Abstract
Objective
The perimenopausal increase in circulating dehydroepiandrosterone sulfate (DHEAS) levels during the menopausal transition (MT) is accompanied by other adrenal steroids that have the potential to alter the estrogen/androgen balance and explain the wide inter-woman range of estrogen-related symptoms experienced during the MT.
Methods
Annual serum samples from the Study of Women’s Health Across the Nation (SWAN), which had previously been analyzed for immunoreactive estradiol (E2), testosterone (T), DHEAS and sex hormone binding globulin (SHBG), were selected based on DHEAS concentration and analyzed for immunoreactive and bioactive estrogens and androgens, including immunoreactive androstenedione (Adione), dehydroepiandrosterone (DHEA) and 5-androstene-3β,17β-diol (androstenediol, Adiol).
Results
A two-fold increase in circulating Adione and T was found to rise in parallel with the rise in circulating DHEAS, while DHEA and Adiol concentrations rose seven to eightfold. Circulating Adiol, which has both androgenic and estrogenic biological activity, was significantly associated (p<0.02) with circulating estrogen bioactivity only when E2 concentrations were low and Adiol levels were high.
Conclusions
The wide range of circulating levels of Adiol and its contribution to total circulating estrogenicity during the MT is consistent with the observed inter-woman difference in symptoms at this time. Therefore, we conclude that Adiol contributes to circulating estrogenicity when E2 production falls at menopause and may contribute significantly to the endocrine changes experienced by midlife women.
Keywords: Androstenediol, estrogenicity, menopause, adrenal
Introduction
A gradual decrease in ovarian function is usually invoked to explain many of the symptoms such as hot flushes that are associated with the menopausal transition (MT). However, a simple and satisfactory explanation for the specific timing of onset of symptoms and the inability to demonstrate a lack of correlation of circulating hormones to the severity of symptoms has been challenging, based on the observed between-woman similarity in circulating estradiol (E2) through most of the MT. Despite this similarity in circulating E2, only some perimenopausal and postmenopausal women become clinically estrogen deficient at and following menopause, while others require estrogen replacement.
Hormone levels in longitudinal blood samples collected in the early follicular phase of the menstrual cycle indicate that circulating E2 levels fall modestly during the early and early-late perimenopause, then decline more rapidly during the two years just before and immediately following menopause1. E2 levels decline to their lowest circulating concentration two years following menopause2. Circulating testosterone (T) levels tend to follow the same timing and trajectory as E2 levels prior to menopause3. Circulating follicle stimulating hormone (FSH) levels begin to rise several years prior to any detected fall in circulating E2 levels and sex hormone-binding globulin (SHBG) concentrations gradually decline during the three years just prior to menopause1.
In contrast to the ovarian steroid hormones, there is an increase in adrenal dehydroepiandrosterone sulfate (DHEAS) levels beginning during the early perimenopause which plateau at menopause4. This rise in DHEAS (from 108 to 112 ug/dL, p=0.0027) has been shown to occur in 85% of women and represents increased adrenal steroidogenesis, since it is observed in women who have undergone bilateral salpingo-oophorectomy5. If this increase in adrenal steroidogenic activity during the MT includes downstream steroids in the delta five steroidogenic pathway, particularly 5-androstene-3β,17β-diol (Adiol), then such a general increase could contribute to the estrogen/androgen ratio since Adiol has both estrogenic and androgenic biological properties6,7. Increased circulating Adiol may therefore be important before and immediately following menopause when circulating E2 levels undergo the most precipitous decline. In addition, if the wide inter-woman range of adrenal steroid production observed during the menopausal transition includes Adiol, then this wide range of potential estrogenic compliments may help explain the wide range of symptoms observed at this time.
The current dogma indicates that the peripheral conversion of prohormones such as dehydroepiandrosteone (DHEA) progressively compensate for the decreased production of steroid hormones from the ovary as the perimenopause progresses. However, recent data3,4 suggest that an increase in the direct secretion of Adiol, androstenedione (Adione) and T may be equally important to the availability of substrates for 17β-hydroxysteroid dehydrogenase and aromatase. Thus, the measurement of the circulating concentrations of adrenal steroids, particularly Adiol with its estrogenic potential, may provide a more appropriate indicator of hormone balance before and following menopause.
There is growing evidence that the rise in adrenal C-19 steroids may dominate the perimenopause steroid hormone profiles and influence the incidence of symptoms as well as the course of health trajectories. With this in mind, the present study was conducted to document the rise of four C-19 adrenal steroids during the menopausal transition, and characterize each of their biological potentials.
