMC at 460 nm by the Victor2 1420 Multilabel Counter (PerkinElmer Life Sciences). The extinction coefficient for AMC at 460 nm was 16,500 m−1 cm−1. The Leu-AMC hydrolyzing activities of wild-type His6-LAP proteins were as follows: His6-LAP-A, 0.59 ± 0.01 μmol min−1 mg−1; His6-LAP-N, 0.24 ± 0.01 μmol min−1 mg−1; His6-LAP1, 0.22 ± 0.01 μmol min−1 mg−1; His6-LAP2, 0.22 ± 0.01 μmol min−1 mg−1. Consistent with previous reports, the His6-LAP-A mutant proteins were peptidase-deficient, with Leu-AMC-hydrolyzing activities less than 0.01 μmol min−1 mg−1 (34).
Thermal Restriction Enzyme Protection Assay
Thermal protection of the restriction enzyme NdeI was performed according to Santhoshkumar and Sharma (39). The reactions contained 1 unit of NdeI (New England Biolabs, Beverly, MA), 1× New England Biolabs restriction enzyme buffer 4, 4% glycerol, LAP (0–2 μm), Pisum sativum Hsp18.1 (0–2 μm), protein A (0–2 μm) (Sigma), or lysozyme (0–48 μm) (Sigma) in a final volume of 13 μl. PsHsp18.1 was kindly donated by Dr. Elizabeth Vierling (University of Massachusetts, Amherst, MA). Because lysozyme (14.7 kDa) is 24 times smaller than the LAP-A hexamer (365 kDa), higher concentrations of lysozyme were used to get equal protein amounts as a negative control. Protein A (42 kDa) is approximately the same size as the LAP monomer (∼55 kDa) and therefore was used in equal molar amounts. NdeI, NdeI-LAP, NdeI-PsHsp18.1, and NdeI-lysozyme mixes were incubated for 90 min in a 43 °C water bath. At this time, 140 ng of plasmid DNA (cLEX-6-H6; 2 μl) was added. Digestion was allowed to occur for 90 min at 37 °C. Digested plasmid DNA was visualized by electrophoresis on a 1% agarose gel stained with ethidium bromide. NdeI digestion of plasmid DNA at 37 °C (without the 43 °C incubation) served as a positive control. cLEX-6-H6 is a cDNA clone encoding a GID-like gibberellin receptor (SGN-E304247; Sol Genomics Network). NdeI cuts at two sites in this 4.8-kb plasmid, releasing fragments 4.6 and 0.2 kb. His6-LAP-A was stored in 50% glycerol to prolong its stability, and for this reason, NdeI-His6-LAP-A reactions had 4% glycerol. To ensure that the thermal protection provided by His6-LAP-A was due to chaperone activity and not higher glycerol levels, His6-LAP-A was purified and stored without glycerol. When tested in the NdeI thermal inactivation assay, the glycerol-free and 50% glycerol His6-LAP-A had similar levels of chaperone activity toward NdeI (supplemental Fig. S1).
Thermal Citrate Synthase Aggregation Assay
Aggregation assay reactions contained 300 nm citrate synthase (Sigma), 50 mm HEPES-KOH (pH 7.5), 5% glycerol, and 0–1200 nm purified His6-LAP in a total volume of 600 μl. The mix was placed in a plastic cuvette and heated in a 43 °C water bath. Light scattering at 360 nm was measured at the indicated times (0–60 min) using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Rockford, IL). As a negative control, lysozyme (1200 nm) or protein A (1200 nm) was added instead of LAP protein in separate reactions. PsHsp18.1 (1200 nm) was used as a positive control.
Luciferase Refolding Assay
Prior to assays, His6-LAP-A wild-type and mutant proteins were dialyzed against Buffer A using “V” series membranes (0.05 μm; Millipore, Billerica, MA) to remove glycerol and imidazole. LAP-N wild-type and mutant proteins were used fresh and were in glycerol-free Buffer A. Firefly luciferase (Luc) refolding was measured according to Siddique et al. (40) with some modifications allowing use of a 96-well format. Heating reactions contained 1 μm QuantiLum recombinant Luc (Promega, Madison, WI) and 0–6 μm His6-LAP, 1 μm PsHsp18.1, 3 μm protein A, or 3 μm lysozyme in 2.5 mm HEPES-KOH (pH 7.5), 5 mm MgCl2, 150 mm KCl, and 2 mm dithiothreitol (DTT) (total volume of 25 μl). Samples were heated for 11 min at 42 °C and chilled on ice for 5 min. One μl of heated samples was added to the reactivation mix that included 24 μl of rabbit reticulocyte lysate (RRL) (Promega), 25 mm HEPES-KOH (pH 7.5), 2 mm ATP, 5 mm MgCl2, 10 mm KCl, and 1 mm DTT; the final volume was 40 μl and had a final concentration of 25 nm Luc. Fifty μl of the Luc assay system (Promega) was added to 10-μl aliquots of the reactivation mix in a 96-well microtiter plate and incubated at 30 °C. For the His6-LAP-A (wild-type and mutants), His6-LAP-N, His6-LAP1, and His6-LAP2 assays, luminescence was measured using a LUMIstar Galaxy luminometer (BMG Labtechnologies, Offenberg, Germany) with an integration time of 10 s. Luminescence for the His6-LAP-N mutant studies was measured using a TriStar LB 941 luminometer (Berthold, Oak Ridge, TN) with an integration time of 10 s. The percentage activity corresponds to the relative luminescence compared with unheated luciferase.
RESULTS
Tomato LAP-A Exhibits Chaperone Activity toward Three Model Substrates
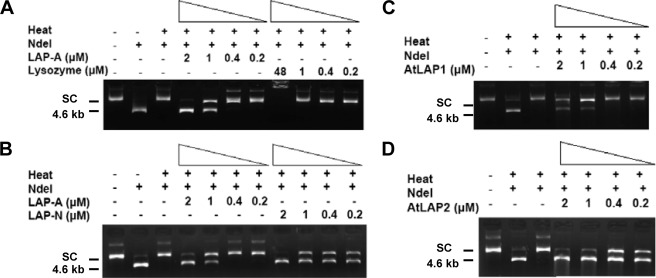
Three assays were used to evaluate the chaperone activity of tomato His6-LAP-A. The ability of His6-LAP-A to prevent protein unfolding was demonstrated in a thermal denaturation assay. The restriction enzyme NdeI was heated for 90 min at 43 °C either alone, with lysozyme or protein A (negative controls), with PsHsp18.1 (positive control), or with His6-LAP-A (0.2–2.0 μm). NdeI activity was measured by cutting of plasmid DNA. NdeI was inactivated after 90 min at 43 °C (Fig. 1A). The addition of 1–2 μm His6-LAP-A protected NdeI from thermal inactivation, whereas lysozyme and protein A did not (Fig. 1A and supplemental Fig. S2A). PsHSP18.1 was able to protect at concentrations as low as 0.2 μm (supplemental Fig. S2B). The level of His6-LAP-A chaperone activity was similar to that reported for the bovine α-crystallins (39).
