DNA methylation is a mechanism for changing the base sequence of DNA without altering its coding function. As a heritable, yet reversible, epigenetic change, it has the potential of altering gene expression and has profound developmental and genetic consequences. The methylation reaction itself is mechanistically complex and involves the flipping of the target cytosine out of the intact double helix, so that the transfer of the methyl group from S-adenosylmethionine can occur in a cleft in the enzyme (1). Cytosine methylation is inherently mutagenic, which presumably has led to the 80% suppression of the CpG methyl acceptor site in eukaryotic organisms, which methylate their genomes. It contributes strongly to the generation of polymorphisms and germ-line mutations, and to transition mutations that inactivate tumor-suppressor genes (2). Despite a 10- to 40-fold increase in the rate of transitions at methylated versus unmethylated cytosines (3), methylation is not only tolerated in several eukaryotes, but is actually required for the embryonic development of mammals (4). The reasons 5-methylcytosine is essential for development remain obscure, but most probably relate to the well documented ability of methylation, particularly the methylation of CpG-rich promoters, to block transcriptional activation. Indeed, there is growing evidence that methylation plays a pivotal role in key developmental processes such as genomic imprinting and stabilization of X-chromosome inactivation. It therefore is not surprising that alterations in this essential epigenetic system might play a role in carcinogenesis (see Fig. 1 for examples).
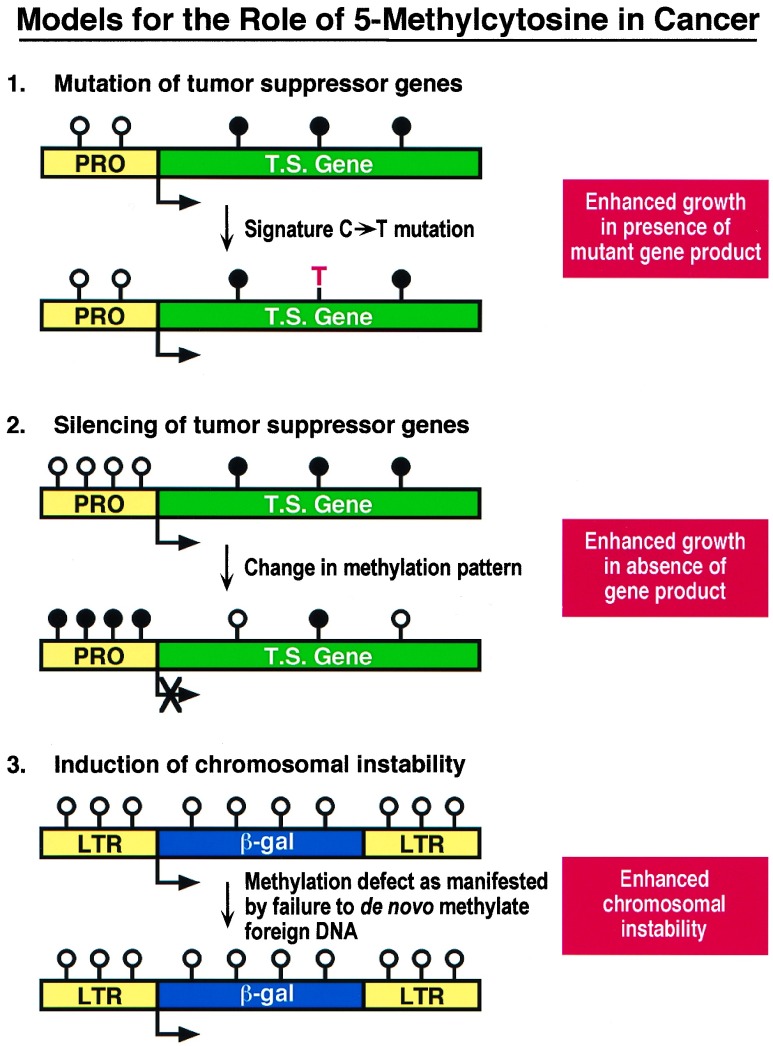
Figure 1.
