Abstract
The average lifespan of humans is increasing, and with it the percentage of people entering the 65 and older age group is growing rapidly and will continue to do so in the next 20 years. Within this age group, cardiovascular disease will remain the leading cause of death, and the cost associated with treatment will continue to increase. Aging is an inevitable part of life and unfortunately poses the largest risk factor for cardiovascular disease. Although numerous studies in the cardiovascular field have considered both young and aged humans, there are still many unanswered questions as to how the genetic pathways that regulate aging in model organisms influence cardiovascular aging. Likewise, in the molecular biology of aging field, few studies fully assess the role of these aging pathways in cardiovascular health. Fortunately, this gap is beginning to close, and these two fields are merging together. We provide an overview of some of the key genes involved in regulating lifespan and health span, including sirtuins, AMP-activated protein kinase, mammalian target of rapamycin, and insulin-like growth factor 1 and their roles regulating cardiovascular health. We then discuss a series of review articles that will appear in succession and provide a more comprehensive analysis of studies carried out linking genes of aging and cardiovascular health, and perspectives of future directions of these two intimately linked fields.
Keywords: aging, cardiovascular disease, calorie restriction, longevity genes
The most important determinant of cardiovascular health is a person’s age. By 2030, approximately 20% of the population will be aged 65 or older. In this age group, cardiovascular diseases (CVD) will result in 40% of all deaths and rank as the leading cause. Furthermore, the cost to treat cardiovascular disease will triple in that time.1,2 Hence, it remains vital that we understand why age is such a critical component of CVD etiology. However, until recently, the fields of cardiovascular disease and molecular biology of aging have remained largely separate. Most rodent studies of atherosclerosis or cardiomyopathies were performed in young mice, whereas studies of genetic and pharmacological interventions that extend lifespan rarely assessed whether CVD or heart function are improved. Fortunately, the situation is changing. In this introductory review, we discuss genetic pathways that have been identified to regulate the process of aging. Furthermore, we provide an overview of changes that occur in the cardiovascular system with age and introduce a series of reviews that will highlight the recent breakthroughs that have been made bridging the gap between the aging and cardiovascular research fields.
Measuring the Impact of Aging on the Heart and Vasculature
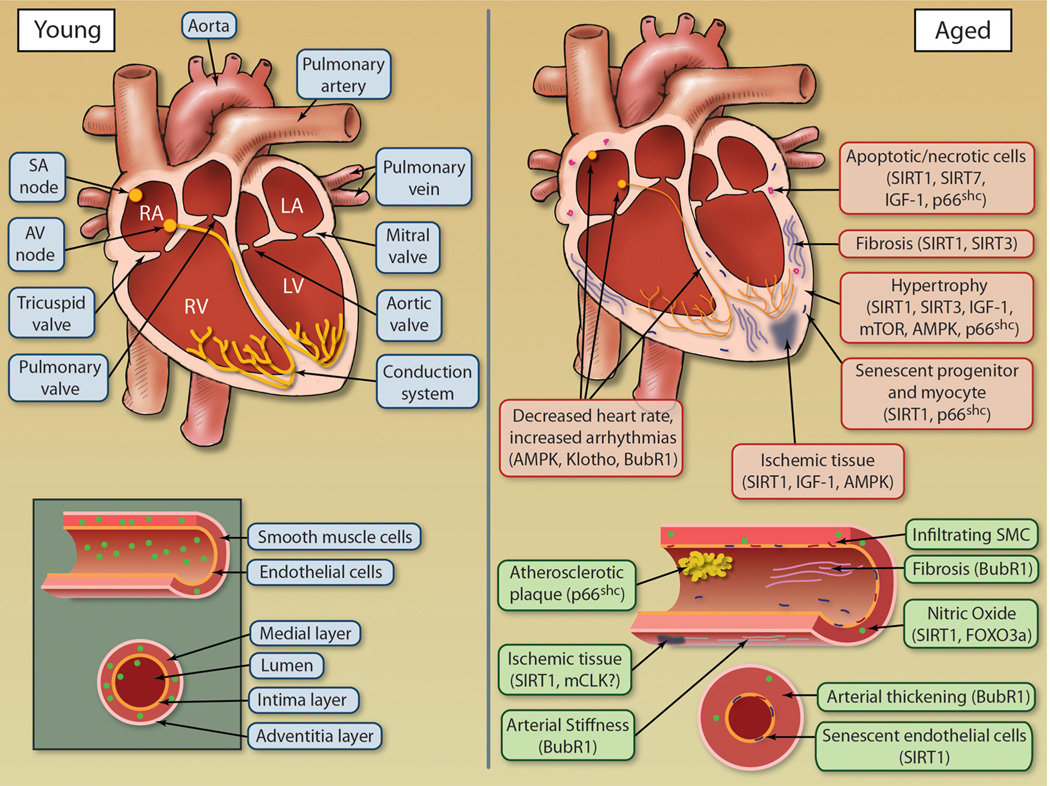