FIGURE 1.
Tomato and Arabidopsis LAPs protect NdeI from thermal inactivation. NdeI (1 unit) was incubated in buffer with 4% glycerol (A–D) in the presence or absence of His6-LAP-A (0.2–2 μm; A and B), lysozyme (0.2–48 μm; A), His6-LAP-N (0.2–2 μm; B), His6-LAP1 (0.2–2 μm; C), or His6-LAP2 (0.2–2 μm; D) for 90 min at 43 °C. At this time, 140 ng of plasmid DNA was added and digested for 90 min at 37 °C. Control lanes show plasmid DNA only and DNA after digestion with unheated NdeI. NdeI cuts at two sites in the 4.8-kb plasmid, releasing fragments of 4.6 and 0.2 kb; only the 4.6-kb fragment is shown on these gels. The monomeric supercoiled plasmid (SC) and multimeric supercoils are observed in undigested DNA samples.
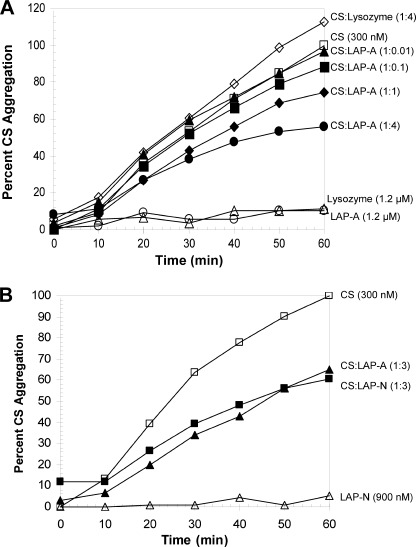
The ability of His6-LAP-A to protect the model substrate citrate synthase (CS) from heat-induced aggregation was tested. CS (300 nm) was heated for 60 min at 43 °C in the presence or absence of His6-LAP-A, and CS aggregation was measured by light scattering. His6-LAP-A protected CS from aggregation in a dose-dependent manner with activity seen with as little as 300 nm His6-LAP-A (Fig. 2A). Neither lysozyme (1.2 μm) nor protein A (1.2 μm) prevented CS aggregation, whereas 1.2 μm His6-LAP-A reduced CS aggregation by ∼50% (Fig. 2A and supplemental Fig. S3). PsHSP18.1 was able to completely protect CS at 1.2 μm (supplemental Fig. S3).
FIGURE 2.
Tomato LAP-A and LAP-N protect CS from thermal aggregation. A, CS (300 nm) was incubated in 50 mm HEPES-KOH (pH 7.5), 5% glycerol, and His6-LAP-A or lysozyme (1200 nm) (♢) at 43 °C for 60 min. The LAP-A concentrations of 0 (□), 3 (▴), 30 (■), 300 (♦), or 1200 nm (●) corresponded to CS/LAP-A ratios of 1:0.01, 1:0.1, 1:1, and 1:4, respectively. Neither His6-LAP-A (○) nor lysozyme (△) aggregated on their own. B, CS was incubated with 900 nm His6-LAP-A (■) or His6-LAP-N (▴). His6-LAP-N did not aggregate on its own (△). Aggregation of CS was determined by measuring light scattering at 360 nm. After 60 min at 43 °C, aggregation of 300 nm CS reached an absorbance of 0.8–1. Data shown are representative of two or more independent experiments. His6-LAP-A (900 nm) reduction of CS aggregation varied in independent assays from 40 to 60%.
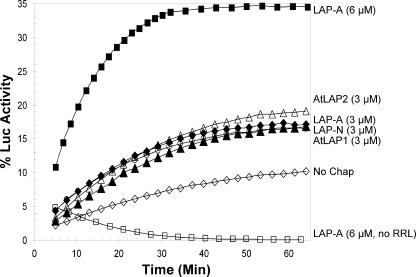
The third chaperone assay assessed if His6-LAP-A could facilitate the refolding of the heat-sensitive Luc to its native state (41). Luc (1 μm) was heated at 42 °C for 11 min alone or with 3–6 μm of His6-LAP-A or 1 μm PsHsp18.1 (positive control). Luc was then allowed to refold in RRL supplemented with 2 mm ATP; RRL is a rich source of ATP-dependent chaperones (HSP70 system) (42). In the absence of His6-LAP-A or PsHsp18.1, less than 5% of Luc activity was detected (Fig. 3). His6-LAP-A was an effective chaperone because 3 and 6 μm His6-LAP-A restored 17 and 35% of Luc activity, respectively. His6-LAP-A-mediated refolding of Luc was dependent on the presence of the RRL. In comparison, 38% activity of Luc was protected by 1 μm PsHsp18.1 (supplemental Fig. S4A; as previously shown in Ref. 41), and protein A did not enable Luc refolding (supplemental Fig. S4B). These data indicated that His6-LAP-A protected Luc from complete denaturation and thereby enabled its refolding by the ATP-dependent chaperones similar to well characterized sHSPs (supplemental Fig. S4A) (19, 20, 22).
FIGURE 3.
Tomato and Arabidopsis LAPs aid refolding of Luc. Luc (1 μm) was heated for 11 min at 42 °C with 3 μm (♦) or 6 μm His6-LAP-A (■), 3 μm His6-LAP-N (▴), 3 μm His6-LAP1 (○), 3 μm His6-LAP2 (△), or no chaperone (no chap, ♢). Luc was allowed to refold in the presence of RRL supplemented with 2 mm ATP. Luc was also heated in the presence of 6 μm His6-LAP-A allowed to refold without RRL (□). Percent activity corresponds to the relative luminescence compared with unheated luciferase. Measurements were taken for three technical replicates. Data are representative of two or more independent experiments. The degree of His6-LAP-A (3 μm) protection of Luc varied in independent experiments, ranging from 14 to 20%. The ability of Luc to refold in the absence of ATP-independent chaperone varied in independent experiments, ranging from 2 to 10%.
