Background: The functional role of the fat-derived plasma protein CTRP9 in ischemic heart disease is unknown.
Results: Systemic delivery of CTRP9 reduces myocardial infarct size and apoptosis following ischemia-reperfusion in mice. CTRP9 protects cardiomyocyte from apoptosis through activation of AMP-activated protein kinase (AMPK).
Conclusion: CTRP9 prevents acute cardiac ischemic injury via an AMPK-dependent mechanism.
Significance: CTRP9 represents a novel target molecule for manipulation of myocardial ischemic injury.
Keywords: Adipokines, AMP-activated Protein Kinase (AMPK), Apoptosis, Ischemia, Myocardial Infarction, CTRP9
Abstract
Ischemic heart disease is the major cause of death in Western countries. CTRP9 (C1q/TNF-related protein 9) is a fat-derived plasma protein that has salutary effects on glucose metabolism and vascular function. However, the functional role of CTRP9 in ischemic heart disease has not been clarified. Here, we examined the regulation of CTRP9 in response to acute cardiac injury and investigated whether CTRP9 modulates cardiac damage after ischemia and reperfusion. Myocardial ischemia-reperfusion injury resulted in reduced plasma CTRP9 levels and increased plasma free fatty acid levels, which were accompanied by a decrease in CTRP9 expression and an increase in NADPH oxidase component expression in fat tissue. Treatment of cultured adipocytes with palmitic acid or hydrogen peroxide reduced CTRP9 expression. Systemic administration of CTRP9 to wild-type mice, before the induction of ischemia or at the time of reperfusion, led to a reduction in myocardial infarct size following ischemia-reperfusion. Administration of CTRP9 also attenuated myocyte apoptosis in ischemic heart, which was accompanied by increased phosphorylation of AMP-activated protein kinase (AMPK). Treatment of cardiac myocytes with CTRP9 protein reduced apoptosis in response to hypoxia/reoxygenation and stimulated AMPK phosphorylation. Blockade of AMPK activity reversed the suppressive actions of CTRP9 on cardiomyocyte apoptosis. Knockdown of adiponectin receptor 1 diminished CTRP9-induced increases in AMPK phosphorylation and survival of cardiac myocytes. Our data suggest that CTRP9 protects against acute cardiac injury following ischemia-reperfusion via an AMPK-dependent mechanism.
Introduction
Ischemic heart disease, including myocardial infarction, is a life-threatening disease that remains the major cause of mortality in the industrialized countries (1, 2). Obesity complications, including glucose intolerance, are closely linked with increased cardiac injury and an adverse prognosis after acute myocardial infarction as well as after reperfused therapy for acute myocardial infarction (3–5). Adipose tissue produces numerous bioactive substances, also known as adipocytokines or adipokines, which directly influence nearby or remote tissues (6–9). It has been suggested that the imbalance in generation of adipocytokines caused by obesity can contribute to the development of obesity-linked metabolic and cardiovascular disorders (7).
Adiponectin is an adipocytokine that has a collagenous domain and a C-terminal globular C1q domain (6). Adiponectin is down-regulated in association with obesity complications, including type 2 diabetes (6). We and others have demonstrated that adiponectin exerts salutary actions on various metabolic and cardiovascular diseases (6, 10–16). In particular, we have shown that adiponectin protects against acute cardiac damage following ischemia-reperfusion (13).
We sought to identify molecules with structural similarities to adiponectin, and BLAST searches for C1q domain sequences of adiponectin revealed that CTRP9 (C1q/TNF-related protein 9) shows the highest amino acid identity to adiponectin. CTRP9 belongs to the adiponectin paralog CTRP family and acts as an adipocytokine that exerts a beneficial effect on glucose metabolism (17). A recent study showed that CTRP9 promotes endothelium-dependent vasodilation (18). CTRP9 is expressed primarily in fat tissue, and its expression is perturbed by obese states (17, 18). Therefore, it is plausible that CTRP9 can modulate obesity-linked metabolic and cardiovascular disorders. However, nothing is known about the role of CTRP9 in ischemic heart disease. Here, we investigated the expression and regulation of CTRP9 in response to myocardial ischemia-reperfusion and examined the impact of CTRP9 on cardiac damage following ischemia-reperfusion in mice. We also investigated the effects of CTRP9 on apoptosis of cardiac myocytes and assessed its potential mechanisms.
EXPERIMENTAL PROCEDURES
Materials
Antibodies to phospho-AMP-activated protein kinase (AMPK)2 (Thr-172), AMPK, acetyl-CoA carboxylase (ACC), phospho-Akt (Ser-473), Akt, phospho-p42/44 ERK (Thr-202/Tyr-204), and ERK were purchased from Cell Signaling Technology. Phospho-ACC (Ser-79) and c-Myc tag antibodies were purchased from Upstate Biotechnology. Anti-tubulin antibody was purchased from Oncogene. Recombinant human CTRP9 protein and anti-mouse CTRP9 antibody were purchased from Adipobioscience. Rat adiponectin receptor (AdipoR) 1 siRNAs, AdipoR2 siRNAs, and unrelated siRNAs were purchased from Thermo Scientific. Compound C was purchased from Calbiochem. Palmitic acid and anti-sarcomeric actinin antibody were purchased from Sigma. Hydrogen peroxide was purchased from Wako Chemicals.
