Abstract
Background and Purpose
Electromagnetic brain stimulation might have value to reduce motor deficits after stroke. Safety and behavioral effects of higher frequencies of repetitive transcranial magnetic stimulation (rTMS) require detailed assessment.
Methods
Using an active treatment-only, unblinded, 2-center study design, patients with chronic stroke received 20 minutes of 20 Hz rTMS to the ipsilesional primary motor cortex hand area. Patients were assessed before, during the hour after, and 1 week after rTMS.
Results
The 12 patients were 4.7±4.9 years poststroke (mean±SD) with moderate–severe arm motor deficits. In terms of safety, rTMS was well tolerated and did not cause new symptoms; systolic blood pressure increased from pre- to immediately post-rTMS by 7 mm Hg (P=0.043); and none of the behavioral measures showed a decrement. In terms of behavioral effects, modest improvements were seen, for example, in grip strength, range of motion, and pegboard performance, up to 1 week after rTMS. The strongest predictor of these motor gains was lower patient age.
Conclusions
A single session of high-frequency rTMS to the motor cortex was safe. These results require verification with addition of a placebo group and thus blinded assessments across a wide spectrum of poststroke deficits and with larger doses of 20 Hz rTMS.
Keywords: plasticity, recovery, stroke, transcranial magnetic stimulation, treatment
Motor deficits are a major contributor to disability after stroke. A number of forms of brain stimulation are under study for improving these deficits. Detailed assessment of these interventions, particularly safety, remains incomplete. The current study examined high-frequency repetitive transcranial magnetic stimulation (rTMS) with an emphasis on safety assessments. Prior studies of high-frequency rTMS to date suggest good safety in healthy subjects1,2 and in patients with stroke.3–5 A secondary aim was to characterize behavioral changes, including “offline” effects, ie, those that endure after the end of stimulation. Favorable neurological effects have been reported after high-frequency rTMS in patients with stroke.3,4 However, a detailed description of the safety and neurological effects of a single 20-Hz rTMS session has not been previously described.
The current study used an active treatment-only, unblinded, 2-center study design to address the hypothesis that in patients with chronic stroke, a single session of 20 Hz rTMS applied to the ipsilesional hand motor area is safe and has favorable “offline” behavioral effects. As an additional secondary aim, predictors of tTMS behavioral effects were evaluated.
Methods
Subjects
Consenting patients were aged 18 to 85 years; stroke that is supratentorial, unilateral, ischemic, or hemorrhagic but not subarachnoid and does not come within 15 mm of the rTMS target; arm motor Fugl-Meyer score = 15 to 55 out of 66; and stroke >11 weeks prior. Exclusion criteria were prestroke Rankin score >1; history of seizure; other focal cortical pathology; Zung Depression score ≥50; decreased alertness, language reception, or attention; pregnant/lactating; advanced systemic disease; terminal illness; coexistent neurological/psychiatric disease; prior TMS; and TMS/MRI contraindication. Local human subjects committees and the US Food and Drug Administration approved the study.
Study Structure
A standard approach was achieved through a detailed manual of operating procedures and regular videoconferences. At Visit 1, history/physical was followed by scoring on 7 behavioral outcome measures and then anatomic/functional MRI scanning. Visit 2, 1 day later, repeated Fugl-Meyer, grip strength, 9-hole peg test, and 2 active ranges of motion; vital signs were taken; rTMS was applied; and a rigidly timed schedule of testing was performed during the postrTMS hour. Patients returned for Visit 3 at 7 days after rTMS.
Safety Outcome Measures
Patients were asked about any new symptoms at Visit 3 and the end of Visit 2; change in vital signs; and decrements in any of the 7 behavioral outcome measures.
Behavioral Outcome Measures
Behavioral outcome measures consisted of the Barthel Index, Fugl-Meyer, Action Research Arm Test, hand grip strength, 9-hole peg test, and active ranges of motion at the affected side wrist and index finger metacarpophalangeal joint.