Aging is associated with a progressive decline in numerous physiological processes, leading to an increased risk of health complications and disease. By delivering oxygenated blood to all tissues in the body, the health of the cardiovascular system is vital for health of every tissue and longevity of the organism as a whole. Aging has a remarkable effect on the heart and arterial system, leading to an increase in CVD including atherosclerosis, hypertension, myocardial infarction, and stroke.3 Aging cardiovascular tissues are exemplified by pathological alterations including hypertrophy, altered left ventricular (LV) diastolic function, and diminished LV systolic reverse capacity, increased arterial stiffness, and impaired endothelial function3,4 (Figure 1). However, the health of the arterial and cardiac systems is not mutually exclusive, as each system greatly affects the other. For instance, an increase in arterial stiffness leads to compensatory mechanisms by the myocardium including LV hypertrophy and fibroblast proliferation, resulting in decreased cardiac output and increase in fibrotic tissue.3,4 Heart rate modulation is also affected by age with a decrease in both rate variability and maximum heart rate.5 Heart rate is influenced not only by the loss of cells in the sinoatrial node (responsible for controlling heart rate) but also by structural changes in the heart, including fibrosis and hypertrophy, which slow propagation of electric impulse throughout the heart.
Figure 1. Age-dependent changes to cardiovascular tissues.
Both the heart and vasculature undergo numerous alterations during aging as a result of deregulation of molecular longevity pathways, leading to compromised function. Illustration credit: Cosmocyte/Ben Smith.
At early ages, the LV diastolic filling rate begins to decline, which is compensated for by increasing arterial contraction to sustain stroke volume and workload, maintaining sufficient ejection fraction.6,7 However, with age, the LV contractility and ejection fraction, as well as sympathetic modulation of heart rate, and response to β-adrenergic receptor activation all decrease.4 A reduction in cardiac output due to decline in function with age stimulates the myocardium to compensate by increasing muscle mass by undergoing cardiac hypertrophy; although this may provide short-term enhancement of cardiac output, the long-term effect of hypertrophy diminishes cardiac function.8 Ventricular hypertrophy is the result of an increase in size of individual cardiomyocytes and can be either physiological, which is reversible (eg, exercise-induced), or pathological which is irreversible (disease-based).9 Hypertrophy in mammalian models such as mouse and rat can be measured with a variety of techniques including echocardiography to measure LV size, histological analysis of heart tissue, or isolation of cardiomyocytes for cell size measurements. The noninvasive nature of echocardiography allows for the unique opportunity to measure alterations in cardiac size and functional parameters longitudinally in an aging study cohort.