Mouse Model of Ischemia-Reperfusion Injury
Male C57BL/6J mice were purchased from Oriental BioService, Inc. We subjected mice at 10–12 weeks of age to myocardial ischemia-reperfusion as described previously (13, 19). Briefly, after anesthetization (50 mg/kg pentobarbital intraperitoneally) and intubation, the left anterior descending coronary artery was ligated for 60 min with a suture using a snare occluder and then loosened. At 24 h after reperfusion, the suture was retied, and Evans blue was systemically injected into mice to determine the non-ischemic tissue. The heart was excised, cut, and incubated with 2,3,5-triphenyltetrazolium chloride to determine infarction. The left ventricular (LV) area, the area at risk (AAR), and the infarct area (IA) were assessed by computerized planimetry using NIH ImageJ. In some experiments, we administered recombinant CTRP9 protein (1.0 μg/g of mouse) or vehicle (PBS) into the right jugular vein at the time of reperfusion. In some experiments, blood and epididymal fat were collected at 3 h after the reperfusion. Plasma free fatty acid levels were measured with enzymatic kits (Wako Chemicals). Study protocols were approved by the Institutional Animal Care and Use Committees at Nagoya University.
Mouse Model of Obesity
To produce diet-induced obesity, male C57BL/6J mice at 8 weeks of age were maintained on a high fat/high sucrose diet (F2HFHSD, Oriental BioService, Inc.) for 14 weeks. Male db/db mice in a background of C57BL/6J were purchased from Charles River Laboratories. Male C57BL/6J mice fed a normal diet (CE-2, CLEA Japan, Inc.) served as controls. Study protocols were approved by the Institutional Animal Care and Use Committees at Nagoya University.
Generation of Adenoviral Vectors
Full-length mouse CTRP9 cDNA was subcloned into an adenoviral shuttle vector, and the adenoviral (Ad) vectors expressing CTRP9 (Ad-CTRP9) were constructed under the control of the CMV promoter (20). Ad-β-gal was used as a control. At 3 days before surgery, Ad-CTRP9 or Ad-β-gal (3.0 × 108 plaque-forming units/mouse) was intravenously injected into the jugular veins of the mice.
Cultures of Cardiac Myocytes
Primary cultures of neonatal rat ventricular myocytes were incubated in DMEM supplemented with 10% fetal calf serum as described previously (21). After 12 h of serum starvation, cardiac myocytes were treated with CTRP9 protein (10 μg/ml) or vehicle for the indicated lengths of time. For hypoxia/reoxygenation studies, cells were exposed to 12 h of hypoxia followed by 24 h of reoxygenation in the presence of CTRP9 protein or vehicle. Hypoxic conditions were generated using an Anaero Pack system (Mitsubishi GAS Chemical Co., Inc.). In some experiments, cells were infected with an adenoviral vector encoding c-Myc-tagged dominant-negative AMPK (Ad-dnAMPK) or Ad-β-gal as a control at a multiplicity of infection of 10 for 24 h (22), followed by treatment with CTRP9 or vehicle. In some experiments, cells were transfected with siRNAs (40 nm) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. In some experiments, cells were pretreated with Compound C (10 μm) or vehicle (Me2SO) for 60 min, followed by incubation with CTRP9 or vehicle.
Cultures of 3T3-L1 Adipocytes
Mouse 3T3-L1 cells (American Type Culture Collection) were cultured in DMEM with 10% fetal bovine serum and then differentiated into adipocytes by DMEM supplemented with 5 μg/ml insulin, 0.5 mm 1-methyl-3-isobutylxanthine, and 1 μm dexamethasone as described previously (23). On 7 day from induction of differentiation, 3T3-L1 adipocytes were treated with hydrogen peroxide (200 μm) or vehicle for 24 h. In some experiments, differentiated 3T3-L1 adipocytes were treated with palmitic acid (250 μm) in 0.5% BSA or vehicle (0.5% BSA) for 24 h.
Determination of mRNA Levels
Gene expression levels were quantified by real-time PCR. Total RNA was extracted from 3T3-L1 adipocytes using an RNeasy mini kit (Qiagen) and from fat tissues using an RNeasy lipid tissue mini kit (Qiagen). cDNA was produced using a SuperScript real-time PCR system (Invitrogen). PCR was performed with a Bio-Rad real-time PCR detection system using SYBR Green I as a double-standard DNA-specific dye. The primers used were 5′-TGGTGAACGTGGTGCCTACA-3′ and 5′-TGCAGTCACATCCCACCCT-3′ for mouse CTRP9, 5′-CTAAGGCCAACCGTGAAAAG-3′ and 5′-GGTACGACCAGAGGCATACA-3′ for mouse β-actin, 5′-TTGGGTCAGCACTGGCTCTG-3′ and 5′-TGGCGGTGTGCAGTGCTATC-3′ for mouse gp91phox, and 5′-GATGTTCCCCATTGAGGCCG-3′ and 5′-GTTTCAGGTCATCAGGCCGC-3′ for mouse p47phox.