Materials and Methods
This study reports results from annual serum samples obtained from the overall SWAN population, which has been previously described8. Briefly, SWAN is a multi-site, longitudinal cohort study that was conducted in community-based groups of women who belonged to one of five ethnic/racial groups. Eligibility criteria for the SWAN longitudinal cohort were: age 42 to 52 years; intact uterus and at least one ovary; no current use of estrogens or other medications known to affect ovarian function; at least one menstrual period in the three months before screening; and self-identification in one of five eligible ethnic groups (Table 1). Institutional Review Board approval was obtained at each study site.
Table 1.
Demographics of Study Subjects
| DHEAS Characteristic | |
|---|---|
| Mean (SD) | |
| Age (years) | 46.2 (2.6) |
| Body mass index (kg/m2) | 26.2 (6.3) |
| Percent (N) | |
| Ethnicity: | |
| African American | 18.3 (26) |
| Caucasian | 61.3 (87) |
| Chinese | 9.9 (14) |
| Hispanic | 1.4 (2) |
| Japanese | 9.2 (13) |
| Menopause status: | |
| Premenopausal | 57.1 (80) |
| Early perimenopausal | 42.9 (60) |
| Hot flashes or night sweats in past 2 weeks (yes) | 38.0 (54) |
| Hot flashes (regardless of night sweats) in past 2 weeks (yes) | 25.4 (36) |
| Current smoking ( yes) | 16.2 (23) |
| Difficulty paying for basics: | |
| Very hard | 7.0 (10) |
| Somewhat hard | 27.5 (39) |
| Not hard at all | 65.5 (93) |
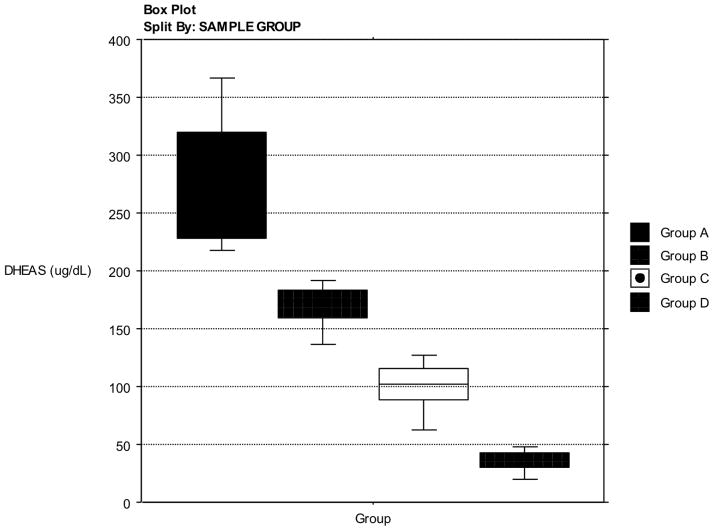
Selection of serum samples (n=144) from the SWAN collections was based solely on the concentration of DHEAS during the MT in order to capture the full range of values in the steroids secreted in concert with DHEAS. The samples selected represented equal numbers for the following groups (n=36 for each group): Greater than one standard deviation (SD) above the mean DHEAS concentration (Group A); between the mean and one SD above the DHEAS mean (Group B); between the mean one SD below the DHEAS mean (Group C); lower than one SD below the DHEAS mean (Group D). Samples were chosen so that they represent the complete range of DHEAS concentrations (Figure 1). Ethnicity, BMI, menopausal status or other demographic characteristics were not considered in selection of samples, only DHEAS concentration. This selection was based on the concept that the rise in DHEAS is common among women and Adiol circulating concentrations would be strongly correlated to DHEAS, and a full range of Adiol concentrations would be captured. These four groups were further divided into the upper and lower halves of the E2 concentrations within each Group (n=18). The eight groups permitted the estimate of the adrenal contribution to the estrogen receptor-alpha ligand load (ERLL) and androgenic bioactivity (AR ligand) when adrenal steroids, using DHEAS as a surrogate, were complimenting circulating E2. All samples were frozen (−80°C) upon collection over the previous ten years. They had been thawed once previous to this study.
Figure 1.
Selection of Samples. Box plot display depicting the distribution of serum samples selected for hormone analysis. The selection was based on serum DHEAS concentration chosen from the full SWAN Sample cohort.