MRI Acquisition and Analysis
Using a Philips 3-T scanner, a T1-weighted whole-brain anatomic image was followed by 2 functional MRI (fMRI) runs, each 96 seconds long contrasting 24 seconds rest with 24 seconds squeezing (25 axials slices with 4-mm thickness/1-mm gap, TR=2000 ms, TE=30 ms). Squeezing was isometric with the affected hand closing on a smooth, inflexible wooden object whose dimensions approximate those of a Jamar dynamometer. An investigator observed patient movements during scanning.
Using SPM2, fMRI images were realigned, normalized to MNI space, and then spatially smoothed (full width at half maximum =8 mm). Images at rest were contrasted with images during task performance with the 2 fMRI series for each task combined when neither was contaminated by excess head motion. Analysis of the primary sensorimotor cortex (“hand area” from http://hendrix.IMdtu.dk/services/jerne/ninf/voi.html) yielded activation volume (P<0.001, uncorrected) and task-related fMRI signal change and a laterality index.6
Application of Repetitive Transcranial Magnetic Stimulation
Each patient’s head was coregistered with his or her MRI using a frameless stereotaxic system. The rTMS target was the posterior precentral gyrus at the hand knob.7,8 Single-pulse TMS (figure-of-8 coil; Magstim 200) of the ipsilesional hemisphere identified resting motor threshold that produced a motor-evoked potential ≥50 µV in the stroke-affected first dorsal interosseus in ≥3/5 stimuli. The patient sat relaxed while 40 rTMS trains of 40 pulses at 20 Hz, separated by an intertrain interval of 28 seconds, were delivered for a total of 1600 pulses using the Magstim Rapid. Stimulation intensity was 90% motor threshold; for the 7 patients with no elicitable motor-evoked potential, default stimulation intensity was 60% device output.
Predicting Behavioral Effects
The ability of 14 variables recorded before rTMS to predict behavioral effect was examined. These measures were demographic (age and time poststroke), behavioral (Zung Depression score, National Institutes of Health Stroke Scale score, Barthel Index, Fugl-Meyer, and grip strength), neurophysiological (motor-evoked potential threshold, using 100% for patients with no elicitable motor-evoked potential), and fMRI (activation volume in contra- and ipsilesional hand sensorimotor area and their laterality index plus task-related fMRI signal change in the same 2 areas and their laterality index).
Data Analysis
Two-tailed parametric statistics were used (JMP; SAS, Cary, NC). Changes over time were evaluated by paired t tests. This was an exploratory study with no corrections made for multiple comparisons.
Results
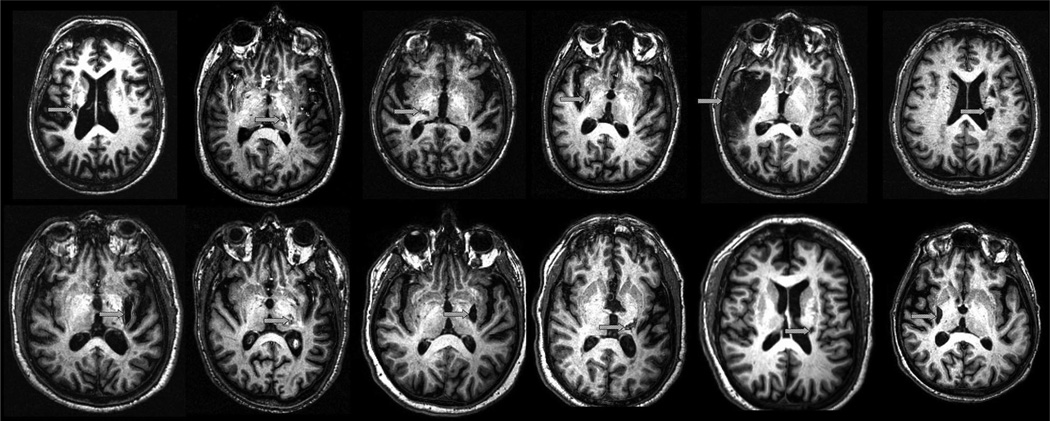
Patient characteristics are described in Table 1. All had received a course of standard rehabilitation therapy during the subacute stroke period. A motor-evoked potential could be evoked in 5 of 12 patients, among whom motor threshold was 76±13% of maximum device output. Stroke topography is presented in Figure 1.