Aging of the vasculature results in increased arterial thickening and stiffness as well as dysfunctional endothelium. Clinically, these changes result in increased systolic pressure and present major risk factors for development of atherosclerosis, hypertension and stroke, and arterial fibrillation.4 Vascular dysfunction associated with aging leads to a variety of age-related pathologies, including loss of adequate tissue perfusion (resulting in ischemia), insufficient vascular growth or regression (resulting in hypertension), or excessive growth and remodeling (resulting in age-related macular degeneration). The vasculature undergoes structure and function alterations with age that are well documented, such as luminal enlargement with wall thickening and a decline in endothelial cell function negatively affecting endothelium-dependent dilation and promoting vascular stiffness.10 In addition, endothelial cells lose their ability to proliferate and migrate after tissue injury.11 Furthermore, endothelial barriers become porous and vascular smooth muscle cells migrate into subendothelial spaces and deposit extracellular matrix proteins that result in intimal thickening. At the molecular level, as endothelial cells age, they exhibit a reduction in endothelial nitric oxide synthetase (eNOS) activity, reducing the abundance of NO.12 NO is a critical vasodilator produced by endothelial cells, regulating vascular tone, in addition to inhibiting vascular inflammation, thrombotic events, and aberrant cellular proliferation.13 Loss of NO also promotes endothelial cell senescence.14 Numerous mechanisms can modulate eNOS activity. However, hemodynamic shear stress, the frictional force acting on endothelial cell surface as a result of blood flow, is one of the most potent inducers of eNOS activity.12 As vessels age, they are exposed to less hemodynamic stress due to reduced blood flow caused by decline in heart function; in addition, endothelial cells become less responsive to shear stress, resulting in a decline in the protective NO.15 Therefore, measuring arterial thickness and stiffness in aged models can lead to further understanding of the role various longevity genes influence vasculature aging. Elastic properties of arteries can be directly measured, as well as histological analysis of luminal enlargement, arterial thickness, and deposition of vascular smooth muscle cells. In addition, measuring eNOS activity in vessels, as well as endothelial senescence, can also serve as a marker of vascular aging.
The heart undergoes complex changes during aging that affect the cellular composition, marked by a decrease in absolute number of cardiomyocytes due to increased apoptosis and necrosis and a decrease in repopulation of cardiomyocytes from cardiac stem cell reserves.16,17 With age, cardiomyocytes become more susceptible to stress, including oxidative stress. Therefore the increase in oxidative stress due to the increase in reactive oxygen species (ROS) production with age results in an overall enhancement in the rate of cardiomyocyte death with age. In cases when cardiomyocytes undergo necrosis, the release of cellular components can affect survival of neighboring cardiomyocytes, in addition to promoting the development of proinflammatory and profibrotic environments in the aging heart. Cardiomyocyte senescence, defined by the increased expression of senescence markers and decreased telomere length, also increases with age.18 However, a recent study suggests that removal of senescence cells alone may not be sufficient to improve cardiac defects observed in some aging models.19 Measuring cardiac-specific senescence, DNA damage, as well as levels of apoptosis and necrosis, coupled with fibrosis measurements in animal models of aging, will lead to a better understanding of the link between aging and CVD. Senescence is coupled with expression of such factors as p53, p21, p16, and senescence-associated β-galactosidase activity, which can be measured by immunoblotting or histological techniques. In addition, the levels of damaged nuclear and mitochondrial DNA can be assessed by quantifying levels of 8-oxoguanine, a common DNA lesion associated with oxidative stress, and thus a marker for fundamental damaged DNA pathways in the heart.20 DNA damage and can also be assessed by determining phosphorylation status of γ-H2Ax, a histone variant that is phosphorylated near DNA break sites and thus forms readily detectable foci in cells.21 Fibrosis is measured primarily by histological methods, using staining techniques such as Masson trichrome. These biomarkers of aging can be used in cardiac tissue to assess how modulation of longevity genes influences the rate and degree of cardiovascular aging at the cellular level.
Although long thought to be postmitotic, cardiomyocytes undergo division and regeneration, and recent work has discovered that cardiac regeneration is a vital mechanism of maintaining cardiovascular health. However, the rate of regeneration in the aged may not be adequate to maintain cardiomyocyte numbers in response to cardiomyocyte loss.22 Regenerated cardiac tissue is thought to involve a small pool of cardiac stem cells and a subset of small incompletely differentiated cardiomyocytes that can reenter the cell cycle.23,24 Regenerative capacity can be measured by assessing the proliferative index, using markers of proliferation such as Ki67 or proliferating cell nuclear antigen. Therefore, long-lived or short-lived progeroid animal models can be tested for alterations in their regenerative capacity to determine how regulatory mechanisms controlling regeneration are involved in the aging cardiovascular system.