Assay Methodology
DHEAS, T and E2 were measured by competitive chemiluminescent immunoassays on the Bayer Diagnostic ACS-180 automated analyzer as reported previously9. The interassay coefficients of variations (CVs) are shown for the various assays in Table 2, and additional details can be found in the supplemental materials (See Appendix, Supplemental Digital Content 1, http://links.lww.com/MENO/A16). Adione and Adiol were analyzed in serum by radioimmunoassay with preceding organic solvent extraction and Celite column chromatography steps10,11. The E2 assay is a semi-automated, competitive immunoassay with manual steps and an off-line incubation. Bioactive androgens were measured by the androgen signal transduction assay, using a human embryonic kidney cell line, stably cotransfected with human androgen receptor (AR), and a luciferase reporter plasmid as previously reported12. We report the following: inter-assay and intra-assay coefficients of variation, 7.4% and 7.5%, respectively.
Table 2.
Assay Precision
| Analyte (unit) | Coefficient of Variation (%) | |
|---|---|---|
| Intraassay | Interassay | |
| DHEAS (ug/mL) | 8.0 (N= 58) | 11.3 (N= 124) |
| Estradiol (pg/mL) | 8.5 (N= 628) | 13.8 (N= 1265) |
| Testosterone (ng/dL) | 4.6 (N= 81) | 11.3 (N= 160) |
| Androstenediol (pg/mL) | 11 (N= 8) | 13 (N= 8) |
| Androstenedione (pg/mL) | 10 (N=8 ) | 8 (N= 8) |
| Bioactive androgens, AR (ng/mL) | 7.5 (N= 36) | 7.4 (N= 9) |
| Bioactive estrogens, ERLL (pg/mL) | 1.8 (N= 15) | 3.3 (N= 10) |
The intraassay and interassay coefficients of variation for the various assays utilized in this study
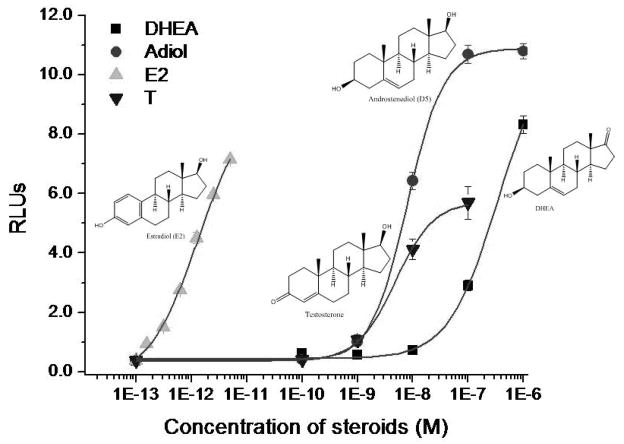
Bioactive estrogens were assessed as estrogen receptor ligand load (ERLL) in a stable signal transduction assay, using human ovarian carcinoma cells (BG1) that have been stably transfected with a luciferase reporter gene plasmid under the regulation of four estrogen-response elements. These transfected BG1 cells were cultured in Alpha Minimum Essential Medium (Alpha-MEM) with 10% FBS. When cells reached 80% confluence, they were trypsinized and well dispersed in phenol-red free DMEM supplemented with 10% DCC-FBS. Equal number of cells at a density of 25,000 cells in 50 ul/well was then added to 96-well tissue culture plates containing 150 uL /well of phenol-red free DMEM supplemented with 10% DCC-FBS. On each of the two subsequent days (Days 2 and 3), the media in each well was removed and replaced with 200 uL of phenol-red free DMEM supplemented with 10% DCC-FBS. On Day 4, media was again removed and replaced with 200 uL phenol red-free DMEM supplemented with 10% DCC-FBS containing increasing concentrations of E2 standards or sample sera. E2 standards were dissolved in absolute alcohol and had a final alcohol content of 0.1% (v/v) to minimize any organic solvent effects. To compensate for any serum matrix effects, DCC-FBS was added to the E2 standard preparation at a proportion equal to that of the serum content of the sample preparation (5% v/v). Serum samples were diluted in DCC-FBS with a final serum content of 5% (v/v). Plates were then incubated for an additional 18 hours. Media was removed, and 100 uL of cell lysis buffer was added to each well and allowed to incubate for 20 minutes. Cell lysates (40 uL) were transferred to 96-well Microfluor II plates (Fisher Scientific, Santa Clara, CA). Luciferin substrate was injected into each well and the luciferase activity induced by the standards and/or test serum was measured by a Veritas Luminometer (Turner Biosystems, Sunnyvale, CA, USA). T, Adiol and E2 (Steraloids, Newport, RI) were measured as relevant dose-responses in the same assay to determine their relative ERLL potency (Figure 2). We report the following: inter-assay and intra-assay coefficients of variation, 3.3% and 1.8%, respectively.
