Abstract
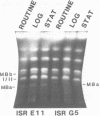
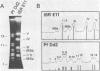
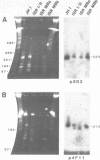
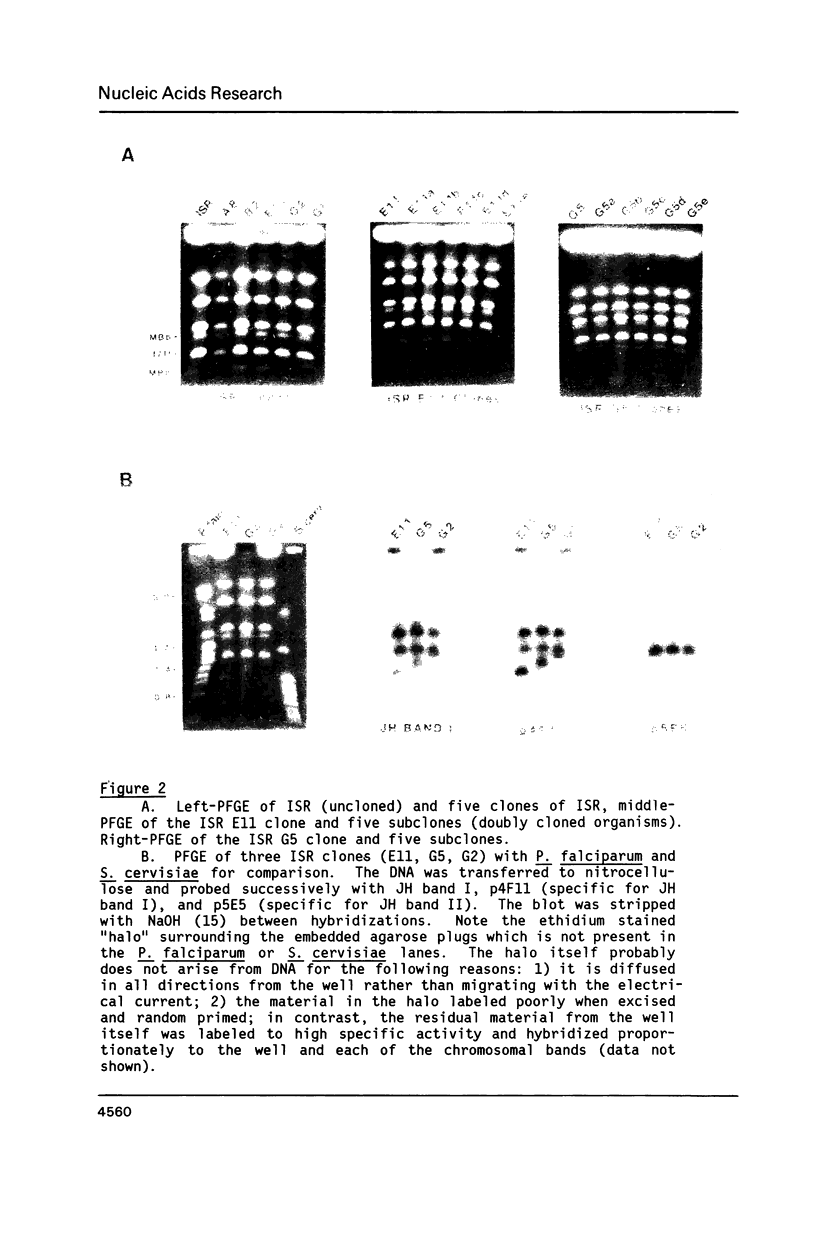
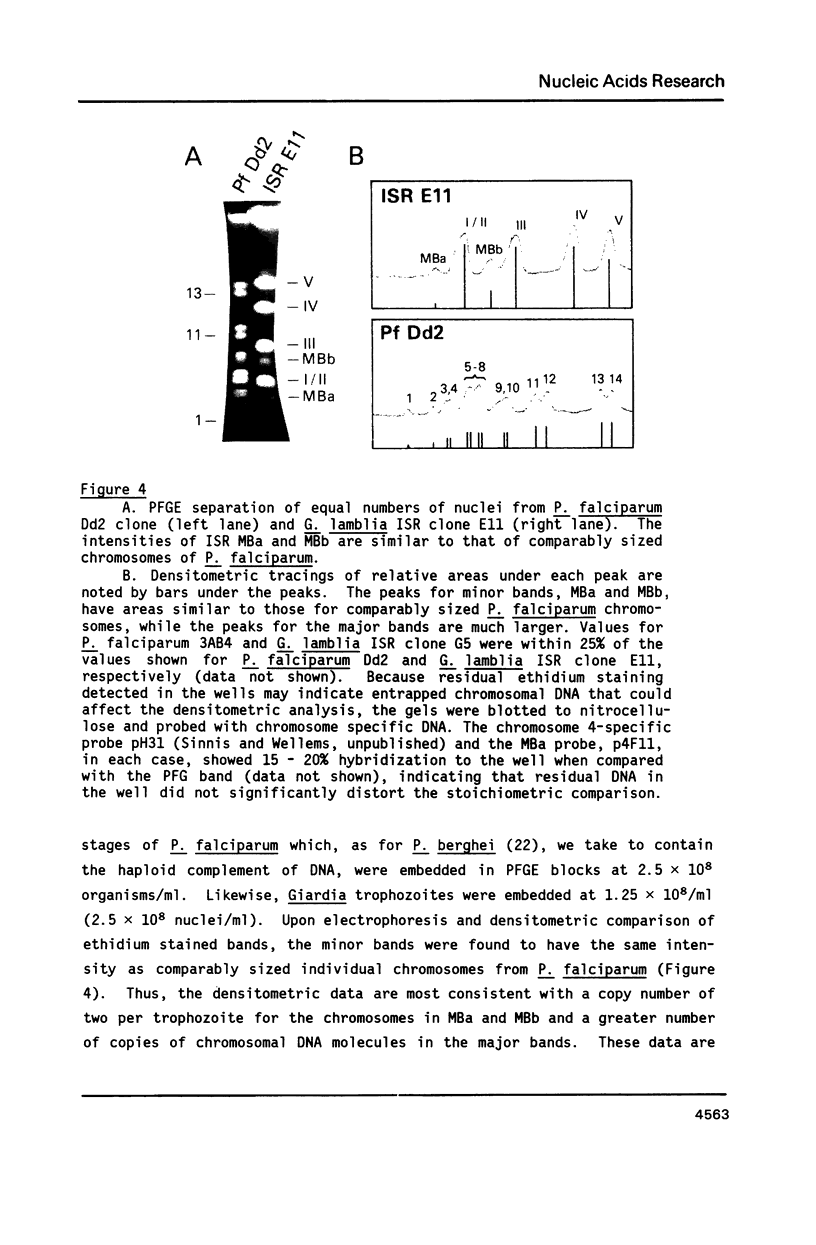
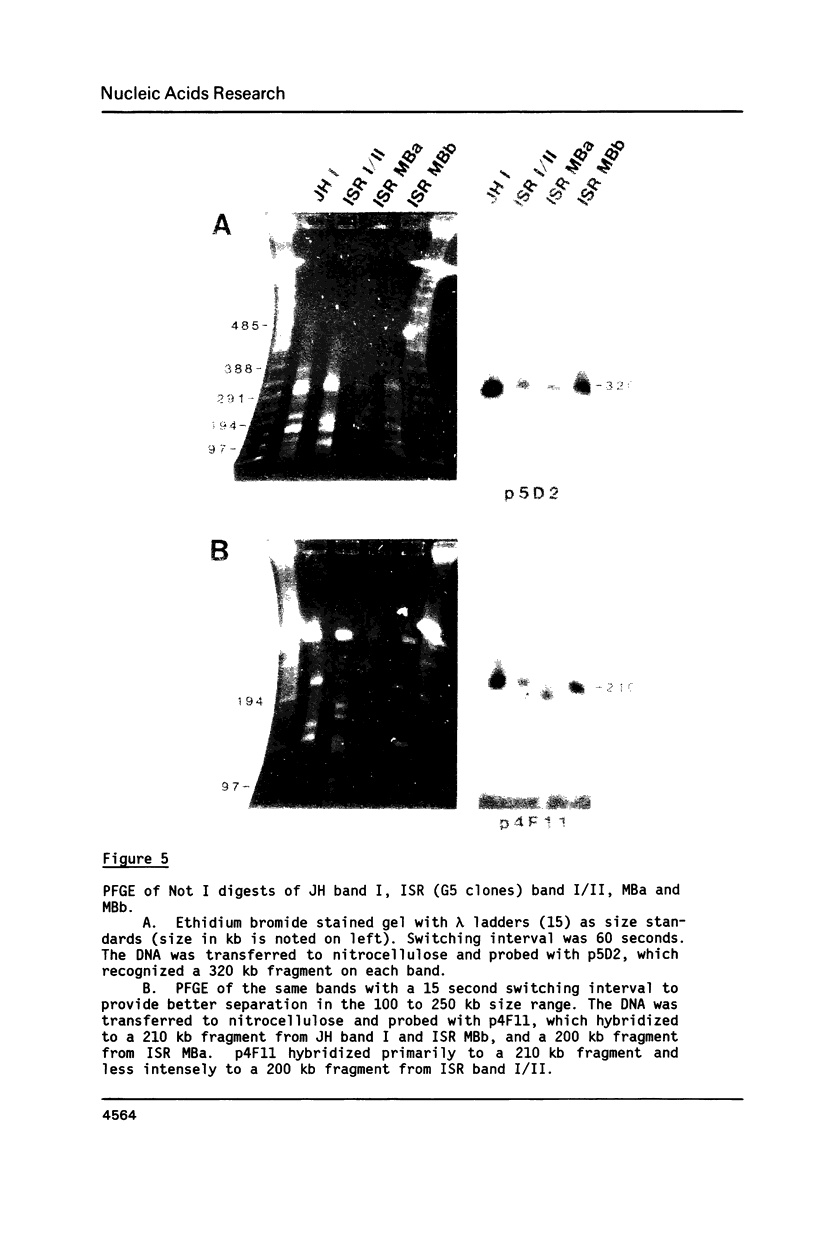
Size variations in homologous chromosomes from six Giardia lamblia isolates have been demonstrated. Four or five intensely stained (major) bands as well as a variable number of lightly stained (minor) bands are present in pulsed field gradient separations. Southern blot analysis with total chromosomal DNA as well as chromosome specific probes indicates that each minor band cross-hybridizes with a major band. Minor bands of doubly cloned organisms appear identical to those of parent clones, indicating that the minor bands do not reflect the presence of variant members within the total population of trophozoites. Densitometric comparisons of chromosome bands from known numbers of Plasmodium falciparum ring stage forms and known numbers of Giardia trophozoites suggest that minor bands MBa and MBb are present in each Giardia trophozoite. Comparison of Not I restriction fragments from the major and minor bands reveals common restriction fragments. Taken together, the data imply that sets of closely related chromosomes occur in the Giardia trophozoite.