Tomato LAP-N and Arabidopsis LAPs Are Molecular Chaperones
Because the stress-inducible LAP-A of tomato displayed chaperone activity based on three independent chaperone assays, the chaperone activities of the tomato LAP-N were tested. Using the NdeI thermal protection assay, chaperone activity was detected using as little as 0.2 μm His6-LAP-N (Fig. 1B). His6-LAP-N was at least 5-fold more active than His6-LAP-A in this assay. In the CS-aggregation assay, His6-LAP-N and His6-LAP-A displayed similar chaperone activity levels, with 900 nm His6-LAP-N and His6-LAP-A preventing ∼40% of CS aggregation (Fig. 2B). In the Luc refolding assay, His6-LAP-N (3 μm) enabled Luc refolding. This level of chaperone activity was similar to His6-LAP-A, with ∼17% of Luc activity being restored in the presence of 3 μm His6-LAP-N (Fig. 3).
To determine if chaperone activity was solely associated with the tomato LAPs or characteristic of plant LAPs, the chaperone activities of Arabidopsis cytosolic LAP1 and plastid-localized LAP2 were tested. LAP1 and LAP2 are orthologs of the tomato LAP-N (14). His6-LAP1 (1–2 μm) prevented thermal inactivation of NdeI; this level of chaperone activity was similar to the tomato His6-LAP-A (Fig. 1, A and C). In contrast, chaperone activity of His6-LAP2 was detected at 0.2 μm, similar to the tomato His6-LAP-N (Fig. 1, B and D). In addition, both His6-LAP1 (3 μm) and His6-LAP2 (3 μm) were able to protect Luc from thermal inactivation and restored Luc activity to 17 or 19% of the unheated control, respectively. These activity levels were similar to the tomato LAPs (Fig. 3). However, LAP1 and LAP2 chaperone activity was not demonstrated using the CS aggregation assay. His6-LAP1 (900 nm) was unable to protect CS from aggregation (supplemental Fig. S5), and the His6-LAP2 protein aggregated on its own and could not be tested for its chaperone activity toward CS.
In Vitro Chaperone Activity of His6-LAP-A Is Independent of Its Peptidase Activity
A bank of mutations in four residues (Glu-347, Lys-354, Asp-429, and Arg-431) of the tomato LAP-A reactive site were characterized previously (34). Glu-347 and Asp-429 correspond to the E. coli and bovine LAP residues that coordinate one of the two zinc ions in the reactive site. Lys-354 and Arg-431 have a role in catalysis. All mutations at these sites inactivate the peptidase activity of His6-LAP-A (34); unexpectedly, some amino acid substitutions prevent assembly of the LAP-A hexamer (disruption mutants), and fast migrating forms are observed, whereas other mutant peptidases assemble into hexameric complexes (non-disruption mutants) (34) (supplemental Fig. S6A). To further characterize the disruption mutants, purified His6-LAP-As were run on a series of native polyacrylamide gels to determine the masses of the oligomeric species present. Disruption mutants had protein complexes migrating with masses of 60, 120, and 192 kDa; these masses were consistent with the disruption mutant LAP-As being a mixture of trimers (165 kDa), dimers (110 kDa), and monomers (55 kDa) (supplemental Fig. S6).
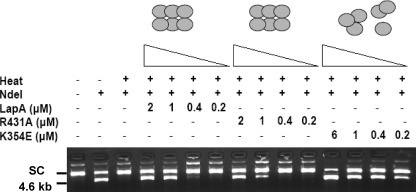
To determine if the chaperone activity of LAP-A was dependent on its peptidase activity, four non-disruption mutant proteins were tested for their chaperone activity. The His6-LAP-A catalytic mutants R431A and K354R and zinc ion-binding mutants D347N and E429V protected NdeI from thermal denaturation (Fig. 4 and supplemental Fig. S7). Their levels of chaperone activity were similar to the wild-type His6-LAP-A with chaperone activity displayed at 1–2 μm. These data indicated that chaperone activity of LAP-A was independent of its peptidase activity and its ability to bind substrate or coordinate zinc ions.
FIGURE 4.
LAP-A mutants protect NdeI from thermal inactivation. NdeI (1 unit) was incubated alone or with 0.2–2 μm His6-LAP-A (wild type), R431A (peptidase-deficient, non-disruption mutant), or K354E (peptidase-deficient, disruption mutant) as described in the legend to Fig. 1. Control lanes show supercoiled (SC) plasmid DNA only and DNA after digestion with unheated NdeI (4.6 kb).
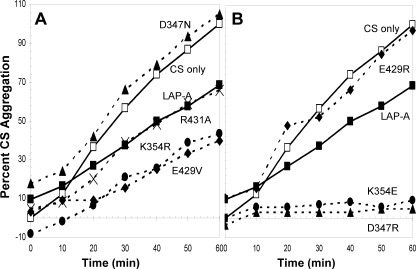
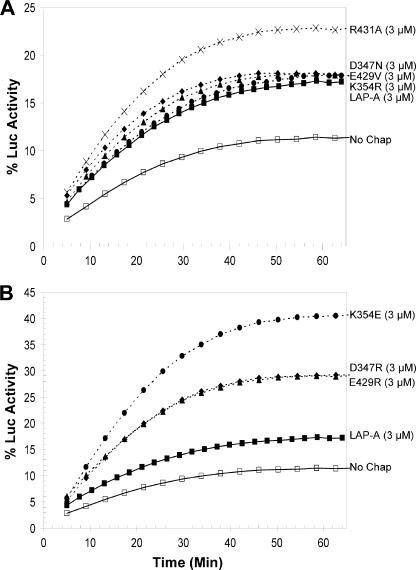
Using the CS aggregation assay, the chaperone activities of R431A, K354R, and E429V were also demonstrated. These proteins reduced CS aggregation by 40–60% relative to unprotected CS (Fig. 5A). In contrast, D347N did not prevent CS aggregation. The Luc refolding assay revealed that all four non-disruption mutants had chaperone activities similar to the WT LAP-A (Fig. 6). Three of the four non-disruption mutant proteins (K354R, E429V, and D347N) restored 17–18% of Luc activity, whereas the R431A protein had greater chaperone activity with ∼22% recovery of Luc activity. Collectively, the three chaperone assays indicated that chaperone activity of LAP-A was independent of its peptidase activity.