Other less understood changes in the heart with age are a decrease in the number and function of sinoatrial nodal pacemaker cells and a concomitant increase in conduction abnormalities. The aging heart undergoes a decrease in heart rate variability and maximum heart rate.5 Consistent with this notion, the aging models budding uninhibited by benzimidazoles related 1 (BubR1) and Klotho have both been described to contain abnormalities leading to arrhythmias and altered conduction system, respectively.19,25 Very few aging models have been assessed for alterations in cardiac conduction. Therefore the use of electrophysiology (EP) studies as well as testing aging models for alterations in EP parameters is a future area of research that could lead to a prominent understanding for the increased incidence of cardiovascular disease with age. EP studies can be carried out in cultured cardiomyocytes and in vivo by intracardiac and surface ECG analysis. Like echocardiography, the noninvasive nature of measuring surface ECGs can allow for longitudinal studies in aging cohorts.
Aging Research Leads to a New View of CVD
In both the aging and cardiovascular fields, it is generally accepted that a low calorie diet combined with exercise will increase the health span of mammals and that adiposity and a sedentary lifestyle have the opposite effect. The traditional view is that CVD results from the accumulation of cholesterol and fatty acids in tissues, which compromise tissue function and stimulate the production of inflammatory cytokines as well as ROS. In the past decade, however, the aging field has proposed that there is another fundamental process at work: a diet high in calories without exercise may be harmful because it suppresses the expression of “longevity genes” that promote cellular defenses against aging and age-related diseases.26
The new concept has its roots in the seminal observation by McCay et al in 1935 that reducing a rat’s caloric intake by 20% to 40% dramatically extends its lifespan.27 Since then, the lifespan-extending properties of calorie restriction (CR) have been observed in yeast, fish, some rodents, dogs, and macaque monkeys, although there are exceptions, including conflicting results in various inbred and outbred strains of mice and wild mice.28–30 In mammals, CR reduces the incidence of most age-related diseases, including cancer, sarcopenia, kidney failure, and CVD.31
Whether or not CR increases the lifespan of primates is debated, in part because of conflicting data from 2 different sites and questions about which types of death should be censored. Data from human studies indicate that Okinawans eat a moderately restricted diet (≈ 1785 kcal/d) and have lower rates of coronary heart disease and a relatively high frequency of centenarians. Consistent with this, individuals on CR diets (0.5–8 years) have a physiology consistent with protection from CVD, including reduced triglycerides, lower blood pressure, reduced inflammatory markers, and decreased oxidative stress.32–34
Many of the fundamental molecular processes involved in CR-mediated protection of the cardiovascular system are known. CR increases mitochondrial function while reducing oxidative stress in the vasculature, in part by inducing expression of the Nfr2 stress response transcription factor, which induces expression of NADPH:quinone oxidoreductase 1, heme oxygenase I, and glutathione S transferase.35–37 CR also reduces inflammation by suppressing the activity of vascular adhesion molecules, prostanoids, and inflammatory cytokines in both rodents38 and humans.39 Endothelial function is enhanced and both atherosclerosis and arterial stiffness are reduced by CR in rodents.40,41 With regard to cardiac function, CR delays the age-related decline in diastolic filling accompanied by reductions in inflammation, cardiomyopathy, cardiac fibrosis, and myocardial degeneration.42 Given all these data, there is considerable interest in understanding how CR works and in finding ways to mimic these effects with pharmacological agents.