Models for the role of 5-methylcytosine in cancer. [1] Signature mutations in the form of C → T transitions at methylated CpG sites are the hallmark of hydrolytic deamination of 5-methylcytosine and commonly produce mutations in tumor suppressor genes such as p53. [2] Abnormal silencing of tumor suppressor genes can occur through the epigenetic effects of DNA methylation at CpG islands, which may be localized to the promoters of growth regulatory genes such as p16, Rb, and VHL. [3] Induction of chromosomal instability may result from the inability of a cell to carry out de novo methylation as proposed in the report by Lengauer et al. (15). Circles represent cytosines located at CpG dinucleotides; solid circles indicate methylated cytosines, open circles indicate unmethylated cytosines. T.S., tumor suppressor gene; PRO, promoter; LTR, long terminal repeat.
Two papers in this issue of the Proceedings add new evidence to support the notion that alterations in the DNA methylation machinery are among the most common changes associated with neoplasia and may have a causative role at an early stage in carcinogenesis. In their report, Ushijima et al. (5) have modified the powerful representation difference analysis (RDA) technique pioneered by Wigler and colleagues (6) to characterize and clone DNA fragments that show methylation changes during murine hepatocarcinogenesis. This method adds to the growing number of genome scanning approaches, including restriction landmark genomic scanning (7) and methylation-sensitive arbitrarily primed PCR (8, 9), which all have identified frequently altered methylation sites in cancer cells. The new methodologies are important in that they not only identify changes, but allow for the cloning of the relevant fragments of DNA. The data from these nondirected approaches all show that it is not simply the overall levels of DNA methylation that are altered in cancer, but rather that profound changes occur in the distribution of methyl groups.
The above studies have been paralleled by approaches focusing on the de novo methylation of the promoter regions of specific tumor suppressor genes. In several cases including Rb, p16, and VHL, the promoters are CpG rich and fulfill the criteria for CpG islands, thus providing a rationale for believing that they might be subject to methylation silencing (10–13). The importance of this work is that it suggests an alternative pathway for the inactivation of tumor suppressors. A weakness is that many of these studies have used data obtained from the analysis of a few restriction enzyme sites whose relevance to gene control remains undetermined. This situation developed because of difficulties in sequencing genomic DNA for 5-methylcytosine distribution. In this regard, it is difficult to overstate the contribution of Frommer et al. (14) who have simplified genomic sequencing by the use of bisulfite treatment, making the analysis of DNA methylation patterns a fairly routine task. The coupling of this precise and focused approach with the nondirected genome scanning techniques such as that developed by Ushijima et al. (5) and others (7–9) is likely to lead to rapid progress in this field.
Almost all studies to date have focused on changes in methylation status of endogenous genes in transformed cells relative to their presumed normal counterparts. In the other related paper in this issue, Lengauer et al. (15) have taken a different approach by introducing exogenous CpG-rich sequences in the form of retroviruses into colon cancer cell lines and have come up with an entirely unexpected result. The authors show that two distinct expression patterns were observed in the transfectants. Five out of 10 lines failed to express the retrovirally encoded β-galactosidase gene, and these lines were all deficient in DNA mismatch repair (MMR−). On the other hand, cells competent for repair (MMR+) efficiently expressed the gene. Importantly, gene expression could be induced in the MMR− cells by subsequent treatment with 5-azacytidine, a well known inhibitor of DNA methylation (16). This result, together with Southern blot analysis showing partial methylation of restriction enzyme sites in MMR− but not MMR+ lines, suggests the existence of two distinct phenotypes, i.e., MMR−, MET+ (where MET+ means methylation proficient) and MMR+, MET−.
The correlation between the MMR phenotype and expression is clear, but it will take some time to resolve the precise role of the observed de novo methylation in gene silencing. The authors confirmed the presence of methylation, using the powerful bisulfite genomic sequencing method discussed earlier, and observed a low, but measurable, level of methylation in the viral long terminal repeat (LTR). The methylation patterns were heterogeneous, and some LTR molecules were unmethylated, which might have contributed to the low levels of expression seen in some of the transfectants.