Western Blot Analysis
Heart tissue and cell samples were prepared in lysis buffer containing 1 mm PMSF (Cell Signaling Technology). The protein concentration was calculated using a BCA protein assay kit (Pierce). Equal amounts of proteins or plasma (2.0 μl) were separated by denaturing SDS-PAGE. Proteins were transferred onto PVDF membrane (GE Healthcare) and probed with the primary antibody, followed by incubation with HRP-conjugated secondary antibody. The ECL Plus system (GE Healthcare) was used for detection of the protein signal. The expression level was determined by measurement of the corresponding band intensities using ImageJ software. Immunoblots were normalized to the tubulin signal.
Determination of Apoptosis
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining of the frozen heart sections or cultured cardiac myocytes was performed using an in situ cell death detection kit (Roche Applied Science) as described previously (13). Cryosections (5 μm thick) were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100. To determine cardiac myocytes, heart sections were stained with anti-sarcomeric actinin antibody. TUNEL-positive cells were counted in three randomly selected fields of the slide, and the experiments were repeated three times in duplicates.
Statistical Analysis
Data are presented as means ± S.E. Group differences were analyzed by Student's unpaired t test or one-way analysis of variance. A p value of <0.05 was considered statistically significant.
RESULTS
Circulating CTRP9 Levels Decline after Myocardial Ischemia-Reperfusion
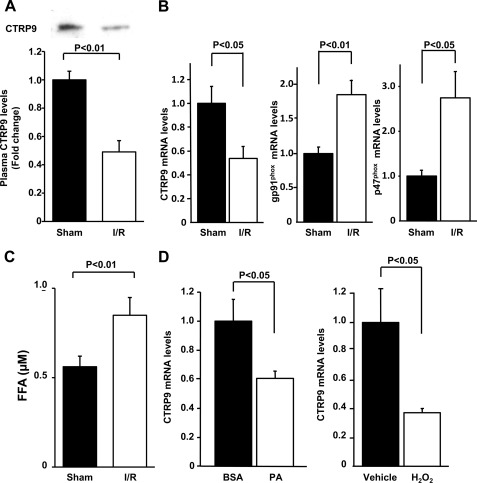
To test whether cardiac ischemia-reperfusion affects plasma CTRP9 levels in wild-type C57BL/6J mice, plasma CTRP9 levels were determined after ischemia-reperfusion or sham surgery by Western blot analysis. Plasma CTRP9 levels significantly declined by 51 ± 8% following ischemia-reperfusion compared with those after sham operation (Fig. 1A). Because CTRP9 is expressed predominantly in adipose tissue (17), CTRP9 mRNA expression in epididymal fat was measured by real-time PCR. Ischemia-reperfusion resulted in a significant reduction in CTRP9 mRNA in fat tissue (Fig. 1B). Because increased oxidative stress in fat tissue causes the dysregulated production of adipocytokines (24), we assessed the mRNA expression of NADPH oxidase components in epididymal fat. The mRNA levels of gp91phox and p47phox were significantly up-regulated in response to myocardial ischemia-reperfusion injury (Fig. 1B). An increase in free fatty acids (FFAs) is linked to increased oxidative stress in fat tissue (24, 25). Thus, we measured plasma FFA levels following ischemia-reperfusion or sham surgery. Ischemia-reperfusion led to a significant elevation of plasma FFA levels compared with the sham operation control (Fig. 1C), consistent with a previous report (26).
FIGURE 1.
Plasma CTRP9 declines following cardiac ischemia-reperfusion injury. A, plasma CTRP9 levels after myocardial ischemia-reperfusion (I/R) or sham surgery. Wild-type mice were subjected to 60 min of ischemia, followed by 24 h of reperfusion or sham operation. CTRP9 protein levels in plasma (2.0 μl) were determined by Western blot analysis. Relative protein levels of CTRP9 were quantified using ImageJ (mean ± S.E., n = 6). B, expression of CTRP9 and NADPH oxidase components in fat tissue. Adipose tissues were collected from wild-type mice at 3 h after ischemia-reperfusion or sham operation. Transcript levels were determined by quantitative real-time PCR and are expressed relative to β-actin levels (mean ± S.E., n = 4). C, plasma FFA levels at 3 h after ischemia-reperfusion or sham operation (mean ± S.E., n = 4). D, effect of palmitic acid and hydrogen peroxide on CTRP9 expression in adipocytes. Differentiated 3T3-L1 adipocytes were treated with palmitic acid (PA; 250 μm) or BSA (left panel) and H2O2 (200 μm) or vehicle (right panel) for 24 h. CTRP9 mRNA levels were assessed by quantitative real-time PCR and are expressed relative to β-actin levels (mean ± S.E., n = 4).
To investigate the possible participation of fatty acids in the regulation of CTRP9 expression in adipocytes, 3T3-L1 adipocytes were stimulated with palmitic acid. Treatment with palmitic acid significantly reduced CTRP9 mRNA levels in 3T3-L1 adipocytes (Fig. 1D). Furthermore, treatment of adipocytes with hydrogen peroxide led to a significant reduction in CTRP9 mRNA expression. These data indicate that myocardial ischemic injury promotes oxidative stress in adipose tissue, leading to reduced production of CTRP9.