Figure 2.
The dose-response curves and molecular structures for estradiol (E2), androstenediol (ADIOL), dehydroepiandrosterone (DHEA) and testosterone (T) using a stably transfected cell line. Relative biological activity of each steroid was assessed using a cell-base signal transduction assay for estrogen receptor-alpha ligand load (ERLL). The concentration for each steroid is shown on the abscissa (M) and the signal transduction strength is shown on the ordinate in relative light units (RLUs).
Data Analysis
One-way analysis of variance was used to test whether Adione, Adiol, and T means differed by DHEAS groups. Spearman correlations and simple linear regression were used to test the strength and direction of associations of circulating Adione, Adiol and T levels with DHEAS as well as ERLL and AR with Adiol. Hormone values were log transformed prior to analysis. A p value of less than 0.05 was considered to be statistically significant. Data analysis was carried out using StatView Version 5.0.1.0 (1998, SAS Institute, Inc,) and SAS version 8.0.
Results
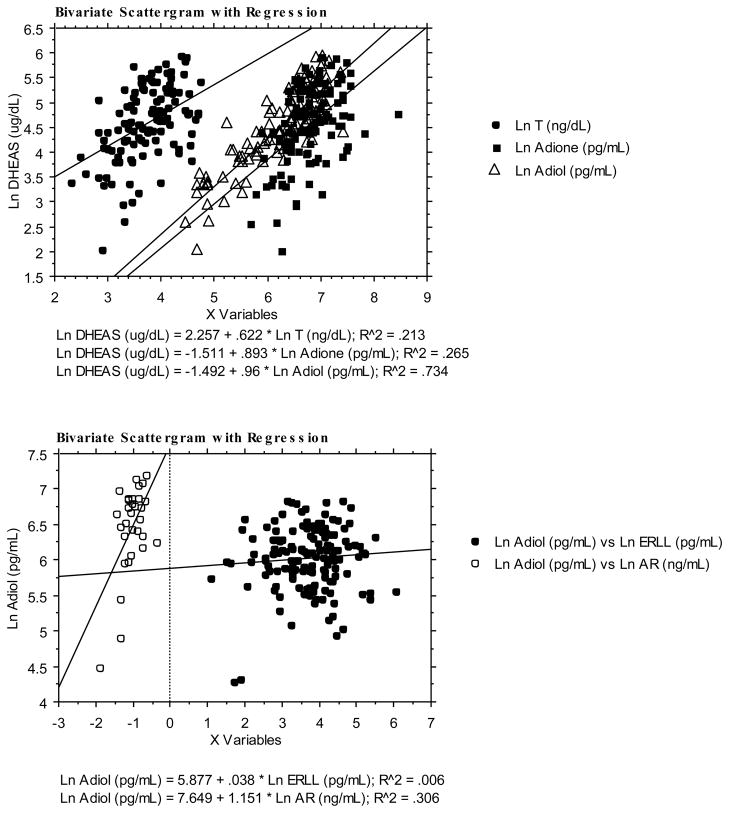
Mean circulating concentrations of all adrenal androgens increase in concert with DHEAS, which has been reported previously3–5. Adiol (from 153 to 1018 pg/mL) and DHEAS (from 35 to 283 ug/dL) increased seven and eight fold, respectively, while T (from 25 to 59 ng/dL) and Adione (from 556 to 1216 pg/ml) increased two fold (Table 3). DHEAS was positively correlated to T, Adione and Adiol (linear regressions in Figure 3 and Table 4). The correlation of DHEAS with Adiol was highest and was a little higher with Adione than with T. Adiol was consistently correlated to androgen bioactivity (linear regressions in Figure 3), but in general it was not correlated to circulating estrogen bioactivity across the entire range of ERLL concentrations.
Table 3.