Table 1.
Patient Characteristics
| n | 12 |
| Age, years | 67 ± 12 |
| Time poststroke, years | 4.7 ± 4.5 |
| Gender | 10 M/2 F |
| Brain side affected by stroke | 7 L/5 R |
| Baseline National Institutes of Health Stroke Scale score |
4 ± 2 |
| Zung depression score (lower is better, depression present with score ≥50) |
35 ± 10 |
| Modified Nottingham sensory score (normal=12, higher is better) |
11 ± 2 |
| Handedness | 11 R/1 L |
| Site of patient enrollment | 8 at Irvine/4 at Boston |
| Hypertension present | 10 |
| Hyperlipidemia present | 10 |
| Diabetes mellitus present | 4 |
Results are mean±SD.
M indicates male; F, female; L, left; R, right.
Figure 1.
On a representative slice from the T1-weighted anatomic image from each of the 12 study patients, an arrow indicates stroke location. Stroke was subcortical in 11 (although directly abutting the cortex in 5 of 11) and cortical in one.
Safety Outcome Measures
There were no witnessed adverse events during rTMS, and patients reported no new symptoms through Visit 3. Systolic blood pressure, assessed in the first minute after rTMS, increased 7 mm Hg as compared with immediately prerTMS (P<0.05, Table 2), a change accounted for by 9 of 12 patients, but no such change was apparent for diastolic blood pressure or pulse. None of the behavioral measures showed a decrement.
Table 2.
Within-Subject Changes Before versus After rTMS
| Measure Evaluated | Pre-rTMS | Immediately Post-rTMS |
1-Week Follow-Up |
|---|---|---|---|
| Barthel Index | 85 ± 14 | 85 ± 14 | |
| Active range of motion, affected wrist extensor, degrees |
63 ± 45 | 71 ± 45† | 69 ± 46 |
| Active range of motion, affected index finger metacarpophalangeal joint, degrees |
38 ± 41 | 43 ± 43* | 39 ± 38 |
| Arm Fugl-Meyer motor score | 34.5 ± 15 | 35.6 ± 15 | 36.0 ± 15* |
| Pegs placed by affected hand | 1 ± 1.9 | 1.8 ± 2.8* | 2.2 ± 3.3† |
| Grip strength, affected hand | 25 ± 18 | 31 ± 18* | 29 ± 21 |
| ARAT score | 19 ± 18 | 21 ± 18 | 21 ± 18 |
| Systolic blood pressure | 128 ± 11 | 135 ± 12* | |
| Diastolic blood pressure | 77 ± 10 | 80 ± 10 | |
| Pulse | 66 ± 9 | 66 ± 8 |
Values are mean±SD. P values reflect paired testing (*P<0.05, †P<0.06, comparison with pre-rTMS values). For data in the immediately post-rTMS column, the time that measurement started after rTMS was rigidly controlled and was 7 minutes for active range of motion, 30 minutes for Fugl-Meyer score, 60 minutes for no. of pegs placed by the affected hand, 60 minutes for grip strength (pounds) by the affected hand, 10 minutes for ARAT score, and 1 minute for the 3 vital signs. All pre-rTMS measures were recorded on the day of rTMS, immediately before brain stimulation, except for the Barthel Index, which was assessed at the baseline examination.
ARAT indicates Action Research Arm Test.
