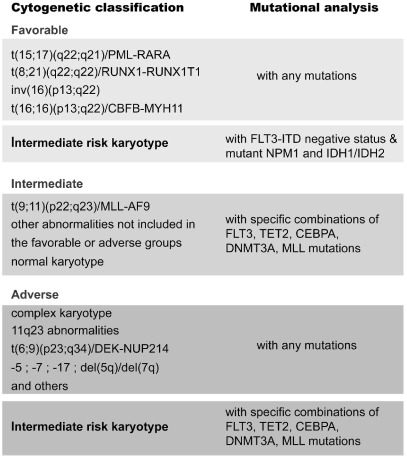
Acute myeloid leukemia (AML) is a genetically complex and heterogeneous leukemia that is caused by the accumulation of multiple genomic lesions that affect key oncogenes and tumor suppressor genes. Historically, chromosomal aberrations, that often result in the generation of fusion genes, have attracted much attention as markers for diagnosis and classification, and also as important prognostic markers.1–3 In this way, t(15;17) or t(8;21) are known to be good prognostic markers while translocations involving 11q23 or t(6;9) are known to be unfavorable prognostic markers. One problem with this cytogenetic classification is that AML patients with normal karyotype were classified with an intermediate prognosis, despite the fact that this is a very heterogeneous group. In recent years, it has become clear that several additional molecular markers can be used to further refine this classification,2 and this has been elegantly extended in a recent study led by Levine.4 Based on molecular analyses, patients with normal karyotype can be further classified into a good prognosis group (such as those lacking FLT3-ITD with NPM1 mutation), an intermediate prognosis group (such as those with FLT3-ITD negative and without TET2 mutations), and a poor prognosis group in which AML patients with, for example, FLT3-ITD positive patients with TET2 mutations are incorporated (Figure 1).1,2,4
Figure 1.
Integration of cytogenetic and molecular markers allows for the assignment of AML patients with normal karyotype to the different risk groups.
These findings open new perspectives for the treatment of AML with normal karyotype, with opportunities to treat the good prognosis group with less severe therapy and the poor prognosis group with more intensive regimens or experimental therapies. A better stratification of AML patients is likely to result in improved outcomes, as has also been obtained over the last decades for young ALL patients.5 While we expect a lot from targeted therapies, recent data from the FTL3 inhibitor AC220 are promising, but again highlight the problem of resistance.6 In a recent study by a team led by Shah, the development of resistance to AC220 has been observed in AML patients treated with this inhibitor.6 Similar to previous findings with imatinib and other tyrosine kinases, resistance to AC220 is acquired by mutations in the kinase domain of FLT3. These studies demonstrate the need for new therapies for the treatment of poor prognosis groups, but also indicate the problems with targeted therapies in acute leukemia.
References
- 1.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 2.Marcucci G, Haferlach T, Döhner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29(5):475–86. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Grimwade D, Simonsson B, Apperley J, Baccarani M, Barbui T, et al. The European LeukemiaNet: achievements and perspectives. Haematologica. 2011;96(1):156–62. doi: 10.3324/haematol.2010.032979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cools J. Improvements in the survival of children and adolescents with acute lymphoblastic leukemia. Haematologica. 2012;97(5):635. doi: 10.3324/haematol.2012.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]