Range of hormone concentrations
| Analytes (unit) | Group A (DHEAS > x̄ +1SD, N=36) | Group B ( x̄ < DHEAS < x̄ +1SD, N=36) | Group C ( x̄ > DHEAS > x̄ − 1SD, N=36) | Group D (DHEAS < x̄ − 1SD, N=36) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SEM | Mean | SD | SEM | Mean | SD | SEM | Mean | SD | SEM | |
| DHEAS (ug/mL) | 283 | 64 | 10.7 | 170* | 20 | 3.3 | 100* | 22 | 3.7 | 35* | 11 | 1.8 |
| Estradiol (pg/mL) | 71 | 45 | 7.5 | 75 | 49 | 8.2 | 82 | 62 | 10.3 | 105 | 128 | 21.3 |
| Testosterone (ng/dL) | 59 | 21 | 3.5 | 51 | 19 | 3.2 | 53 | 47 | 7.8 | 25* | 11 | 1.8 |
| Androstenediol (pg/mL) | 1018 | 234 | 39.0 | 863 | 241 | 40.2 | 581* | 280 | 46.7 | 153* | 56 | 9.3 |
| Androstenedione (pg/mL) | 1216 | 362 | 60.3 | 989 | 306 | 51.0 | 1022 | 649 | 108.2 | 556* | 203 | 33.8 |
| Bioactive androgens, AR (ng/mL) | 0.39 | 0.18 | 0.0 | 0.39 | 0.08 | 0.0 | 0.38 | 0.11 | 0.0 | 0.22* | 0.06 | 0.0 |
| Bioactive estrogens, ERLL (pg/mL) | 54 | 56 | 9.3 | 54 | 34 | 5.7 | 62 | 55 | 9.2 | 63 | 82 | 13.7 |
p<0.05
The mean value, standard deviation and standard error of the mean of the hormones for each of the four groups of serum samples selected for hormone analysis as described in Figure 1. Groups are significantly different when compared to the higher group (*p<0.05). Highest E2 is not significantly greater than the lowest E2
Figure 3. Linear regression analysis for DHEAS, AR ligands and ER ligands.
Upper Panel: Linear regression analysis of log transforms of circulating concentrations of testosterone (T, closed circles), androstenedione (Adione, closed squares) and androstenediol (Adiol, open triangles) versus the log transform of dehydroepiandrosterone sulfate (DHEAS). Compared to T, both Adione and Adiol are more strongly related to DHEAS.
Lower panel: The log transform of all circulating Adiol concentrations are highly associated with the estimate of circulating androgenicity (open circles) while there is no relationship between the log transform of Adiol and circulating estrogenicity when all Adiol measurements are included.
Table 4.
Rho values for Spearman Rank Correlation coefficients (ρ) and R2 Coefficients of determination for selected inter hormonal relationships
| Hormone | Ln DHEAS (ug/mL) | Ln Androstenediol (pg/mL) | ||
|---|---|---|---|---|
| ρ | R2 | ρ | R2 | |
| Ln Estradiol (pg/mL) | 0.041 | 0.003 | 0.152 | 0.003 |
| Ln Testosterone (ng/dL) | 0.469 | 0.213 | 0.587 | 0.407 |
| Ln Androstenediol (pg/mL) | 0.822 | 0.616 | - | - |
| Ln Androstenedione (pg/mL) | 0.503 | 0.265 | 0.573 | 0.360 |
| Ln Bioactive androgens, AR (ng/mL) | 0.326 | 0.260 | 0.380 | 0.308 |
| Ln Bioactive estrogens, ERLL (pg/mL) | 0.041 | 0.012 | 0.016 | 0.006 |
Circulating T was highly correlated to bioactive androgens throughout its range as previously described12 (data not shown). Prior to menopause, SHBG concentrations declined and showed a modest, positive correlation with E2 (ρ = 0.222) and negative correlation with T (ρ = −0.113), while Adiol (ρ = −0.067) and Adione (ρ = −0.083) both showed weak negative correlations (data not shown).
Circulating Adiol concentrations were significantly correlated with bioactive estrogen concentrations (ERLL) only in the lowest quartile of E2 and the highest quartile of adrenal steroids (ρ =0.38, p=0.02) when compared with the mean of all correlations (ρ =0.016, p=0.846). This correlation was weaker than the correlation between E2 and ERLL, (ρ =0.941, p =<0.0001), the mean of all correlations for all E2 quartiles. The highest concentrations of E2 showed an inhibition by the highest levels of Adiol indicating that when E2 is highest and presenting the greatest physiological impact, higher circulating levels of Adiol may reduce the effectiveness of E2 by competing with E2 for ER binding.