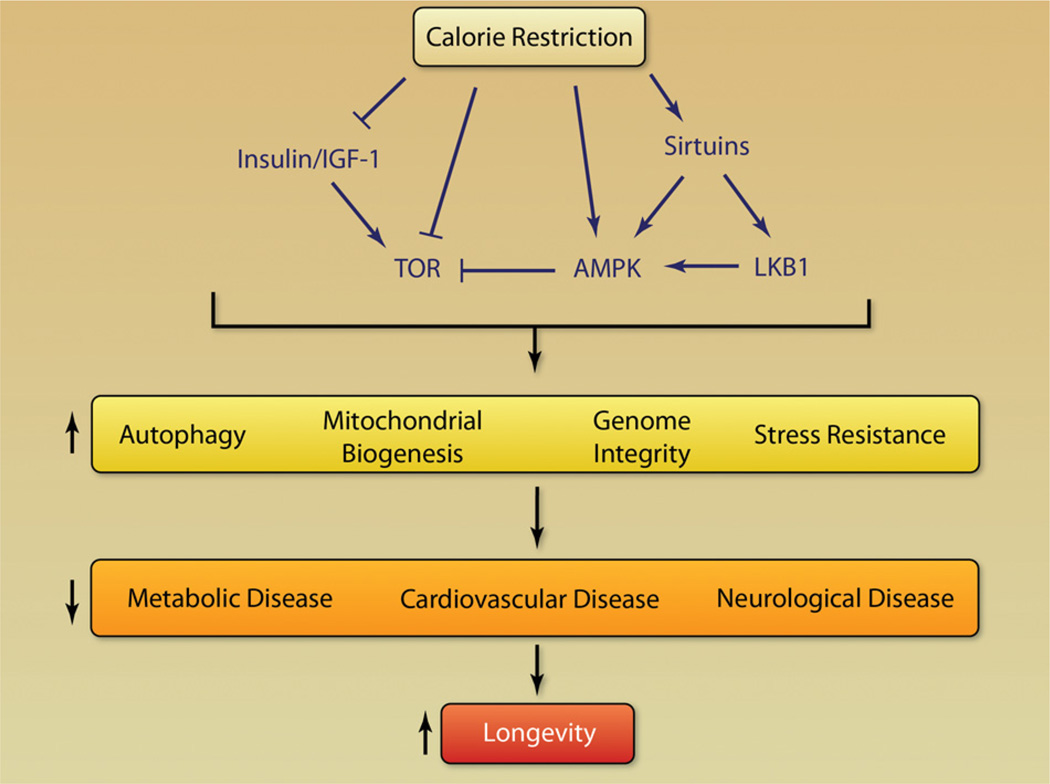
Considerable progress has been made in the past decade toward understanding not only how but also why CR works. The original idea that CR works passively by suppressing metabolic rate or reducing damage caused by ROS is being replaced by a fundamentally different model in which CR triggers an active defense response that evolved to promote survival during harsh conditions (Figure 2). At the center of this response are so-called “longevity regulatory” pathways, which include insulin/insulin-like growth factor 1 (IGF-1), the mammalian target of rapamycin (mTOR), AMP-activated kinase (AMPK), and nicotinamide adenine dinucleotide (NAD)+-dependent deacetylases (sirtuins). Although researchers still argue about which longevity gene is most important for the health benefits of CR, the emerging picture is that these genes form a network of redundant pathways and both positive and negative feedbacks (Figures 2 and 3). How these pathways improve health and control one another is the most intense area of research at present in the aging field.
Figure 2. Pathway leading from calorie restriction to longevity.
Calorie restriction modulates factors in the longevity network, leading to alterations in cellular responses to stress, affecting common diseases of aging and influencing longevity.
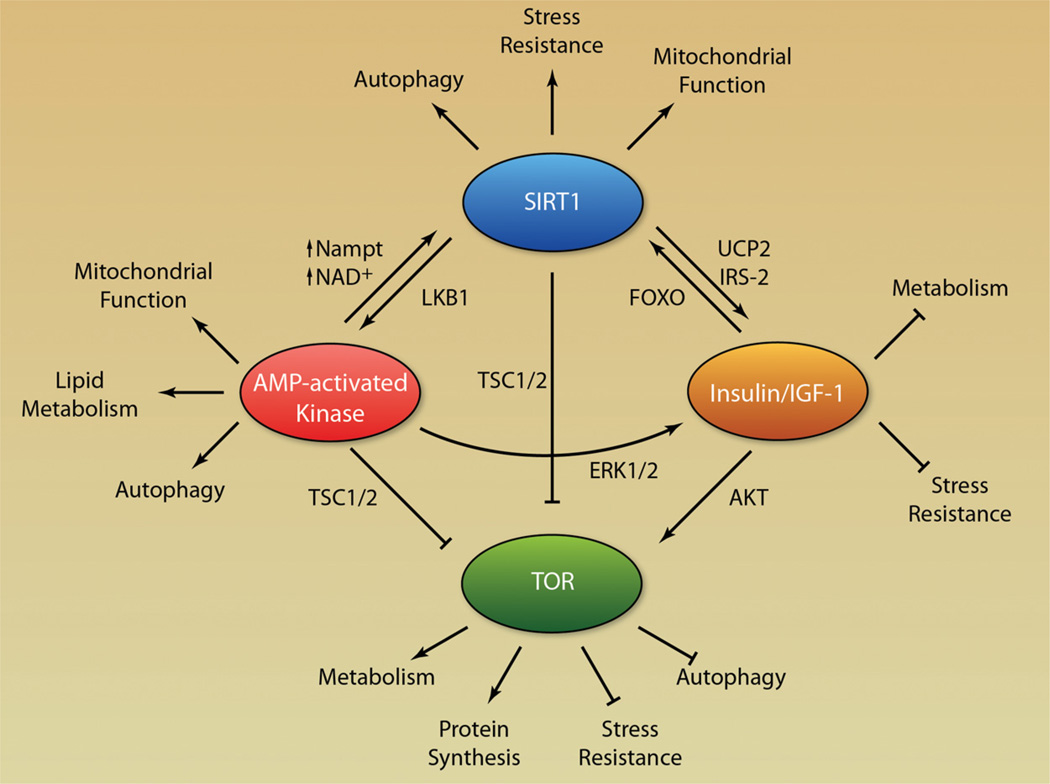
Figure 3. Model for a longevity network.