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman D. M., Reddy L. V., Donelson J. E., Kirchhoff L. V. Trypanosoma cruzi exhibits inter- and intra-strain heterogeneity in molecular karyotype and chromosomal gene location. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):115–123. doi: 10.1016/0166-6851(87)90041-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend D. S. The fine structure of Giardia muris. J Cell Biol. 1966 May;29(2):317–332. doi: 10.1083/jcb.29.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., Schittini M., Keithly J. S., Warburton P. W., Cantor C. R., Van der Ploeg L. H. Karyotype analysis of Leishmania species and its use in classification and clinical diagnosis. Science. 1986 May 9;232(4751):762–765. doi: 10.1126/science.3961502. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Metge D. W., Santi D. V. Plasmid migration using orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1987 Oct 26;15(20):8387–8398. doi: 10.1093/nar/15.20.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C. J., van der Klooster P. F., van der Kaay H. J., van der Ploeg M., Overdulve J. P. DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol Biochem Parasitol. 1986 Aug;20(2):173–182. doi: 10.1016/0166-6851(86)90029-0. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Matsumoto T., Niwa O., Klco S., Fan J. B., Yanagida M., Cantor C. R. An electrophoretic karyotype for Schizosaccharomyces pombe by pulsed field gel electrophoresis. Nucleic Acids Res. 1987 Jun 11;15(11):4481–4489. doi: 10.1093/nar/15.11.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Smits M., Ponnudurai T., Vermeulen A., Meuwissen J. H., Langsley G. Chromosome-sized DNA molecules of Plasmodium falciparum. Science. 1985 Aug 16;229(4714):658–661. doi: 10.1126/science.3895435. [DOI] [PubMed] [Google Scholar]
- Wei R., Wilkinson H., Pfeifer K., Schneider C., Young R., Guarente L. Two or more copies of Drosophila heat shock consensus sequence serve to activate transcription in yeast. Nucleic Acids Res. 1986 Oct 24;14(20):8183–8188. doi: 10.1093/nar/14.20.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Walliker D., Smith C. L., do Rosario V. E., Maloy W. L., Howard R. J., Carter R., McCutchan T. F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987 Jun 5;49(5):633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- Wiesehahn G. P., Jarroll E. L., Lindmark D. G., Meyer E. A., Hallick L. M. Giardia lamblia: autoradiographic analysis of nuclear replication. Exp Parasitol. 1984 Aug;58(1):94–100. doi: 10.1016/0014-4894(84)90024-9. [DOI] [PubMed] [Google Scholar]