FIGURE 5.
LAP-A mutants protect CS from thermal aggregation. CS aggregation assays were performed as described in the legend to Fig. 2. A, CS was heated at 43 °C with 900 nm His6-LAP-A (■); with the non-disruption mutant protein E429V (♦), D347N (▴), K354R (●), or R431A (×); or alone (□). B, CS was heated with 900 nm His6-LAP-A (■); with the disruption mutant protein E429R (♦), D347R (▴), or K354E (●); or alone (□). Data shown are representative of at least two independent experiments.
FIGURE 6.
LAP-A mutants aid refolding of Luc. A, Luc (1 μm) was heated for 11 min at 42 °C with 3 μm His6-LAP-A (■); with one of the non-disruption mutants E429V (♦), D347N (▴), K354R (●), or R431A (×); or alone (no chap, □). B, Luc (1 μm) was heated for 11 min with 3 μm His6-LAP-A (■); with one of the disruption mutants E429R (♦), D347R (▴), or K354E (●); or alone (no chap, □). Luc was allowed to refold, and its activity was measured as described in the legend to Fig. 3. Data shown are representative of at least two independent experiments.
Disruption of His6-LAP-A Hexameric Structure Increases in Vitro Chaperone Activity
Because LAP-A peptidase activity is dependent on its hexameric structure, we tested if His6-LAP-A chaperone activity was also dependent on its oligomeric integrity. Three disruption mutant proteins that abolished either catalysis (K354E) or zinc ion binding (D347R and E429R) were tested for chaperone activity (34). In contrast to the non-disruption mutants, all three disruption mutants had increased chaperone activity toward NdeI (Fig. 4 and supplemental Fig. S7). The E429R protein protected NdeI activity at 0.4 μm, whereas the K354E and D357R proteins protected NdeI at concentrations as low as 0.2 μm. These data were in marked contrast with oligomeric structure mutants of HSP16.6 from Synechocystis, where the oligomeric stability of HSP16.6 is required for chaperone activity in vitro (43, 44).
LAP-A disruption mutants were tested for their ability to prevent CS aggregation. Consistent with the NdeI assay, the K354E and D347R proteins had increased chaperone activity toward CS (Fig. 5B). At 900 nm, the K354E and D347R proteins completely protected CS from heat-induced aggregation compared with the WT His6-LAP-A, which reduced CS aggregation by 40%. However, the E429R protein was unable to protect CS from aggregating. In the Luc refolding assay, the K354E protein was the most active chaperone, with only 3 μm of K354E protein refolding ∼40% of Luc (Fig. 6B). The E429R and D347R proteins (3 μm) aided in the refolding of ∼30% of the Luc. Although the E429R and D347R proteins were not as active as the K354E protein, all disruption mutant proteins displayed more chaperone activity than either the WT His6-LAP-A or the four non-disruption mutants (Fig. 6A).
Active Site Mutation of His6-LAP-N Alters Chaperone Activity and Heat Stability
Despite their strong sequence conservation in the C-terminal catalytic domain (80% identity), His6-LAP-A and His6-LAP-N have distinct substrate specificities (14, 34) and distinct protein stabilities, suggesting that LAP-A and LAP-N may have differences in their structure that could influence the dependence/independence of LAP-N chaperone and peptidase activities. To this end, eight substitution mutants of the LAP-N residue Lys-357 were characterized. LAP-N Lys-357 is equivalent to LAP-A Lys-354 (14). Five of the His6-LAP-N Lys-357 mutations (Lys-357 → Glu, Arg, Met, Gly, and Thr) had corresponding His6-LAP-A Lys-354 mutations. The His6-LAP-N wild-type and mutant proteins were expressed in E. coli and purified, and their peptidase activity and oligomeric states were determined.
Consistent with the peptidase deficiencies of the Lys-354 mutants of His6-LAP-A (34), all eight His6-LAP-N Lys-357 mutants were severely impaired in their ability to cleave Leu-AMC, with ≤1.4% of wild-type His6-LAP-N activity (supplemental Table 4). However, when quaternary structures were examined, His6-LAP-N and His6-LAP-A mutants were distinct. All eight of the His6-LAP-N Lys-357 mutant proteins (Lys-357 → Glu, Arg, Met, Gly, Thr, Cys, Pro, and Leu) assembled into hexamers (supplemental Fig. S8). This contrasts with the His6-LAP-A mutants, where only K354R assembled into a stable hexamer, and the other mutants had full (Lys-354 → Glu and Gly) or partial (Lys-354 → Met, Thr, Cys, Pro, and Leu) disassembly of the hexamer (34) (supplemental Fig. S6).
Although tests for increases in chaperone activity in a disruption mutant could not be performed for LAP-N, K357E and K357R allowed direct comparison with His6-LAP-A mutants K354E and K354R. Therefore, the molecular chaperone activities of the mutants K357E and K357R were compared with that of wild-type His6-LAP-N to determine if the potent LAP-N chaperone activity was independent of its peptidase. In the NdeI thermal protection assay, wild-type, K357E, and K357R His6-LAP-N proteins had similar chaperone activity levels, with thermal protection observed with as little as 0.2 μm His6-LAP-N (supplemental Fig. S9). Unlike the K354E His6-LAP-A disassembly mutant, which has enhanced chaperone activity, the K357E His6-LAP-N did not display molecular chaperone activity in the CS assay or Luc refolding assay (supplemental Figs. S10 and S11). Finally, the K357R protein aggregated in the CS assay, and its chaperone activity could not be assessed; furthermore, K357R was inactive in the Luc refolding assay because activity levels were similar to the RRL control (supplemental Figs. S10 and S11).
DISCUSSION
Based on their ability to protect proteins from thermal denaturation and aggregation and aid in the refolding of denatured proteins in vitro, plant LAPs are molecular chaperones. LAPs lack ATP-binding domains (45), and their in vitro chaperone activity was independent of ATP. Together, these data point to plant LAPs acting like “holdases” similar to sHSPs (19, 20, 46). However, LAPs share no sequence similarity with sHSPs. In particular, they lack the α-crystallin domain that is essential for sHSP chaperone activity (19). Therefore, LAPs represent a new class of chaperone proteins within plants.
