Abstract
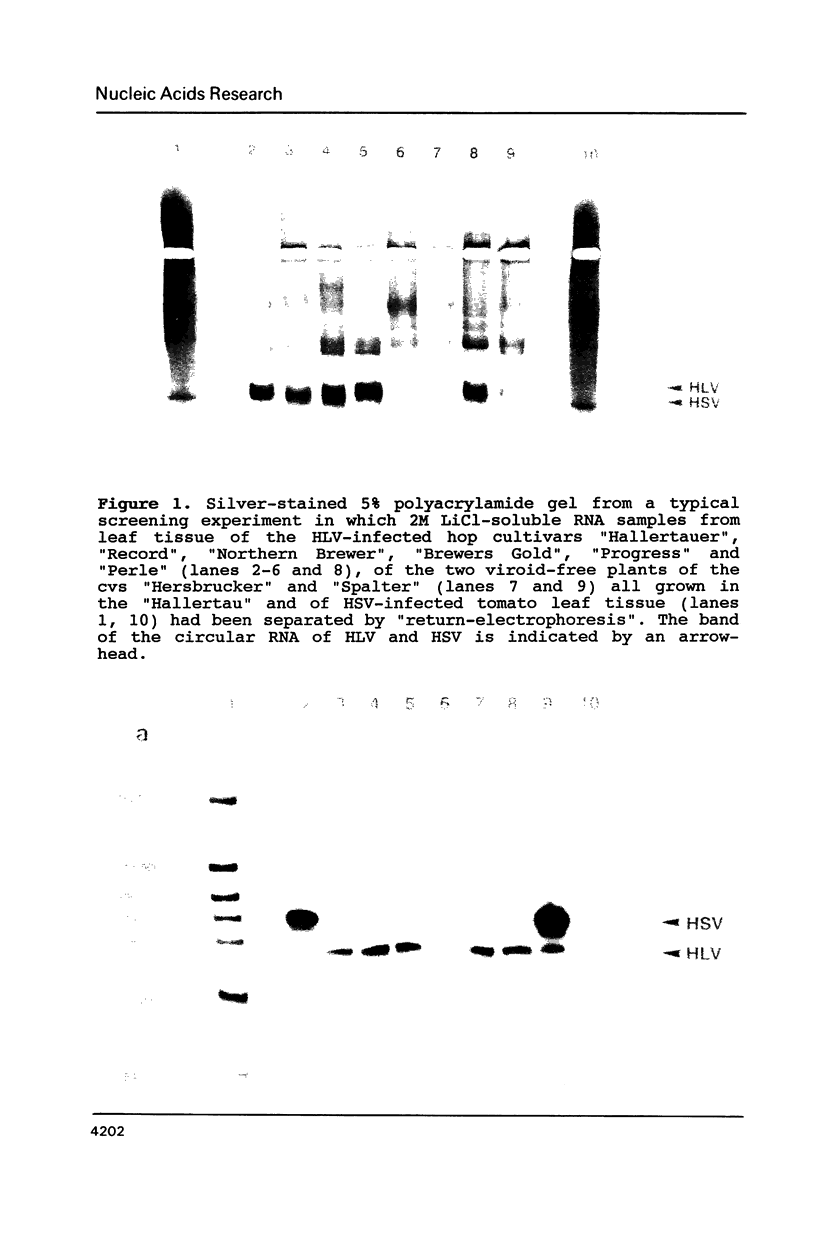
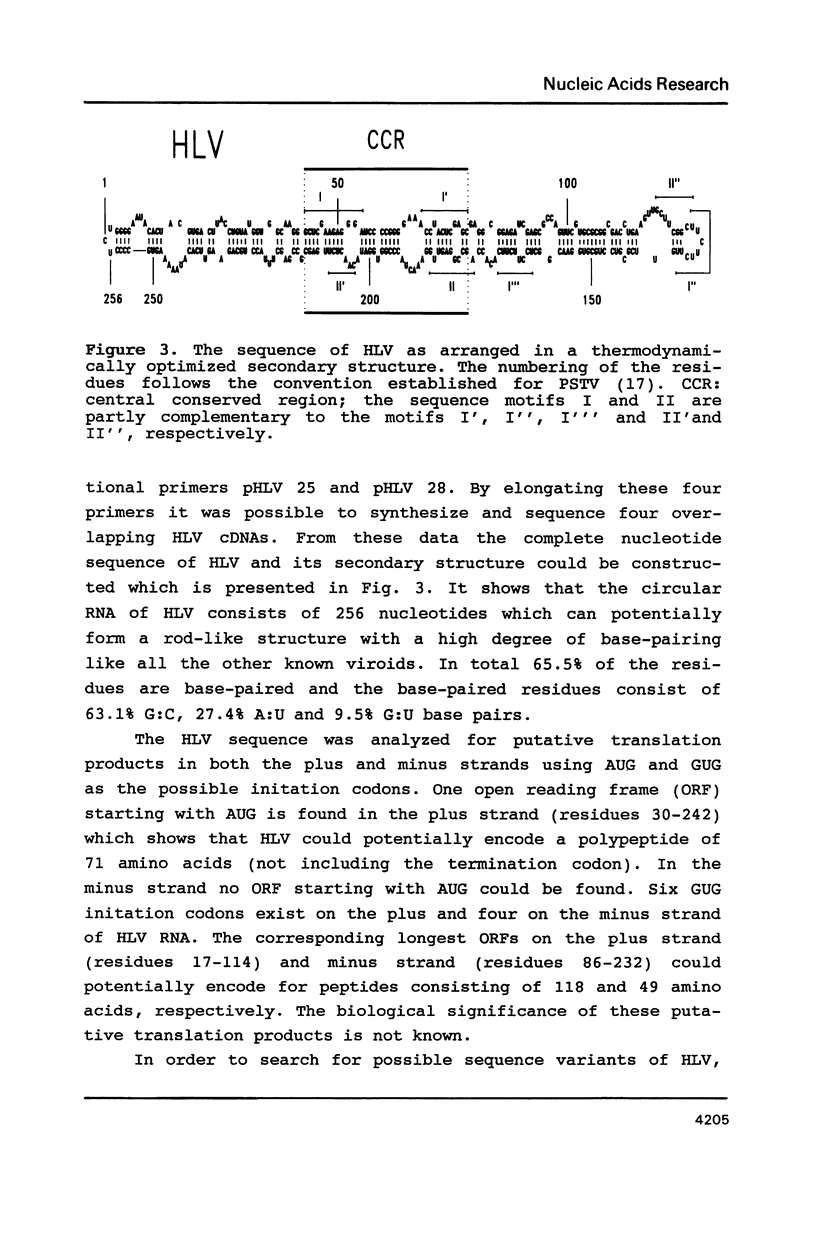
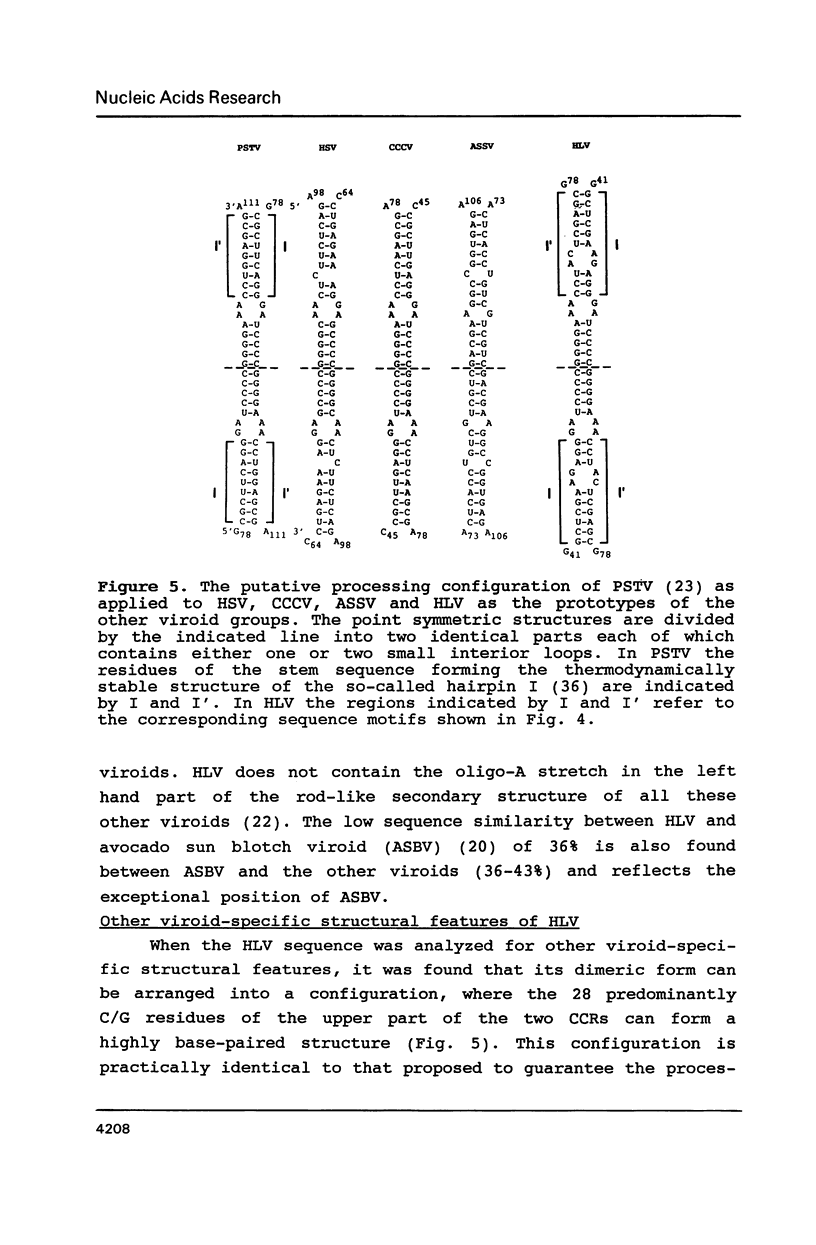
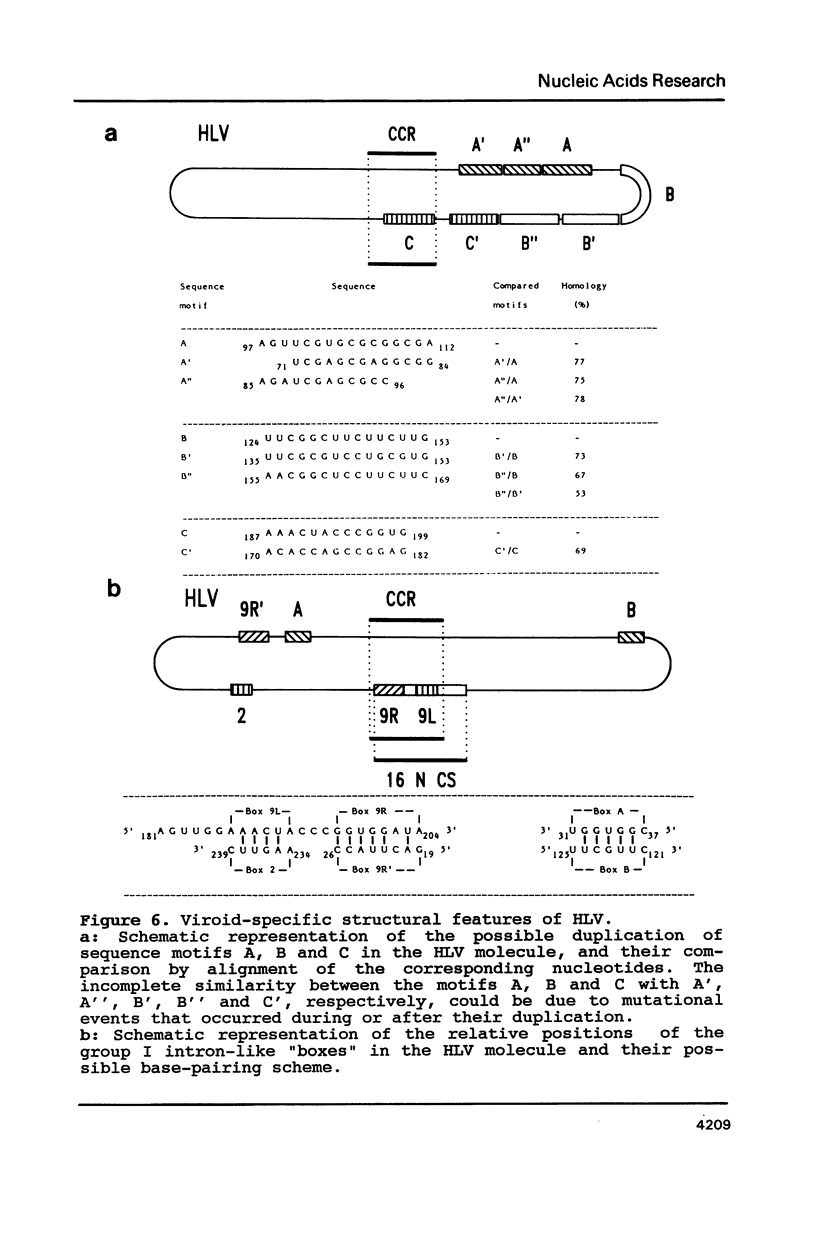
A new viroid which does not seem to produce any symptoms of disease, and is therefore tentatively named hop latent viroid (HLV) was found to occur worldwide in hops. HLV proved to be infectious when mechanically inoculated onto viroid- and virus-free hops. The viroid nature of HLV was also substantiated by sequence analysis which revealed that HLV is a circular RNA consisting of 256 nucleotides, that can be arranged into the viroid-specific, rod-like secondary structure. HLV also contains the central conserved region typical for most of the presently known viroids. However HLV does not contain the viroid-specific oligo(A) stretch in the upper left part of its rod-like molecule. Because of this feature and a sequence similarity with the prototypes of the other viroid groups below 55%, HLV can be regarded as the first member of a new viroid group.