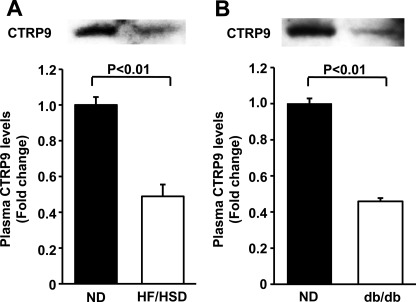
Because obese states such as insulin resistance and hyperinsulinemia are associated with an increased risk of cardiovascular disease (3, 5, 27, 28), we assessed the plasma levels of CTRP9 in mouse models of obesity with insulin resistance and hyperinsulinemia. Plasma CTRP9 levels were significantly decreased by 51 ± 2% in high fat/high sucrose diet-induced obese mice compared with lean wild-type mice fed a normal chow diet (Fig. 2A), consistent with a previous report showing reduced plasma levels of CTRP9 in high fat diet-induced obese mice (18). Likewise, the plasma levels of CTRP9 were significantly reduced by 54 ± 3% in leptin receptor-deficient db/db mice compared with normal diet-fed wild-type mice (Fig. 2B).
FIGURE 2.
Circulating CTRP9 levels are reduced in mouse models of obesity. A and B, plasma CTRP9 levels in diet-induced obese and db/db mice, respectively. Wild-type mice at 8 weeks of age were maintained on a high fat/high sucrose diet (HF/HSD) or a normal chow diet (ND) for 14 weeks (A). CTRP9 protein in plasma (2.0 μl) was analyzed by Western blotting. CTRP9 protein levels were quantified using ImageJ (mean ± S.E., n = 4). Plasma CTRP9 levels in control mice fed a normal chow diet and db/db mice at 8 weeks of age were determined by Western blot analysis and quantified using ImageJ (mean ± S.E., n = 3) (B).
Systemic Delivery of CTRP9 Reduces Infarct Size in Response to Ischemia-Reperfusion
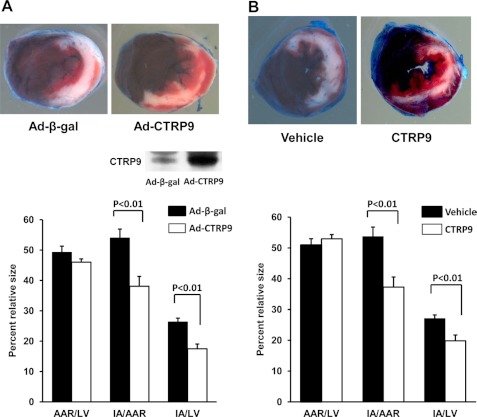
To examine the impact of CTRP9 on acute ischemic injury in the heart, Ad-CTRP9 or Ad-β-gal as a control was intravenously injected into wild-type C57BL/6J mice. Systemic delivery of adenoviral vectors leads to transgene expression in the liver, but not the myocardium (19). Administration of Ad-CTRP9 significantly increased plasma CTRP9 levels by a factor of 3.3 ± 0.4 (mean ± S.E. (n = 4); p < 0.01) at day 3 after adenoviral injection compared with Ad-β-gal as assessed by Western blot analysis (Fig. 3A). At 3 days after adenoviral injection, mice were subjected to ischemia-reperfusion injury. Fig. 3A shows representative photographs of heart tissues stained with Evans blue dye to delineate the AAR and 2,3,5-triphenyltetrazolium chloride to delineate the IA at 24 h after ischemia-reperfusion surgery. Delivery of Ad-CTRP9 significantly attenuated the IA/AAR and IA/LV ratios by 30 ± 6 and 34 ± 6%, respectively (Fig. 3A). In contrast, the AAR/LV ratio did not differ between the two experimental groups.
FIGURE 3.
Systemic delivery of CTRP9 attenuates infarct size in mice following myocardial ischemia-reperfusion. A, effect of Ad-CTRP9 on myocardial infarct size after ischemia-reperfusion. At 3 days after intravenous injection of Ad-CTRP9 or Ad-β-gal (total of 3.0 × 108 plaque-forming units), wild-type mice were subjected to 60 min of ischemia, followed by 24 h of reperfusion. Hearts were stained with Evans blue, followed by 2,3,5-triphenyltetrazolium chloride. Representative pictures of the heart sections at 24 h after ischemia-reperfusion are shown in the upper panels. Quantitative analysis of infarct size after ischemia-reperfusion injury is shown in the lower panel. The LV area, AAR, and IA were measured (mean ± S.E., n = 8). The inset shows a representative blot for CTRP9 in plasma (2.0 μl) at 3 days after injection of Ad-CTRP9 or Ad-β-gal. B, effect of CTRP9 protein on myocardial infarct size following ischemia-reperfusion. Wild-type mice were treated with recombinant CTRP9 protein (1.0 μg/g of mouse) or vehicle at the time of reperfusion. Hearts were stained with Evans blue and subsequently with 2,3,5-triphenyltetrazolium chloride at 24 h after ischemia-reperfusion. Representative pictures of myocardial tissues at 24 h after ischemia-reperfusion are shown in the upper panels. Quantitative analysis of the infarct size of each experimental group after ischemia-reperfusion injury is shown in the lower panel (mean ± S.E., n = 8).