Positive and negative feedback regulation between genes involved in lifespan and age-related diseases including sirtuins, AMPK, IGF-1, and TOR.
There is increasing evidence that longevity pathways are not only important for understanding how CR works, they may also underlie the health benefits of weight loss and exercise. If this is true, then an individual who reduces caloric intake and starts exercising is improving their health because they are triggering a billion-year-old defense response that evolved to keep organisms alive during adversity. Conversely, when an individual gains adiposity and leads a sedentary lifestyle, they are negatively affecting their cardiovascular system by adding stress to the system and are also inhibiting these natural defense pathways.
The logical extension of this idea is that it should be possible to mimic the beneficial effects of dieting and exercise by tweaking the right pathways, using small molecules. Studies with “CR mimetics” such as resveratrol and metformin (which activate the SIRT1-AMPK system) or rapamycin (which inhibits mTOR), show that it is possible for a rodent to be obese and sedentary while maintaining the physiology of a lean animal.43–47 Recent work has also identified a secreting hormone termed irisin, which, when increased, induces energy expenditure in the absence of exercise, positively influencing obesity and glucose homeostasis.48 However, the overall effect of irisin on cardiovascular disease remains largely unexplored.
Longevity Genes and CVD
A number of longevity genes have been identified in model organisms, including the yeast Saccharomyces cerevisiae, the nemotode Caenorhabditis elegans, and the fly Drosophila malanogaster. Many of these genes and their related pathways have been subsequently assessed for their role in regulating longevity in mice, as well as to begin assessing their roles in CVD.
SIR2
Sirtuins are evolutionary conserved enzymes that function as NAD+-dependent deacetylases and ribosyltransferases. Mammals have 7 sirtuin members, which have diverse localizations in the cell and regulate a variety of cellular functions including DNA damage repair, cell cycle, metabolic response to nutrient availability, and protection from neurological degeneration.49 Sirtuins have been observed to modulate aging in yeast to mammals.50–55 However, a direct role for sirtuins in regulating lifespan in worms and flies has been challenged.56 Yet, numerous studies have maintained sirtuins as a key component of the longevity network (Figure 3). SIRT1, which is localized primarily in the nucleus, has been observed to regulate the AMPK pathway through deacetylation of the liver kinase B1.57,58 In addition, SIRT1 regulates the Insulin/IGF-1 pathway through modulation of UCP2 expression and direct regulation of the IGF-1 signaling pathway.55,59,60 SIRT1 also interacts with TSC1/2, inhibiting mTOR activity.61,62 In relation to cardiovascular development and disease, SIRT1 knockout mice have greater injury in response to ischemia reperfusion studies, and injury is attenuated in SIRT1 transgenic mice.63 Cardiac-specific SIRT1-overexpressing mice show delayed age-dependent cardiomyopathies and reduction of stress-induced apoptosis. However, extensive SIRT1 overexpression (≈ 20 fold) resulted in oxidative stress, apoptosis, and cardiomyopathy.64,65 Interestingly, SIRT1 is involved in both pressure overload-induced, and Angiotensin II, cardiac hypertrophy in addition to hypertrophy of vascular smooth muscle.66,67 SIRT1 has been observed to regulate blood vessel growth both in zebrafish and in mice by regulating notch signaling.68,69 Arterial stiffness is also regulated by SIRT1 through preventing hyperphosphatemia-induced arterial calcification.70 SIRT3 is localized in the mitochondria, and knockout mice exhibit both age-dependent and exercise/pressure overload–induced cardiac hypertrophy.71,72 SIRT7 knockout mice develop cardiac hypertrophy and inflammatory cardiomyopathy and are also characterized by an increase in fibrosis.54 Cardiomyocytes from SIRT7 knockout mice have decreased resistance to oxidative stress and an increase in apoptosis. Although a number of effects of sirtuins on cardiovascular health have been described, the molecular mechanisms by which sirtuins regulate the heart and vasculature are only beginning to be understood. Furthermore, sirtuins have been shown to be involved in some of the beneficial effects of CR and resveratrol, suggesting that positive influence of CR and resveratrol in CVD may be modulated in part through sirtuin proteins.