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colpan M., Schumacher J., Brüggemann W., Sänger H. L., Riesner D. Large-scale purification of viroid RNA using Cs2SO4 gradient centrifugation and high-performance liquid chromatography. Anal Biochem. 1983 May;131(1):257–265. doi: 10.1016/0003-2697(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Dinter-Gottlieb G. Viroids and virusoids are related to group I introns. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6250–6254. doi: 10.1073/pnas.83.17.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto J., Koganezawa H. Nucleotide sequence and secondary structure of apple scar skin viroid. Nucleic Acids Res. 1987 Sep 11;15(17):7045–7052. doi: 10.1093/nar/15.17.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Good L., Simonetti J., Zuker M. Some simple computational methods to improve the folding of large RNAs. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):45–52. doi: 10.1093/nar/12.1part1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese P., Osorio-Keese M. E., Symons R. H. Coconut tinangaja viroid: sequence homology with coconut cadang-cadang viroid and other potato spindle tuber viroid related RNAs. Virology. 1988 Feb;162(2):508–510. doi: 10.1016/0042-6822(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Owens R. A., Diener T. O. Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6234–6238. doi: 10.1073/pnas.80.20.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A. M., Rezaian M. A. Grapevine yellow speckle viroid: structural features of a new viroid group. Nucleic Acids Res. 1988 Feb 11;16(3):849–864. doi: 10.1093/nar/16.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y. Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983 Sep 24;11(18):6185–6197. doi: 10.1093/nar/11.18.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic Acid hybridization. Science. 1981 Aug 7;213(4508):670–672. doi: 10.1126/science.213.4508.670. [DOI] [PubMed] [Google Scholar]
- Puchta H., Ramm K., Sänger H. L. Nucleotide sequence of a hop stunt viroid isolate from the German grapevine cultivar 'Riesling'. Nucleic Acids Res. 1988 Mar 25;16(6):2730–2730. doi: 10.1093/nar/16.6.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Tabler M., Sänger H. L. Synthesis of (+) and (-) RNA molecules of potato spindle tuber viroid (PSTV) in isolated nuclei and its impairment by transcription inhibitors. Biosci Rep. 1985 Mar;5(3):251–265. doi: 10.1007/BF01119595. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Infectivity studies on different potato spindle tuber viroid (PSTV) RNAs synthesized in vitro with the SP6 transcription system. EMBO J. 1985 Sep;4(9):2191–2199. doi: 10.1002/j.1460-2075.1985.tb03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