To further assess whether exogenous CTRP9 is therapeutic for myocardial infarction, we intravenously administered a single dose of recombinant CTRP9 protein to wild-type mice at the time of reperfusion. The administration of CTRP9 protein reduced the IA/AAR and IA/LV ratios by 31 ± 2 and 27 ± 1%, respectively, compared with the vehicle (Fig. 3B). Thus, CTRP9 supplementation, either before ischemia-reperfusion or at the time of reperfusion, is effective in reducing cardiac ischemic damage.
CTRP9 Suppresses Myocyte Apoptosis in Vivo and in Vitro
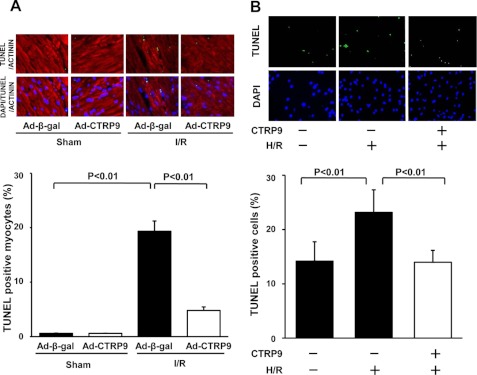
To investigate the effect of CTRP9 on apoptosis in the heart, TUNEL staining was performed in the heart at 24 h after ischemia-reperfusion or sham operation. Fig. 4A shows representative fluorescence photographs of TUNEL-positive nuclei in the heart. Quantitative analysis demonstrated that Ad-CTRP9 treatment significantly reduced the frequencies of TUNEL-positive myocytes in the myocardium of mice following ischemia-reperfusion. In contrast, little or no TUNEL-positive myocytes were observed in the hearts of control or CTRP9-treated mice after sham operation.
FIGURE 4.
Effect of CTRP9 on myocardial apoptosis. A, CTRP9 reduces myocyte apoptosis in the heart at 24 h after ischemia-reperfusion. Wild-type mice were treated with Ad-CTRP9 or Ad-β-gal and subjected to ischemia-reperfusion (I/R) or sham surgery. The upper panels show representative photographs of heart sections stained by TUNEL (green) and with sarcomeric actinin (red) and DAPI (blue). Quantitative analyses of TUNEL-positive myocytes are shown in the lower panel (mean ± S.E., n = 6). B, CTRP9 attenuates apoptosis of cardiac myocytes under conditions of hypoxia/reoxygenation. Neonatal rat cardiac myocytes were treated with CTRP9 protein (10 μg/ml) or vehicle and subjected to normoxia or hypoxia/reoxygenation (H/R). The upper panels show representative photographs of cardiac myocytes stained by TUNEL (green) and with DAPI (blue). The lower panel shows quantitative analysis of TUNEL-positive cells (mean ± S.E., n = 4).
To examine the effect of CTRP9 on apoptosis at the cellular level, neonatal rat cardiac myocytes were subjected to normoxia or hypoxia/reoxygenation in the presence of recombinant CTRP9 protein or vehicle. Treatment with CTRP9 protein significantly suppressed the frequencies of TUNEL-positive cells after hypoxia/reoxygenation (Fig. 4B).
AMPK Is Involved in Anti-apoptotic Action of CTRP9
AMPK activation in the heart is protective against cardiac ischemic injury (29). Thus, the activating phosphorylation levels of AMPK at Thr-172 in the heart were assessed by Western blot analysis. Systemic delivery of CTRP9 resulted in a significant increase in AMPK phosphorylation in the ischemic heart following reperfusion (Fig. 5A). In contrast, CTRP9 administration had no effects on AMPK phosphorylation in the heart after sham operation. Furthermore, treatment of cardiac myocytes with CTRP9 enhanced the phosphorylation of AMPK (Fig. 5B). CTRP9 treatment also stimulated the phosphorylation of ACC, a downstream target of AMPK, in cardiac myocytes. Because CTRP9 has been reported to activate Akt and ERK in cultured myotubes (17), we tested whether CTRP9 modulates the phosphorylation status of Akt and ERK in cultured cardiac myocytes. CTRP9 treatment did not affect the phosphorylation of Akt at Ser-473 (0.8 ± 0.2-fold change, not significant) and ERK at Thr-202/Tyr-204 (0.9 ± 0.2-fold change, not significant) in cardiac myocytes compared with vehicle treatment.
FIGURE 5.