The adrenal contribution to ERLL was evaluated by determining the ratio of the mean ERLL to the mean immunoreactive E2 concentration in each of the eight groups of eighteen samples described above (Table 5). ERLL and AR showed normal distributions within each group, therefore we did not log transform nor use techniques of nonparametric analysis. In general the ratios were 0.49 to 0.76 indicating that the numerator (estimate of bioactive E2 + all other bioactive estrogens) was only slightly and consistently lower than the direct measure of total immunoreactive E2. In contrast, the ratio dropped to 0.64 and 0.49 (p < 0.02) in the group in which DHEAS concentrations were below one standard deviation of the grand mean of DHEAS concentration. Since the portion of E2 in the numerator was still the same as that in the denominator, this drop in the ratio indicates that the biologically active portion of non-E2 estrogenicity was reduced when DHEAS and Adiol concentrations were low (Table 5).
Table 5.
The ratio of ERLL/E2†
| Ratio ERLL/E2 | Higher E2, N=18 (SD) | Lower E2, N=18 (SD) |
|---|---|---|
| DHEAS Group A | 0.75 (0.09) | 0.76 (0.09) |
| DHEAS Group B | 0.74 (0.07) | 0.72 (0.08) |
| DHEAS Group C | 0.76 (0.08) | 0.68 (0.08) |
| DHEAS Group D | 0.64 (0.07) | 0.49* (0.09) |
This ratio provides a dimensionless estimate of the relative contribution of circulating E2 to the total estrogen receptor-alpha ligand load (ERLL) in serum samples segregated by the DHEAS concentrations. The highest concentrations of DHEAS are in Group A and the lowest are in Group D. The ratio for most groups is 0.7 or above indicating a relative constant contribution of non-E2 components in the estimate of ERLL. The ratio drops when DHEAS is low, indicating low Adiol, to 0.64 and 0.49 indicating a drop in non-E2 components. In the DHEAS Group D, lower E2 is significantly different when compared to the higher E2 (*p<0.05).
Discussion
The rise in circulating DHEAS observed in most women during the MT is accompanied by a simultaneous rise in circulating, T, Adione and Adiol levels. This increase of adrenal androgens during the menopausal transition may be important by providing a reservoir of substrate for peripheral conversion into other bioactive steroids. In addition an increase in bioactive androgens from a direct adrenal secretion may be equally, if not more, important than peripheral conversion of weaker ligands to stronger ones.
Adiol has both estrogenic and androgenic activities6,7 and is approximately 1,000 times less bioactive than E2 and T, as indicated by cell-based ERLL and AR12 bioassays, respectively. However, the circulating concentration of T is increasingly greater than that of E2 as menopause approaches, thus the relative contribution of Adiol to estrogenicity will increase while its androgenic potential remains the same or decreases.
The strong overall correlation between circulating immunoreactive E2 and bioactive estrogens (ERLL) in this study confirms that E2 is the predominant circulating bioactive estrogen and contributes the greatest to total estrogenic bioactivity. Similarly T is the predominate androgen12 (Figure 3). These relationships support the concept that the estimates of serum ER and AR signal transduction are reasonable measures of total circulating bioactive sex steroids. The decreasing correlation of E2 to ERLL and increased correlation of Adiol to ERLL, when circulating E2 concentrations decline and Adiol levels rise confirms that the Adiol contribution to circulating estrogenicity increases when the concentration ratio of circulating E2 to Adiol is sufficiently low.
While the estrogenic bioactivity of Adiol is low compared to E2 - estimated to be 0.1% cross-bioactivity in vitro (Figure 2) - its greater than 100-fold higher circulating concentration during the MT (10 to 1015 pg/mL) and lower binding to SHBG compared to E2 or T13 enhances its biological importance. Circulating Adiol concentrations can reach 3,800 pM (1.1 ng/mL) in some women at or near menopause (Table 3) when E2 concentrations are estimated to fall to 6–10 pM (<5 to 10 pg/mL)2. This shift in the ratio from approximately 1:10 (E2:Adiol ) prior to the menopausal transition to 1:>100 (E2 to Adiol) at and following menopause may result in a functional shift in the effective estrogenic potential of E2 and Adiol. Such a biological effect has been suggested at the tissue level as Adiol has been shown to induce endometrial proliferation in anovulatory patients when circulating E2 levels are low14.