Both the Ushijima and Lengauer studies extend previous observations that methylation patterns are commonly altered in tumor cells, but do not address the question of how the de novo methylation of CpG islands occurs. De novo methylation of CpG islands occurs in both endogenous sequences and retrovirally introduced LTRs during development (17). Unfortunately, virtually nothing is known about how this is accomplished in normal or transformed cells, but it may well require the participation of an as-yet unknown de novo methylase in addition to the copying or “maintenance” enzyme isolated and characterized by Bestor and colleagues (18). The existence of such an enzyme seems almost a certainty, because ES cells with a complete knockout of the maintenance enzyme still show residual methylation, including methylation of endogenous LTR sequences (19). An alternative route to altered methylation in cancer cells may be mediated by disregulation of the recently described demethylase activity (20). Further progress in understanding how methylation patterns are acquired and altered is obviously going to require a characterization of the basic enzymology for DNA modification.
What then is the relationship between the MMR and MET phenotypes? One highly plausible explanation would have been that unresolved mismatches created by the MMR deficiency could serve as foci for de novo methylation. There is a strong rationale for this hypothesis, because Smith et al. (21) have clearly demonstrated that mismatches at CpG sites serve as highly efficient substrates for the human maintenance methylase. This does not, however, seem to be the case in the colon cancer cell lines, because restoration of mismatch repair by transfer of the appropriate chromosome to the MMR− cells did not abrogate their abilities to de novo methylate the viral LTR. Lengauer et al. (15) come up with an ingenious alternative explanation to account for their observations and explain the generation of genomic instability in the 85% of colon cancers that have an MMR+, yet MET−, phenotype. They propose that the as-yet uncharacterized “methylation defect” (MET−) directly facilitates the gain and loss of whole chromosomes leading to the genomic instability necessary for cancer development and progression. On the other hand, cells that are MMR− are hypothesized to have a normal methylation proficiency and generate the required instability by the alternative pathway of mismatch repair deficiency.
The hypothesis that DNA methylation and chromosomal integrity and segregation are linked has some experimental support. Feinberg et al. (22) found that colon tumors often have reduced 5-methylcytosine contents relative to normal tissues. They also observed that the three patients in their study with the highest levels of the modified base in their normal colonic epithelia all had Lynch syndrome (HNPCC). This observation, made before the discovery that HNPCC was due to the MMR− phenotype, suggests that the in vitro data obtained by Lengauer et al. (15) may indeed reflect the in vivo situation. Evidence that methylation may promote retention of chromosomes also has been provided by Hsieh (23), who found that methylated plasmids are maintained with higher efficiencies than their unmethylated counterparts in transfected cells. However, other observations seem, at first sight, to be contradictory to this hypothesis. First, DNA hypermethylation (read de novo methylation) rather than hypomethylation precedes allelic loss in neural tumors (24). Also, the elegant studies of Laird et al. (25) demonstrated that lowering the level of maintenance methylase by genetic or pharmacological means led to a significant decrease rather than an increase in the number of polyps in mice harboring the min mutation. Perhaps the difference lies in the nature of the “methylation defect” in the two systems. The methylation pattern present in a cell is likely to be determined by the interplay of a de novo methylase, a maintenance methylase, and possibly a demethylase. The specific player is known in the transgenic experiments but unknown in the tumor systems. Until the relationships between these activities are understood, it may not be possible to resolve this apparent discrepancy. Also, because methylation presumably plays multiple roles in carcinogenesis (Fig. 1), it may be that different pathways are selected with differing degrees of penetration in diverse situations.
The two new studies published today therefore add interesting new dimensions to our understanding of the importance of this essential epigenetic modification in cancer. They extend the role of methylation in cancer beyond the fact that DNA methylation contributes directly to mutagenesis by once again pointing out the importance and prevalence of methylation changes, particularly de novo methylation in the initiation and progression of cancer. Also, they raise intriguing questions as to whether these common methylation defects contribute on a more macro scale to the gross chromosomal imbalances that are the hallmark of all solid tumors.