Each of the tomato and Arabidopsis LAPs displayed distinct activity profiles with the three model substrates in vitro (supplemental Table 1). LAP-A chaperone activity was detected in all three assays. The ability of LAP-A to protect NdeI from denaturation and CS from aggregation was similar to that reported for the chaperone α-crystallin (39), although the ability of LAP-A to enable Luc refolding was ∼4-fold lower than the well characterized PsHsp18.1 (31, 39, 41). The neutral LAPs (LAP-N, LAP1, and LAP2) demonstrated varied chaperone activity toward two or more model substrates. Based on the Luc refolding assay, all three neutral LAPs and LAP-A had similar chaperone activity levels. Whereas LAP2 protected NdeI at the same level as LAP-A (1 μm), LAP-N, LAP1, and PsHsp18.1 had increased chaperone activity toward NdeI (0.2 μm). Finally, of the neutral LAPs, only LAP-N was able to protect CS from aggregation, and its activity was comparable with that of LAP-A. Collectively, these data indicated that the molecular chaperone activity is functionally conserved within the plant LAPs. At the present time, it is unclear why the LAP-A and the neutral LAPs (LAP-N, LAP1, and LAP2) have differences in their relative chaperone activities as measured with three model substrates in vitro. This may reflect differences in substrate specificity in vivo and may even suggest different mechanisms of action; it is not uncommon for chaperones within the same family to show such variation (47).
Study of LAP-A active site mutants demonstrated that the chaperone activity of LAP-A is independent of its peptidase activity (supplemental Table 2). All seven of the LAP-A active site mutants showed chaperone activity toward at least two of the model protein substrates. This demonstrates that key residues involved in catalysis and zinc ion interaction were not involved in protein interactions required for chaperone activity. These data suggest that a different region of LAP-A is important for chaperone substrate interaction. This molecular strategy is similar to that used by plastid ATP-dependent proteases, where the peptidase and chaperone domains are independent (48).
Analyses of LAP-N active site mutants revealed unanticipated conformational and chaperone activity differences from analogous mutations in LAP-A. LAP-N wild type, K357E, and K357R were functional chaperones in the NdeI assay. However, unlike the K354E LAP-A protein that displayed enhanced chaperone activity in all three chaperone assays, the K357E mutation reduced LAP-N activity in the Luc refolding and eliminated LAP-N activity in the CS aggregation assays. Furthermore, K357R LAP-N did not display chaperone activity in the Luc assays, and it aggregated spontaneously in the CS assay. These data suggest that, unlike in LAP-A, mutations in the catalytic site of LAP-N impacted chaperone activity. Because the chaperone substrate binding residues are not known in any LAP, it is unclear if Lys-357 is directly involved in chaperone binding or if disruption of Lys-357 significantly alters the structure of LAP-N. If structural changes occur, they must occur at the secondary or tertiary levels because these mutations did not affect the oligomeric structure of LAP-N. In fact, unlike LAP-A, where a majority of substitutions at Lys-354 cause hexamer disassembly, none of the LAP-N Lys-357 mutants disrupted the LAP-N hexamer. The sensitivity of the LAP-N chaperone to mutations in a catalytic residue may be consistent with the facts that LAP-N is less stable in vitro than LAP-A (14) and the peptidase substrate specificity of LAP-N is distinct from that of LAP-A (14, 30, 34, 35).
The LAP-A disruption mutants demonstrated that LAP-A chaperone activity was independent of LAP-A oligomeric stability (supplemental Table 3). In fact, loss of LAP-A hexameric structure increased LAP-A chaperone in every assay with one exception; the E429R protein was unable to protect CS against heat-induced aggregation. Reduction of the E429R protein's chaperone activity toward CS is not simply due to a mutation at this residue because E429V protein had chaperone activity. Therefore, specificity of chaperone activity must be due to yet undetermined conformational changes within LAP-A. The LAP-A data are in marked contrast to the Synechocystis HSP16.6, which requires oligomeric structure for chaperone activity (43, 44).
Currently, it is unclear why some substitutions in the active site of LAP-A perturb the enzyme's oligomeric structure. There is no correlation of charge or hydrophobicity with residue substitutions and LAP-A disassembly (34). It is even more intriguing that those same substitutions do not disrupt the oligomeric structure of LAP-N. Although animal and microbial LAP crystal structures are known (49–52), they are not adequate to predict the overall structure of LAPs due to the divergence in the C-terminal catalytic domains (49–59%) and more highly diverged N-terminal domains (14). Therefore, to determine the structural changes that occur in the disruption mutants, crystal structures of the WT and mutant LAPs of tomato will be needed.
The enhancement of LAP-A chaperone activity upon disruption of its oligomeric structure is consistent with the exposure of new residues or domains that interact with the protein substrate(s). In this manner, the chaperone activity of LAP-A resembles that of the sHSP class of chaperones. Current sHSP models propose that under ambient conditions, sHSPs form large oligomeric structures. During heat stress, these sHSP oligomers either increase their subunit exchange rate or dissociate into smaller complexes, which have chaperone activity (47, 53). These structural changes are presumed to increase exposure of hydrophobic residues, which bind to and protect the protein substrates. Consistent with the sHSP model, it is predicted that when the hexamer of LAP-A is disrupted, more hydrophobic residues will be exposed on the smaller oligomers (monomers, dimers, and trimers); these residues could enable association with unfolded protein substrates.
Given that both the tomato (LAP-A and LAP-N) and Arabidopsis LAPs (LAP1 and LAP2) have substantive molecular chaperone activity in vitro, it is possible that LAPs may function as chaperones in vivo. However, due to the differences in their subcellular localizations, the peptidase/chaperone substrates of the Arabidopsis LAP1, which is located in the cytosol (33), are likely to be distinct from those of the plastid-localized LAP-A, LAP-N, and LAP2 (6, 18). In addition, it is possible that the stress-induced LAP-A has a different set of peptidase/chaperone substrates from LAP-N and LAP2. Although the roles of LAP-N, LAP1 and LAP2 are not known, LAP-A has an important role in plant defense with potential roles in planta and in caterpillar digestive tracks.