AMPK signaling is essential for CTRP9-stimulated survival of cardiac myocytes. A, CTRP9 promotes AMPK phosphorylation in the ischemic heart. After systemic delivery of Ad-CTRP9 or Ad-β-gal, wild-type mice were subjected to ischemia-reperfusion (I/R) or sham operation. The phosphorylation levels of AMPK (P-AMPK) were analyzed by Western blotting. The relative phosphorylation levels of AMPK were quantified using ImageJ and are expressed relative to the tubulin signal (mean ± S.E., n = 4). B, CTRP9 stimulates AMPK signaling in cardiac myocytes. Cardiac myocytes were treated with CTRP9 protein (10 μg/ml) or vehicle for 15 min. The phosphorylation levels of ACC (P-ACC), AMPK, Akt (P-Akt), and ERK (P-ERK) were determined by Western blot analysis. The relative phosphorylation levels of ACC and AMPK were quantified using ImageJ (mean ± S.E., n = 4). Immunoblots were normalized to the tubulin signal. C and D, effect of AMPK inactivation on CTRP9-stimulated ACC phosphorylation (C) and survival of cardiac myocytes (D). After transduction with Ad-dnAMPK or Ad-β-gal, cardiac myocytes were treated with CTRP9 or vehicle for 15 min (C). The phosphorylation of ACC was determined by Western blotting. The relative phosphorylation levels of ACC were quantified using ImageJ (mean ± S.E., n = 4). Immunoblots were normalized to the tubulin signal. Cardiac myocytes were cultured in the presence of CTRP9 or vehicle under conditions of hypoxia/reoxygenation (D). TUNEL-positive nuclei were counted and quantified (mean ± S.E., n = 4). E, effect of AMPK inhibitor on anti-apoptotic actions of CTRP9. Cardiac myocytes were pretreated with Compound C (10 μm) or vehicle and stimulated with or without CTRP9. TUNEL-positive nuclei were counted and quantified (mean ± S.E., n = 4).
To test whether AMPK signaling participates in CTRP9-stimulated survival of cardiac myocytes, cells were transduced with Ad-dnAMPK or Ad-β-gal. Transduction with Ad-dnAMPK abolished CTRP9-stimulated phosphorylation of ACC in cardiac myocytes (Fig. 5C). Transduction with Ad-dnAMPK also blocked the inhibitory effects of CTRP9 on apoptosis of cardiac myocytes under conditions of hypoxia/reoxygenation (Fig. 5D). Similarly, pretreatment with the AMPK inhibitor Compound C reversed the anti-apoptotic effects of CTRP9 (Fig. 5E). These data indicate that CTRP9 promotes the survival of cardiac myocytes through activation of AMPK.
Role of Adiponectin Receptors in CTRP9-mediated Survival Signaling
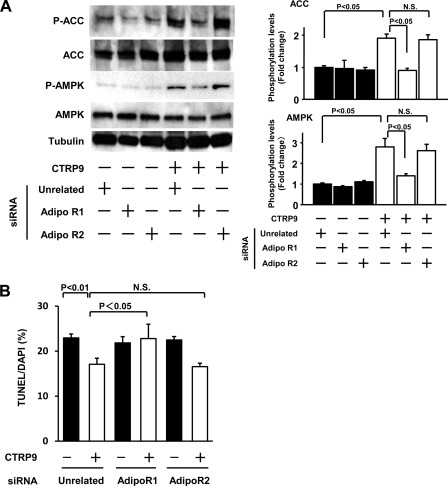
AdipoR1 has been reported to act as a receptor for CTRP9 in endothelial cells (18). To analyze the possible participation of AdipoR1 in myocyte response to CTRP9, cardiac myocytes were transfected with siRNAs targeting AdipoR1 or AdipoR2 or unrelated siRNAs, followed by stimulation with CTRP9 or vehicle. Transfection of cardiac myocytes with siRNAs against AdipoR1 or AdipoR2 led to reductions in AdipoR1 and AdipoR2 mRNA expression of 84 and 82%, respectively. Ablation of AdipoR1, but not knockdown of AdipoR2, suppressed CTRP9-induced increases in AMPK phosphorylation and myocyte survival (Fig. 6, A and B).
FIGURE 6.
Contribution of AdipoR1 to CTRP9-induced survival signaling in cardiac myocytes. A, effect of AdipoR1 ablation on CTRP9-stimulated AMPK signaling. Cardiac myocytes were transfected with siRNAs against AdipoR1 or AdipoR2 or with unrelated siRNAs, followed by stimulation with CTRP9 protein (10 μg/ml) or vehicle for 15 min. The phosphorylation of ACC (P-ACC) and AMPK (P-AMPK) was determined by Western blotting. The relative phosphorylation levels of ACC and AMPK were quantified using ImageJ (mean ± S.E., n = 3). Immunoblots were normalized to the tubulin signal. B, knockdown of AdipoR1 blocks CTRP9-induced survival of cardiac myocytes. After transfection with siRNAs against AdipoR1 or AdipoR2 or with unrelated siRNAs, cardiac myocytes were cultured in the presence of CTRP9 protein (10 μg/ml) or vehicle under conditions of hypoxia/reoxygenation. TUNEL-positive nuclei were counted and quantified (mean ± S.E., n = 3). N.S., not statistically significant.
DISCUSSION
In this study, we have provided the first evidence that CTRP9 protects against acute myocardial ischemic injury in vivo. Overproduction of circulating CTRP9 before the induction of ischemia led to a reduction in myocardial infarct size following ischemia-reperfusion in wild-type mice. Moreover, a single dose of recombinant CTRP9 protein delivered at the time of reperfusion minimized the myocardial infarct size. The beneficial action of CTRP9 was associated with decreased myocyte apoptosis in the heart. Treatment of cardiac myocytes with CTRP9 protein resulted in a reduction in apoptosis in response to hypoxia/reoxygenation. Because circulating CTRP9 levels significantly declined in wild-type mice following myocardial ischemia-reperfusion, the replenishment of CTRP9 may be effective in reducing myocardial damage after ischemia-reperfusion.