References
- 1.Klimasauskas S, Kumar S, Roberts R J, Cheng X. Cell. 1994;76:357–369. doi: 10.1016/0092-8674(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 2.Jones P A. Cancer Res. 1996;56:2463–2467. [PubMed] [Google Scholar]
- 3.Yang A S, Gonzalgo M L, Zingg J-M, Millar R P, Buckley J D, Jones P A. J Mol Biol. 1996;258:240–250. doi: 10.1006/jmbi.1996.0246. [DOI] [PubMed] [Google Scholar]
- 4.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 5.Ushijima T, Morimura K, Hosoya Y, Okonogi H, Tatematsu M, Sugimura T, Nagao M. Proc Natl Acad Sci USA. 1997;94:2284–2289. doi: 10.1073/pnas.94.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisitsyn N, Lisitsyn N, Wigler M. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 7.Kawai J, Hirose K, Fushiki S, Hirotsune S, Ozawa N, Hara A, Hayashizaki Y, Watanabe S. Mol Cell Biol. 1994;14:7421–7427. doi: 10.1128/mcb.14.11.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalgo M L, Liang G, Spruck C H, Zingg J-M, Rideout W M, Jones P A. Cancer Res. 1997;57:594–599. [PubMed] [Google Scholar]
- 9.Huang, T. H.-M., Laux, D. E., Hamlin, B. C., Tran, P., Tran, H. & Lubahn, D.-B. (1997) Cancer Res. in press. [PubMed]
- 10.Greger V, Debus N, Lohmann D, Hopping W, Passarge E, Horsthemke B. Hum Genet. 1994;94:491–496. doi: 10.1007/BF00211013. [DOI] [PubMed] [Google Scholar]
- 11.Herman J G, Latif F, Weng Y, Lerman M I, Zbar B, Liu S, Samid D, Duan D-S, Gnarra J R, Linehan W M, Baylin S. Proc Natl Acad Sci USA. 1994;91:9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Zulueta M, Bender C M, Yang A S, Nguyen T, Beart R W, Van Tornout J M, Jones P A. Cancer Res. 1995;55:4531–4535. [PubMed] [Google Scholar]
- 13.Merlo A, Herman J G, Mao L, Lee D J, Gabrielson E, Burger P C, Baylin S B, Sidransky D. Nat Med. 1995;1:686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- 14.Frommer M, McDonald L E, Millar D S, Collis C M, Watt F, Grigg G W, Molloy P L, Paul C L. Proc Natl Acad Sci USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lengauer C, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1997;94:2545–2550. doi: 10.1073/pnas.94.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones P A. Cell. 1985;40:485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- 17.Jahner D, Stuhlmann H, Stewart C L, Harbers K, Lohler J, Simon I, Jaenisch R. Nature (London) 1982;298:623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- 18.Bestor T H, Verdine G L. Curr Opin Cell Biol. 1994;6:380–389. doi: 10.1016/0955-0674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 19.Lei H, Oh S, Okano M, Jutterman R, Goss K A, Jaenisch R, Li E. Development. 1996;122:3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 20.Weiss A, Keshet I, Razin A, Cedar H. Cell. 1996;86:709–718. doi: 10.1016/s0092-8674(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 21.Smith S S, Laayoun A, Lingeman R G, Baker D J, Riley J. J Mol Biol. 1994;243:143–151. doi: 10.1006/jmbi.1994.1640. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg A P, Gehrke C W, Kuo K C, Ehrlich M. Cancer Res. 1988;48:1159–1161. [PubMed] [Google Scholar]
- 23.Hsieh C-L. Mol Cell Biol. 1994;14:5487–5494. doi: 10.1128/mcb.14.8.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makos M, Nelkin B D, Chazin V R, Cavenee W K, Brodeur G M, Baylin S B. Cancer Res. 1993;53:2715–2718. [PubMed] [Google Scholar]
- 25.Laird P W, Jackson-Grusby L, Fazeli A, Dickinson S L, Jung W E, Li E, Weinberg R, Jaenisch R. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]