The tomato LAP-A is induced by herbivory, wounding, salinity, and water deficit and is one of the most abundant proteins in the chloroplast stroma after these stresses (16, 37); furthermore, LAP-A modulates the late branch of wound signaling downstream of jasmonic acid perception (8). LAP-A is well adapted to the alkaline environment of the stroma, exhibiting maximal peptidase activity at a pH of 9.0 (30). In addition to this extreme pH environment, during herbivory there are rapid ion fluxes, changes in redox potential, increases in reactive oxygen species, and often desiccation at the wound site that also threatens protein integrity (54). Therefore, it is not surprising that plants up-regulate some chaperones in response to insect feeding and wounding (55–58).
To dissect the roles of LAP peptidase and chaperone activities, transgenic tomatoes expressing mutant LAP proteins are being analyzed to reveal if LAP-A acts as a chaperone within the leaf and whether its chaperone activity, peptidase activity, or both are important for the role of LAP-A in defense in tomato. Of particular importance will be understanding if LAP-A undergoes transitions in oligomeric forms in vivo (i.e. monomer/dimer/trimer versus hexamer), which might regulate relative chaperone and peptidase activities in planta. Therefore, LAP-A may represent a new mechanism of stress adaptation (to alkaline environments). LAP-A could act like other chaperones that switch from their primary activity to chaperone activity in response to changes in their environments, such as low pH, temperature extremes, and reactive oxygen species (24, 26, 27, 59, 60).
Many defense proteins exert their effects within the insect gut by inhibiting the activity of digestive enzymes or reducing the quality of the leaf diet by removing essential amino acids or cross-linking proteins (61–64). Lepidopteran midguts are alkaline (65), and due to LAP-A hyperstability within insect digestive track and frass and its alkaline pH optima (30, 66), it has been proposed that LAP-A may degrade peptides within the insect midgut (30, 32). Although preliminary data indicate that artificial diets supplemented with LAP-A do not affect insect growth and development (8), it is also possible that LAP-A works in cooperation with other anti-nutritive enzymes. For example, because LAP-A can also readily hydrolyze N-terminal Arg, it is possible that LAP-A could act in concert with wound-induced arginase to deplete essential Arg from the insect diet (61). However, given the discovery of the chaperone activity of LAP-A, it is also possible that LAP-A protects defense proteins that are poorly adapted to the alkaline environment of the insect midgut. Alternatively, LAP-A may aid in maintaining the conformation, and thereby assuring the maximal activity, of defense proteins with alkaline pH optima, such as threonine deaminase and arginase, which deplete essential amino acids in the insect midgut (61, 66).
The discovery that LAP-A moonlights as a molecular chaperone in vivo may have important ramifications for plant defense, and this will need to be tested genetically once the chaperone domains are identified and LAP-A chaperone mutants can be created. In addition, understanding the identity of in vivo peptidase and chaperone substrates will determine if these diverse functions target overlapping or unique processes. This study also demonstrates that the in vitro molecular chaperone activity is conserved within the plant LAPs, suggesting that the novel roles for the neutral LAPs (LAP-N, LAP-1, and LAP-2) may be revealed. Interestingly, bacterial and archaeal orthologs of the Arabidopsis LAP2 and LAP3 have been predicted to have been co-inherited with HSP70s (AraNet, score = 2.05), suggesting that LAP chaperone function may have an ancient evolutionary origin. Future studies will determine if chaperone activity is an evolutionarily conserved function displayed in LAPs from other kingdoms. Because two microbial enzymes, with structures distinct from LAPs, have dual aminopeptidase and chaperone activities (25, 31), it is intriguing to speculate that aminopeptidase and chaperone activities co-evolved. If confirmed, LAPs will add to the growing diversity of multifunctional aminopeptidases.
Supplementary Material
Acknowledgments
We thank Walling laboratory members and Charles Jang for helpful discussions and Eman Basha for advice on luciferase assays and PsHsp18.1.
This work was supported by National Science Foundation Grants IOB 0645230 and MCB 0313579 (to L. L. W.).

This article contains supplemental Tables 1–4 and Figs. S1–S11.
- LAP
- leucine aminopeptidase
- LAP-A
- acidic leucine aminopeptidase
- LAP-N
- neutral leucine aminopeptidase
- sHSP
- small heat shock protein
- Luc
- luciferase
- CS
- citrate synthase
- AMC
- amino methyl coumarin
- RRL
- rabbit reticulocyte lysate.
REFERENCES
- 1. Barrett A. J., Rawlings N. D., Woessner J. F. (1998) Handbook of Proteolytic Enzymes, Academic Press, Inc., San Diego [Google Scholar]
- 2. Meinnel T., Serero A., Giglione C. (2006) Impact of the N-terminal amino acid on targeted protein degradation. Biol. Chem. 387, 839–851 [DOI] [PubMed] [Google Scholar]
- 3. van Endert P. (2011) Post-proteasomal and proteasome-independent generation of MHC class I ligands. Cell Mol. Life Sci. 68, 1553–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graciet E., Wellmer F. (2010) The plant N-end rule pathway. Structure and functions. Trends Plant Sci. 15, 447–453 [DOI] [PubMed] [Google Scholar]
- 5. Varshavsky A. (2011) The N-end rule pathway and regulation by proteolysis. Protein Sci. 20, 1298–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walling L. L. (2006) Recycling or regulation? The role of amino-terminal modifying enzymes. Curr. Opin. Plant Biol. 9, 227–233 [DOI] [PubMed] [Google Scholar]