A wider range in circulating Adiol provides a more variable source of potential estrogenic support than E2, particularly when circulating E2 levels are low (Table 3). Since the circulating concentration of adrenal-derived Adiol is relatively constant compared to the day-to-day variations in ovarian-derived E2, the Adiol effect will depend largely on the occurrence of low E2 concentrations when Adiol levels are high. When circulating E2 concentrations at and following menopause are reduced dramatically2, then many women would likely exhibit symptoms of E2 deficiency if there are no other sources of estrogenic support. Since some women do not exhibit such symptoms, it follows then that another source of estrogenicity likely exists.
The lower increase in Adione and T compared to Adiol suggests that there is a modest conversion of endogenous adrenal androgens to estrogens (or other metabolites) compared to the formation of Adiol during the MT (Table 3). This observation also suggests one of two potential mechanisms for the greater increase in Adiol compared to T and Adione. One possibility is that a relatively low activity of peripheral 3β-hydroxysteroid dehydrogenase prevents the Δ5 to Δ4 conversion of non-aromatizable to aromatizable androgens, and/or lower activity of peripheral 17β-hydroxysteroid dehydrogenase that converts Adiol back to DHEA. Alternatively, the observed difference in adrenal secretory activity represented by a preferential increase in the adrenal C-19 Δ5 steroid pathway may arise from increased P450 17c activity, thus increasing DHEAS and Adiol synthesis and secretion. Since some women do not manifest an increase in circulating adrenal steroids, particularly Adiol, this may partially explain some of the differences in symptoms and phenotypes that have been previously attributed to an E2 to T balance15. Similarly, the failure of DHEA supplementation to have significant health benefits supports the concept that the marked benefits attributed to higher endogenous circulating DHEA levels16, 17 are actually the effect of the increased Adiol that accompanies higher circulating DHEAS.
The present data also suggests a relatively constant adrenal contribution to circulating estrogenicity. Because both the immunoassay and ER signal transduction assay are relatively specific measures of E2 and based on the same standard, the ratio of ERLL divided by total circulating (measured) E2 provides an estimate of the portion of biologically active ER ligands which is a direct contribution of E2. If all of the circulating E2 was bioactive and no other estrogens were in the system then this ratio would be unity. The consistent finding of ratios between 0.75 and 0.76 when DHEAS concentrations are higher than one standard deviation above the DHEAS mean indicates that there is a relatively constant portion of non-E2 circulating estrogens that are contributing to total bioactive circulating ER ligands. However, this ratio drops to 0.64 and 0.49 when samples from the lowest group of DHEAS containing samples are evaluated, indicating that the non-E2 contribution to the ERLL measure of bioactive estrogens has fallen (Table 5). The decline is most probably due to the fall in circulating T and Adione which are aromatized directly to E2 and estrone, respectively, in peripheral tissues.
This report has both weaknesses and strengths. First, as a descriptive study, it is largely observational and designed to address the single hypothesis that the rise in DHEAS may signal a rise in other adrenal steroids. However, the positive findings in this study are hypothesis-generating and promote several new and provocative concepts. Clearly future studies will be needed to determine the relationship between changes in adrenal steroid hormone levels and the occurrence and severity of symptoms. This was simply beyond the scope of this investigation. Second this study employed relatively novel bioassays as experimental tools to provide additional insights into the endocrinology of the MT. While these bioassays have not yet been validated in terms of providing clinically relevant information they have been validated as useful laboratory research tools as demonstrated here and elsewhere12. As such, they can currently be used to provide support for new concepts that are biologically plausible and therefore potentially clinically relevant. Currently these assays are labor intensive, have not been broadly accepted as standardized methods and remain largely research tools. The new data provided by these methods, however, can lead to additional and more thorough investigations in the future. Finally, the current study suggests but does not demonstrate that the changes in circulating adrenal steroids are a consequence of adrenal steroid hormone production rates. Ruling out changes in metabolism or clearance rate was beyond the scope of this study, however, there is no evidence to suggest that such changes occur during the MT and could have lead to the range of circulating concentrations described here.