Behavioral Outcome Measures
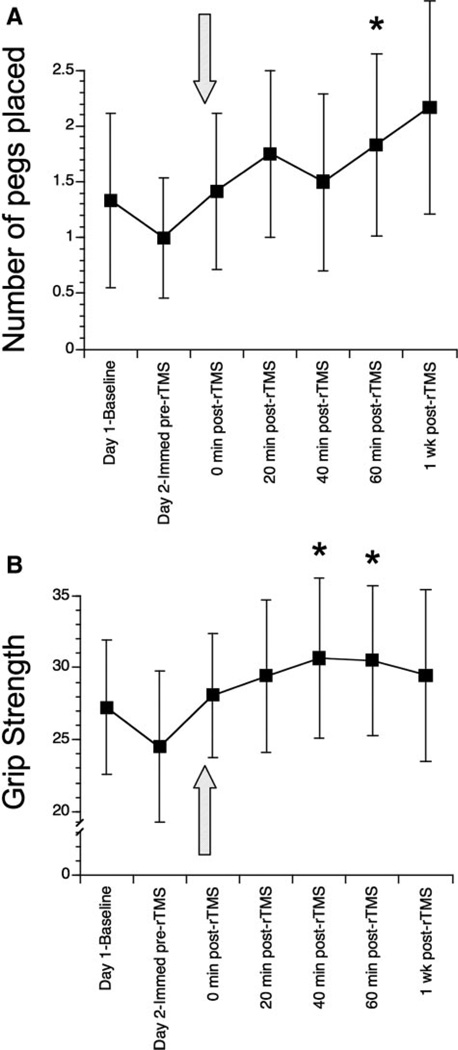
None of the 5 measures changed across the 2 baseline examinations. A significant within-subject change after rTMS was found for 5 of 7 measures (Table 2; Figure 2). Fugl-Meyer increased 30 minutes after rTMS (P=0.071), which reached significance (P<0.02) 1 week later, the latter accounted for by an increase in 8 of 12 patients. Affected hand pegboard testing (Figure 2A) increased from pre-rTMS to 60 minutes post-rTMS (P<0.05), accounted for by 5 of 12 patients; a trend (P=0.057) at Day 7 suggested retention. Grip strength (Figure 2B) at 40 and 60 minutes post-rTMS increased (P<0.03 each) versus pre-rTMS, accounted for by 8 to 9 patients. The active ranges of motion, assessed 7 minutes after rTMS, increased 7° (P=0.051) in the affected wrist extensor and 5° (P<0.02) in the affected index finger metacarpophalangeal joint, each accounted for by 7 of 12 patients. Barthel Index and Action Research Arm Test did not change over time.
Figure 2.
A, Number of pegs placed in the 9-hole pegboard over 60 seconds by the affected hand. B, Grip strength is the maximum force of squeezing by the affected hand on a Jamar dynamometer in pounds. For both A and B, the pre-rTMS baseline was stable, showing no significant change over time. The arrow indicates timing of rTMS application. Values are mean±SEM *P<0.05 versus immediately pre-rTMS, paired testing.
Of the 10 patients with usable fMRI data, 2 showed mirror movements in the unaffected hand and 5 showed adventitial foot movements. Mean activation volumes were 1916±972 mm3 and 982±836 mm3 within the ipsi- and contralesional hand sensorimotor cortex, respectively, with laterality index averaging 0.33±0.51. Respective values for task-related fMRI signal change were 0.84±0.57% and 0.66±0.51%, laterality index averaging 0.13±0.54. These values were fed into the predictive models.
Predicting Behavioral Effects
The dependent measure was an increase in grip strength 1 hour after rTMS, present in 9 patients. Of the 14 variables examined, one had significant (P<0.05) predictive value: age (r=−0.77, P<0.004).
Discussion
In patients with chronic stroke, a single 20-minute session of 20 Hz rTMS applied to the ipsilesional hand motor area was safe, although with a mild systolic blood pressure increase.