- 7. Peer W. A. (2011) The role of multifunctional M1 metallopeptidases in cell cycle progression. Ann. Bot. 107, 1171–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fowler J. H., Narváez-Vásquez J., Aromdee D. N., Pautot V., Holzer F. M., Walling L. L. (2009) Leucine aminopeptidase regulates defense and wound signaling in tomato downstream of jasmonic acid. Plant Cell 21, 1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sánchez-Morán E., Jones G. H., Franklin F. C., Santos J. L. (2004) A puromycin-sensitive aminopeptidase is essential for meiosis in Arabidopsis thaliana. Plant Cell 16, 2895–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peer W. A., Hosein F. N., Bandyopadhyay A., Makam S. N., Otegui M. S., Lee G. J., Blakeslee J. J., Cheng Y., Titapiwatanakun B., Yakubov B., Bangari B., Murphy A. S. (2009) Mutation of the membrane-associated M1 protease APM1 results in distinct embryonic and seedling developmental defects in Arabidopsis. Plant Cell 21, 1693–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ross S., Giglione C., Pierre M., Espagne C., Meinnel T. (2005) Functional and developmental impact of cytosolic protein N-terminal methionine excision in Arabidopsis. Plant Physiol. 137, 623–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor A. (1985) Leucine aminopeptidase activity is diminished in aged hog, beef, and human lens. Prog. Clin. Biol. Res. 180, 299–302 [PubMed] [Google Scholar]
- 13. Colloms S. D. (2004) Leucyl aminopeptidase PepA. in Handbook of Proteolytic Enzymes, 2nd Ed. (Barrett A. J., Rawlings N. D., Woessner J. F., eds) pp. 905–908, Elsevier Academic Press, San Diego [Google Scholar]
- 14. Tu C. J., Park S. Y., Walling L. L. (2003) Isolation and characterization of the neutral leucine aminopeptidase (LapN) of tomato. Plant Physiol. 132, 243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao W. S., Pautot V., Holzer F. M., Walling L. L. (2000) Leucine aminopeptidases. The ubiquity of LAP-N and the specificity of LAP-A. Planta 210, 563–573 [DOI] [PubMed] [Google Scholar]
- 16. Chao W. S., Gu Y. Q., Pautot V. V., Bray E. A., Walling L. L. (1999) Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol. 120, 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartl M., Merker H., Schmidt D. D., Baldwin I. T. (2008) Optimized virus-induced gene silencing in Solanum nigrum reveals the defensive function of leucine aminopeptidase against herbivores and the shortcomings of empty vector controls. New Phytol. 179, 356–365 [DOI] [PubMed] [Google Scholar]
- 18. Narváez-Vásquez J., Tu C. J., Park S. Y., Walling L. L. (2008) Targeting and localization of wound-inducible leucine aminopeptidase A in tomato leaves. Planta 227, 341–351 [DOI] [PubMed] [Google Scholar]
- 19. Tyedmers J., Mogk A., Bukau B. (2010) Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11, 777–788 [DOI] [PubMed] [Google Scholar]
- 20. Kampinga H. H., Craig E. A. (2010) The HSP70 chaperone machinery. J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vierling E. (1991) The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 579–620 [Google Scholar]
- 22. Wang W., Vinocur B., Shoseyov O., Altman A. (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9, 244–252 [DOI] [PubMed] [Google Scholar]
- 23. Hotokezaka Y., Tobben U., Hotokezaka H., Van Leyen K., Beatrix B., Smith D. H., Nakamura T., Wiedmann M. (2002) Interaction of the eukaryotic elongation factor 1A with newly synthesized polypeptides. J. Biol. Chem. 277, 18545–18551 [DOI] [PubMed] [Google Scholar]
- 24. Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Two enzymes in one. Two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 25. Malki A., Caldas T., Abdallah J., Kern R., Eckey V., Kim S. J., Cha S. S., Mori H., Richarme G. (2005) Peptidase activity of the Escherichia coli Hsp31 chaperone. J. Biol. Chem. 280, 14420–14426 [DOI] [PubMed] [Google Scholar]
- 26. Spiess C., Beil A., Ehrmann M. (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97, 339–347 [DOI] [PubMed] [Google Scholar]
- 27. Lee J. R., Lee S. S., Jang H. H., Lee Y. M., Park J. H., Park S. C., Moon J. C., Park S. K., Kim S. Y., Lee S. Y., Chae H. B., Jung Y. J., Kim W. Y., Shin M. R., Cheong G. W., Kim M. G., Kang K. R., Lee K. O., Yun D. J. (2009) Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein, AtTDX. Proc. Natl. Acad. Sci. U.S.A. 106, 5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neuwald A. F., Aravind L., Spouge J. L., Koonin E. V. (1999) AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 [PubMed] [Google Scholar]
- 29. Suzuki C. K., Rep M., van Dijl J. M., Suda K., Grivell L. A., Schatz G. (1997) ATP-dependent proteases that also chaperone protein biogenesis. Trends Biochem. Sci. 22, 118–123 [DOI] [PubMed] [Google Scholar]
- 30. Gu Y. Q., Holzer F. M., Walling L. L. (1999) Overexpression, purification, and biochemical characterization of the wound-induced leucine aminopeptidase of tomato. Eur. J. Biochem. 263, 726–735 [DOI] [PubMed] [Google Scholar]
- 31. Lee S., Kim J. S., Yun C. H., Chae H. Z., Kim K. (2009) Aspartyl aminopeptidase of Schizosaccharomyces pombe has a molecular chaperone function. BMB Rep. 42, 812–816 [DOI] [PubMed] [Google Scholar]
- 32. Pautot V., Holzer F. M., Chaufaux J., Walling L. L. (2001) The induction of tomato leucine aminopeptidase genes (LapA) after Pseudomonas syringae pv. tomato infection is primarily a wound response triggered by coronatine. Mol. Plant Microbe Interact. 14, 214–224 [DOI] [PubMed] [Google Scholar]
- 33. Bartling D., Weiler E. W. (1992) Leucine aminopeptidase from Arabidopsis thaliana. Molecular evidence for a phylogenetically conserved enzyme of protein turnover in higher plants. Eur. J. Biochem. 205, 425–431 [DOI] [PubMed] [Google Scholar]
- 34. Gu Y. Q., Walling L. L. (2002) Identification of residues critical for activity of the wound-induced leucine aminopeptidase (LAP-A) of tomato. Eur. J. Biochem. 269, 1630–1640 [DOI] [PubMed] [Google Scholar]
- 35. Gu Y. Q., Walling L. L. (2000) Specificity of the wound-induced leucine aminopeptidase (LAP-A) of tomato activity on dipeptide and tripeptide substrates. Eur. J. Biochem. 267, 1178–1187 [DOI] [PubMed] [Google Scholar]
- 36. Bryan J. K. (1977) Molecular weights of protein multimers from polyacrylamide gel electrophoresis. Anal. Biochem. 78, 513–519 [DOI] [PubMed] [Google Scholar]
- 37. Gu Y. Q., Pautot V., Holzer F. M., Walling L. L. (1996) A complex array of proteins related to the multimeric leucine aminopeptidase of tomato. Plant Physiol. 110, 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu Y. Q., Chao W. S., Walling L. L. (1996) Localization and post-translational processing of the wound-induced leucine aminopeptidase proteins of tomato. J. Biol. Chem. 271, 25880–25887 [DOI] [PubMed] [Google Scholar]