A recent report demonstrated that CTRP9 promotes vascular relaxation via an AdipoR1/AMPK signaling pathway in endothelial cells (18). In the present study, CTRP9 enhanced AMPK activation in cardiac myocytes and ischemic heart. Blockade of AMPK activity abolished the suppressive effects of CTRP9 on cardiomyocyte apoptosis. Moreover, ablation of AdipoR1 diminished CTRP9-stimulated AMPK activation and survival of cardiac myocytes, suggesting that CTRP9 protects cardiomyocytes from apoptosis through AdipoR1. Taken together, these data indicate that CTRP9 can exert beneficial actions on the cardiovascular systems partly through the AdipoR1/AMPK-dependent mechanism.
The regulation of CTRP9 expression has been poorly understood. Our data show that acute ischemic insult in the heart caused a decrease in plasma CTRP9 levels and an increase in plasma FFA levels. Myocardial ischemic injury also resulted in a reduction of CTRP9 expression in adipose tissue, which was accompanied by increased expression of NADPH oxidase components. Furthermore, treatment of cultured adipocytes with palmitic acid or an inducer of oxidative stress led to attenuation of CTRP9 expression. The excess of FFAs can stimulate oxidative stress in adipocytes, leading to dysregulation of adipocytokine production (24, 25). Thus, these data suggest that elevated plasma FFA levels induced by acute cardiac insult may contribute to enhanced oxidative stress and decreased production of CTRP9 expression in fat tissue, thereby resulting in reduced levels of circulating CTRP9.
In conclusion, our study demonstrates that CTRP9 functions as a novel modulator of acute ischemic damage in the heart through activation of AMPK in cardiac myocytes. Activation of myocardial AMPK has been shown to display salutary effects on various heart diseases, including ischemic heart disease (13, 29, 30). Because CTRP9 is an adipocytokine that is expressed abundantly in adipose tissue (17), CTRP9 seems to affect cardiac AMPK signaling in an endocrine manner. Thus, the CTRP9/AMPK signaling axis may represent a functional link between fat tissue and the heart and also contribute to the cardioprotective actions. Furthermore, our data show that circulating levels of CTRP9 in mice are down-regulated in association with obesity, insulin resistance, and hyperinsulinemia, which are causally linked with the increased prevalence of cardiovascular disease. Collectively, the therapeutic approaches to enhance CTRP9 production can be valuable for prevention or treatment of acute myocardial infarction.
Acknowledgments
We gratefully acknowledge the technical assistance of Yoko Inoue and Miho Sakai.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and grants from the Takeda Science Foundation (to N. O.), by grants from the Uehara Memorial Foundation (to N. O. and R. S.), and by Grant-in-aids for Young Scientists B from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to R. S. and K. O.).
- AMPK
- AMP-activated protein kinase
- dnAMPK
- dominant-negative AMPK
- ACC
- acetyl-CoA carboxylase
- AdipoR
- adiponectin receptor
- LV
- left ventricular
- AAR
- area at risk
- IA
- infarct area
- Ad
- adenoviral
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- FFA
- free fatty acid.
REFERENCES
- 1. Roger V. L., Go A. S., Lloyd-Jones D. M., Adams R. J., Berry J. D., Brown T. M., Carnethon M. R., Dai S., de Simone G., Ford E. S., Fox C. S., Fullerton H. J., Gillespie C., Greenlund K. J., Hailpern S. M., Heit J. A., Ho P. M., Howard V. J., Kissela B. M., Kittner S. J., Lackland D. T., Lichtman J. H., Lisabeth L. D., Makuc D. M., Marcus G. M., Marelli A., Matchar D. B., McDermott M. M., Meigs J. B., Moy C. S., Mozaffarian D., Mussolino M. E., Nichol G., Paynter N. P., Rosamond W. D., Sorlie P. D., Stafford R. S., Turan T. N., Turner M. B., Wong N. D., Wylie-Rosett J. (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123, e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunt S. A., Abraham W. T., Chin M. H., Feldman A. M., Francis G. S., Ganiats T. G., Jessup M., Konstam M. A., Mancini D. M., Michl K., Oates J. A., Rahko P. S., Silver M. A., Stevenson L. W., Yancy C. W., Antman E. M., Smith S. C., Jr., Adams C. D., Anderson J. L., Faxon D. P., Fuster V., Halperin J. L., Hiratzka L. F., Jacobs A. K., Nishimura R., Ornato J. P., Page R. L., Riegel B. (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112, e154–e235 [DOI] [PubMed] [Google Scholar]
- 3. Orlander P. R., Goff D. C., Morrissey M., Ramsey D. J., Wear M. L., Labarthe D. R., Nichaman M. Z. (1994) The relation of diabetes to the severity of acute myocardial infarction and post-myocardial infarction survival in Mexican-Americans and non-Hispanic whites. The Corpus Christi Heart Project. Diabetes 43, 897–902 [DOI] [PubMed] [Google Scholar]
- 4. Woodfield S. L., Lundergan C. F., Reiner J. S., Greenhouse S. W., Thompson M. A., Rohrbeck S. C., Deychak Y., Simoons M. L., Califf R. M., Topol E. J., Ross A. M. (1996) Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J. Am. Coll. Cardiol. 28, 1661–1669 [DOI] [PubMed] [Google Scholar]