Conclusion
These data provide a novel, potential explanation for the wide between-woman differences in phenotypes that are expressed during the MT by considering the contribution of circulating bioactive adrenal steroid hormone levels. This report shows that circulating Adiol, a weak bioactive estrogen and androgen, rises in concert with the rise in DHEAS during the MT, to reach biologically effective concentrations. In addition, the current data provide a plausible, alternative explanation for the strong association between higher endogenous DHEAS and superior administrative function18 and the lack of efficacy of DHEA interventions.19 Circulating Adiol rises to a range of concentrations that are more variable compared to the relatively consistent decline in circulating E2. Future studies are essential to determine the degree that the observed differences in Adiol concentrations correspond to and are associated with differences in incidence and severity of symptoms and the trajectories of health outcomes. Indirect evidence support the concept of an Adiol estrogenic role. A clinical effect of an adrenal contribution to circulating estrogenicity is suggested by the observation of a decline in non-E2 components when Adiol levels are low. Furthermore, the positive correlation of higher levels of circulating Adiol to ER signal transduction suggest the possibility that other estrogenic compounds could be involved was not explored. This shift is most evident when circulating E2 concentrations are low. Together these observations indicate that direct adrenal secretion of bioactive steroids and/or peripheral conversion of prohormones provide additional estrogenic support during and immediately following the MT.
Supplementary Material
Acknowledgments
Funding:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Rachel Wildman, PI 2010 - present; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Project Officer
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Watertown, MA -Sonja McKinlay, PI 1995 – 2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
The author have no conflicts of interest to declare.
References
- 1.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–22. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 2.Labrie FMD, Martel C, Balser J. 2010 Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: role of the ovary? Menopause. 2011;18(1):30–43. doi: 10.1097/gme.0b013e3181e195a6. [DOI] [PubMed] [Google Scholar]
- 3.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87(8):3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 4.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley BL. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94(8):2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vug M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause. 2011;18(5):494–498. doi: 10.1097/gme.0b013e3181fb53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JB. Adrenal androgens and human breast cancer: a new appraisal. Breast Cancer Research & Treatment. 1998;51(2):183–8. doi: 10.1023/a:1006050720900. [DOI] [PubMed] [Google Scholar]
- 7.Adams JB, Martyn P, Lee FT, Phillips NS, Smith DL. Metabolism of 17 beta-estradiol and the adrenal-derived estrogen 5-androstene-3 beta,17 betAdiol (hermaphrodiol) in human mammary cell lines. Annals of the New York Academy of Sciences. 1990;595:93–105. doi: 10.1111/j.1749-6632.1990.tb34285.x. [DOI] [PubMed] [Google Scholar]
- 8.Sowers M, Crawford S, Morgenstein D. Design and sampling methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Wren J, Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; 1999. [Google Scholar]
- 9.England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48(9):1584–1586. [PubMed] [Google Scholar]
- 10.Goebelsmann U, Horton R, Mestman JH, Arce JJ, Nagata Y, Nakamura RM, Thorneycroft IH, Mishell DR., Jr Male pseudohermaphroditism due to testicular 17-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1973;36:867. doi: 10.1210/jcem-36-5-867. [DOI] [PubMed] [Google Scholar]
- 11.Dorgan JF, Stanczyk FZ, Longcope C, Stephenson HE, Jr, Chang L, Miller R, Franz C, Falk RT, Kahle L. Relationship of serum dehydroepiandrosterone (DHEA), DHEA sulfate, and 5-androstene-3b,17b-diol to risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1997;6:177–181. [PubMed] [Google Scholar]
- 12.Chen J, Thirkill TL, Lohstroh PN, et al. Bromodichloromethane inhibits human placental trophoblast differentiation. Toxicological Sciences. 2004;78(1):166–74. doi: 10.1093/toxsci/kfh046. [DOI] [PubMed] [Google Scholar]
- 13.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 14.Plaza F, Gabler F, Romero C, Vantman D, Valladares L, Vega M. The conversion of dehydroepiandrosterone into androst-5-ene-3beta,17beta-diol (androstenediol) is increased in endometria from untreated women with polycystic ovarian syndrome. Steroids. 2010;75(12):810–7. doi: 10.1016/j.steroids.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poortman J. Proceedings: Interpretation of data on production and secretion rate measurements of DHEA and DHEAS. Horm Res. 1975;6 (5–6):290–1. [PubMed] [Google Scholar]
- 17.James K, Premchand N, Skibinska A, Skibinski G, Nicol M, Mason JI. IL-6, DHEA and the ageing process. Mech Ageing Dev. 1997;93(1–3):15–24. doi: 10.1016/s0047-6374(96)01807-6. [DOI] [PubMed] [Google Scholar]
- 18.Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 19.Davis SRM, Panjari M, Stanczyk FZ. DHEA Replacement for Postmenopausal Women. The Journal of clinical endocrinology and metabolism. 2011;96(6):1642–1653. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.