- 39. Santhoshkumar P., Sharma K. K. (2001) Analysis of α-crystallin chaperone function using restriction enzymes and citrate synthase. Mol. Vision 7, 172–177 [PubMed] [Google Scholar]
- 40. Siddique M., Gernhard S., von Koskull-Döring P., Vierling E., Scharf K. D. (2008) The plant sHSP superfamily. Five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones 13, 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee G. J., Vierling E. (1998) Expression, purification, and molecular chaperone activity of plant recombinant small heat shock proteins. Methods Enzymol. 290, 350–365 [DOI] [PubMed] [Google Scholar]
- 42. Frydman J., Nimmesgern E., Ohtsuka K., Hartl F. U. (1994) Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370, 111–117 [DOI] [PubMed] [Google Scholar]
- 43. Giese K. C., Basha E., Catague B. Y., Vierling E. (2005) Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity. Proc. Natl. Acad. Sci. U.S.A. 102, 18896–18901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giese K. C., Vierling E. (2002) Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J. Biol. Chem. 277, 46310–46318 [DOI] [PubMed] [Google Scholar]
- 45. Walling L. L. (2004) Leucyl aminopeptidase (plant). in Handbook of Proteolytic Enzymes, 2nd Ed. (Barrett A. J., Rawlings N. D., Woesner J. F., eds) pp. 901–904, Elsevier Academic Press, San Diego [Google Scholar]
- 46. Sun Y., MacRae T. (2005) Small heat shock proteins. Molecular structure and chaperone function. Cell Mol. Life Sci. 62, 2460–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Basha E., Jones C., Wysocki V., Vierling E. (2010) Mechanistic differences between two conserved classes of small heat shock proteins found in the plant cytosol. J. Biol. Chem. 285, 11489–11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sakamoto W. (2006) Protein degradation machineries in plastids. Annu. Rev. Plant Biol. 57, 599–621 [DOI] [PubMed] [Google Scholar]
- 49. Huynh K. H., Natarajan S., Choi J., Song N. H., Kim J. G., Lee B. M., Ahn Y. J., Kang L. W. (2009) Cloning, expression, crystallization, and preliminary x-ray crystallographic analysis of leucine aminopeptidase (LAP) from the pepA gene of Xanthomonas oryzae pv. oryzae. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65, 952–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kale A., Pijning T., Sonke T., Dijkstra B. W., Thunnissen A. (2010) Crystal structure of the leucine aminopeptidase from Pseudomonas putida reveals the molecular basis for its enantioselectivity and broad substrate specificity. J. Mol. Biol. 398, 703–714 [DOI] [PubMed] [Google Scholar]
- 51. Sträter N., Sun L., Kantrowitz E. R., Lipscomb W. N. (1999) A bicarbonate ion as a general base in the mechanism of peptide hydrolysis by dizinc leucine aminopeptidase. Proc. Natl. Acad. Sci. U.S.A. 96, 11151–11155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGowan S., Oellig C. A., Birru W. A., Caradoc-Davies T. T., Stack C. M., Lowther J., Skinner-Adams T., Mucha A., Kafarski P., Grembecka J., Trenholme K. R., Buckle A. M., Gardiner D. L., Dalton J. P., Whisstock J. C. (2010) Structure of the Plasmodium falciparum M17 aminopeptidase and significance for the design of drugs targeting the neutral exopeptidases. Proc. Natl. Acad. Sci. U.S.A. 107, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. (2005) Some like it hot. The structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 12, 842–846 [DOI] [PubMed] [Google Scholar]
- 54. Bostock R. M. (2005) Signal cross-talk and induced resistance. Straddling the line between cost and benefit. Annu. Rev. Phytopathol. 43, 545–580 [DOI] [PubMed] [Google Scholar]
- 55. Halitschke R., Gase K., Hui D., Schmidt D. D., Baldwin I. T. (2003) Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol. 131, 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lawrence S. D., Novak N. G., Ju C. J., Cooke J. E. (2008) Potato, Solanum tuberosum, defense against Colorado potato beetle, Leptinotarsa decemlineata (Say). Microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. J. Chem. Ecol. 34, 1013–1025 [DOI] [PubMed] [Google Scholar]
- 57. Pautot V., Holzer F. M., Reisch B., Walling L. L. (1993) Leucine aminopeptidase. An inducible component of the defense response in Lycopersicon esculentum (tomato). Proc. Natl. Acad. Sci. U.S.A. 90, 9906–9910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reymond P., Weber H., Damond M., Farmer E. E. (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12, 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hong W., Jiao W., Hu J., Zhang J., Liu C., Fu X., Shen D., Xia B., Chang Z. (2005) Periplasmic protein HdeA exhibits chaperone-like activity exclusively within stomach pH range by transforming into disordered conformation. J. Biol. Chem. 280, 27029–27034 [DOI] [PubMed] [Google Scholar]
- 60. Tapley T. L., Franzmann T. M., Chakraborty S., Jakob U., Bardwell J. C. (2010) Protein refolding by pH-triggered chaperone binding and release. Proc. Natl. Acad. Sci. U.S.A. 107, 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen H., Wilkerson C. G., Kuchar J. A., Phinney B. S., Howe G. A. (2005) Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. U.S.A. 102, 19237–19242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Felton G. W., Donato K., Delvecchio R. J., Duffey S. S. (1989) Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J. Chem. Ecol. 15, 2667–2694 [DOI] [PubMed] [Google Scholar]
- 63. Johnson R., Narvaez J., An G., Ryan C. (1989) Expression of proteinase inhibitors I and II in transgenic tobacco plants. Effects on natural defense against Manduca sexta larvae. Proc. Natl. Acad. Sci. U.S.A. 86, 9871–9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang J. H., Wang L., Giri A., Baldwin I. T. (2006) Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell 18, 3303–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dow J. A. (1992) pH Gradients in lepidopteran midgut. J. Exp. Biol. 172, 355–375 [DOI] [PubMed] [Google Scholar]
- 66. Chen H., Gonzales-Vigil E., Wilkerson C. G., Howe G. A. (2007) Stability of plant defense proteins in the gut of insect herbivores. Plant Physiol. 143, 1954–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.