- 5. Carrabba N., Valenti R., Parodi G., Santoro G. M., Antoniucci D. (2004) Left ventricular remodeling and heart failure in diabetic patients treated with primary angioplasty for acute myocardial infarction. Circulation 110, 1974–1979 [DOI] [PubMed] [Google Scholar]
- 6. Ouchi N., Kihara S., Funahashi T., Matsuzawa Y., Walsh K. (2003) Obesity, adiponectin, and vascular inflammatory disease. Curr. Opin. Lipidol. 14, 561–566 [DOI] [PubMed] [Google Scholar]
- 7. Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berg A. H., Scherer P. E. (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96, 939–949 [DOI] [PubMed] [Google Scholar]
- 9. Ouchi N., Higuchi A., Ohashi K., Oshima Y., Gokce N., Shibata R., Akasaki Y., Shimono A., Walsh K. (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., Eto K., Akanuma Y., Froguel P., Foufelle F., Ferre P., Carling D., Kimura S., Nagai R., Kahn B. B., Kadowaki T. (2002) Adiponectin stimulates glucose utilization and fatty acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 [DOI] [PubMed] [Google Scholar]
- 12. Shinmura K., Tamaki K., Saito K., Nakano Y., Tobe T., Bolli R. (2007) Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 116, 2809–2817 [DOI] [PubMed] [Google Scholar]
- 13. Shibata R., Sato K., Pimentel D. R., Takemura Y., Kihara S., Ohashi K., Funahashi T., Ouchi N., Walsh K. (2005) Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 11, 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouchi N., Kihara S., Arita Y., Maeda K., Kuriyama H., Okamoto Y., Hotta K., Nishida M., Takahashi M., Nakamura T., Yamashita S., Funahashi T., Matsuzawa Y. (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100, 2473–2476 [DOI] [PubMed] [Google Scholar]
- 15. Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., Komuro R., Ouchi N., Kihara S., Tochino Y., Okutomi K., Horie M., Takeda S., Aoyama T., Funahashi T., Matsuzawa Y. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- 16. Ohashi K., Parker J. L., Ouchi N., Higuchi A., Vita J. A., Gokce N., Pedersen A. A., Kalthoff C., Tullin S., Sams A., Summer R., Walsh K. (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285, 6153–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong G. W., Krawczyk S. A., Kitidis-Mitrokostas C., Ge G., Spooner E., Hug C., Gimeno R., Lodish H. F. (2009) Identification and characterization of CTRP9, a novel secreted glycoprotein from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 23, 241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng Q., Yuan Y., Yi W., Lau W. B., Wang Y., Wang X., Sun Y., Lopez B. L., Christopher T. A., Peterson J. M., Wong G. W., Yu S., Yi D., Ma X. L. (2011) C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor 1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler. Thromb. Vasc. Biol. 31, 2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shibata R., Sato K., Kumada M., Izumiya Y., Sonoda M., Kihara S., Ouchi N., Walsh K. (2007) Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc. Res. 74, 471–479 [DOI] [PubMed] [Google Scholar]
- 20. Ouchi N., Oshima Y., Ohashi K., Higuchi A., Ikegami C., Izumiya Y., Walsh K. (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 283, 32802–32811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pimentel D. R., Amin J. K., Xiao L., Miller T., Viereck J., Oliver-Krasinski J., Baliga R., Wang J., Siwik D.A., Singh K., Pagano P., Colucci W. S., Sawyer D. B. (2001) Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 89, 453–460 [DOI] [PubMed] [Google Scholar]
- 22. Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enomoto T., Ohashi K., Shibata R., Higuchi A., Maruyama S., Izumiya Y., Walsh K., Murohara T., Ouchi N. (2011) Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J. Biol. Chem. 286, 34552–34558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimizu I., Yoshida Y., Katsuno T., Tateno K., Okada S., Moriya J., Yokoyama M., Nojima A., Ito T., Zechner R., Komuro I., Kobayashi Y., Minamino T. (2012) p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 15, 51–64 [DOI] [PubMed] [Google Scholar]
- 26. Yue T. L., Bao W., Jucker B. M., Gu J. L., Romanic A. M., Brown P. J., Cui J., Thudium D. T., Boyce R., Burns-Kurtis C. L., Mirabile R. C., Aravindhan K., Ohlstein E. H. (2003) Activation of peroxisome proliferator-activated receptor α protects the heart from ischemia/reperfusion injury. Circulation 108, 2393–2399 [DOI] [PubMed] [Google Scholar]
- 27. DeFronzo R. A., Abdul-Ghani M. (2011) Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am. J. Cardiol. 108, 3B–24B [DOI] [PubMed] [Google Scholar]
- 28. Reilly M. P., Rader D. J. (2003) The metabolic syndrome: more than the sum of its parts? Circulation 108, 1546–1551 [DOI] [PubMed] [Google Scholar]
- 29. Russell R. R., 3rd, Li J., Coven D. L., Pypaert M., Zechner C., Palmeri M., Giordano F. J., Mu J., Birnbaum M. J., Young L. H. (2004) AMP-activated protein kinase mediates ischemic glucose uptake and prevents post-ischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 114, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan A. Y., Soltys C. L., Young M. E., Proud C. G., Dyck J. R. (2004) Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 279, 32771–32779 [DOI] [PubMed] [Google Scholar]