Results are overall consistent with prior studies of rTMS at 3 to 20 Hz in patients with stroke.3–5 Khedr et al found that 10 sessions of 3 Hz rTMS to the motor cortex improved disability and overall neurological status to a greater extent than sham rTMS did in patients with subacute stroke.3 Kim et al found that a single session of 10 Hz rTMS to the motor cortex improved motor learning more than sham rTMS did in patients with chronic stroke.4
The current focus was 20 Hz because some evidence suggests that motor cortex facilitation increases in parallel with the hertz at which rTMS is applied, possibly on the basis of increases in cortical excitability and metabolism,1,9,10 the latter linked with potential for providing greater behavioral gains. However, higher rTMS frequencies might also carry greater risk for adverse events such as seizure,1 although no serious adverse events were found here.
Although the focus of this study was safety, motor assessments found favorable changes in arm motor function that persisted at least 1 hour, and in some cases 1 week, after rTMS completion. These motor gains showed a significant and negative relationship with age. This is consistent with the negative association that increased age has in studies of the natural history of stroke recovery.11,12
A strength of the current study was effective implementation of a protocol that required multiparameter MRI, single-pulse TMS, rTMS, and behavioral assessments in patients with stroke at 2 sites that span a continent. Weaknesses of the current study include absence of a control intervention and thus blinded outcomes assessment and the possibility of Type I error. Brief electromyographic bursts, a potential harbinger of seizure induction,13 were not measured in the current study. Anatomic (current study) versus physiological3–5,13 methods of defining the rTMS target might be compared in a future study. Finally, the interaction between rTMS and concomitant secondary therapies such as occupational therapy or pharmacological intervention also warrants further study, especially in a maximally diverse stroke population. The current results suggest safety and support further studies of 20 Hz rTMS in patients with stroke.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Center for Research Resources, National Institutes of Health M01 RR000827-29 from the University of California Irvine General Clinical Research Centers Program, and MO1 RR01032 from the Harvard–Thorndike General Clinical Research Center at Beth Israel Deaconess Medical Center, R01 EB005047 and K24 RR018875. M.A.-A. was funded by Fundacion para la Investigacion y Desarrollo del Complexo Hospitalario Universitario de Vigo (FICHUVI) and the Clinical Investigator Training Program.
Footnotes
Disclosures
None.
References
- 1.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 2.Hallett M, Wassermann EM, Pascual-Leone A, Valls-Sole J. Repetitive transcranial magnetic stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:105–113. [PubMed] [Google Scholar]
- 3.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, Yoo WK, Hallett M. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 5.Malcolm MP, Triggs WJ, Light KE, Gonzalez Rothi LJ, Wu S, Reid K, Nadeau SE. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86:707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer S, Nelles G, Benson R, Kaplan J, Parker R, Kwong K, Kennedy D, Finklestein S, Rosen B. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 7.Cramer S, Benson R, Burra V, Himes D, Crafton K, Janowsky J, Brown J, Lutsep H. Mapping individual brains to guide restorative therapy after stroke: rationale and pilot studies. Neurol Res. 2003;25:811–814. doi: 10.1179/016164103771953899. [DOI] [PubMed] [Google Scholar]
- 8.Yousry T, Schmid U, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 9.Gangitano M, Valero-Cabre A, Tormos J, Mottaghy F, Romero J, Pascual-Leone A. Modulation of input–output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2002;113:1249–1257. doi: 10.1016/s1388-2457(02)00109-8. [DOI] [PubMed] [Google Scholar]
- 10.Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on (14)c-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- 11.Kennard M. Age and other factors in motor recovery from precentral lesions in monkeys. Am J Physiol. 1936;115:138–146. [Google Scholar]
- 12.Jongbloed L. Prediction of function after stroke: a critical review. Stroke. 1986;17:765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- 13.Lomarev MP, Kim DY, Richardson SP, Voller B, Hallett M. Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clin Neurophysiol. 2007;118:2072–2075. doi: 10.1016/j.clinph.2007.06.016. [DOI] [PubMed] [Google Scholar]