IGF-1/Growth Hormone
IGF-1 was one of the initial genes to be identified as a longevity gene, where loss of IGF-1 was shown to extend lifespan of C elegans.73 Subsequently, genes involved in the IGF-1 pathway were characterized to modulate lifespan as well, confirming the role of this pathway in lifespan regulation. IGF-1 signaling modulates other factors in the longevity network by inducing mTOR activity through regulation of Akt activity74 (Figure 3). Mice deficient in IGF-1 die shortly after birth, whereas some live to adulthood but are deficient in growth and are dwarfed. However, IGF-1 receptor–deficient mice die postnatally due to respiratory failure.75 Overexpression of IGF-1 in the heart prevented myocardial cell death after infarction and reduced ventricular dilation, hypertrophy, and diabetic cardiomyopathy.76–79 However, in a second overexpression study, IGF-1 led to cardiac hypertrophy and failure80 as well as to diminished recovery of heart function after acute ischemic challenge.81 Interestingly, liver-specific IGF-1 knockout antagonized oxidative stress and cell death in cardiomyocytes induced under potent oxidant treatment with paraquat.82 These differences probably arise from the duality of autocrine and paracrine effects of IGF-1. In Drosophila, which have a primitive cardiovascular system, deletion of the IGF-1 homologue (InR) delayed the effects of aging on the fly cardiovascular system.83 Although a significant role for the IGF-1 pathway in regulating cardiovascular health has been defined, further studies are needed to elucidate the mechanism and clarify the discrepancies that have been observed.
Forkhead Transcription Factors
Forkhead transcription factors (FOXOs) play a role in regulating expression of genes involved in cell growth, proliferation, differentiation, and longevity.84 FOXOs are also downstream effectors of the IGF-1 signaling cascade and are regulated by sirtuin-mediated deacetylation (Figure 3). Deletion of FOXO1 in mice leads to embryonic lethality, exhibiting deficient vascular and cardiac growth, including underdeveloped dorsal aorta and impaired cardiac looping.85,86 However, a balance of FOXO1 is critical, as overexpression of FOXO1 in a cardiac-specific manner leads to embryonic lethality, with impaired cardiomyocyte proliferation, reduced heart size, myocardium thickness, and subsequent heart failure.87 Expression of FOXO3a in mouse hearts resulted in reduction of cardiomyocyte size, suggesting that this FOXO factor functions to reduce hypertrophy.88 FOXO3a deficiency has been shown to increase eNOS expression and enhances postnatal vessel formation and maturation.89 Although recent studies suggest the disparity between the expression of various members of the FOXO family, more research is necessary to understand the role of these transcription factors in regulating cardiovascular development, function, and disease as well as to elucidate how FOXO factors interact with the longevity network in cardiovascular tissues.
Clock 1
Clock 1 (CLK-1)/mammalian CLK-1 (MCLK1) is a hydroxylase localized to the mitochondria that is necessary for the biosynthesis of ubiquinone (coenzyme Q), the essential electron transporter of the mitochondrial respiratory chain.90 Inactivation of clk-1 in C elegans and partial inactivation in mice extends lifespan of both organisms. MCLK-1 heterozygous mice have yet to be assessed for effects on cardiovascular system function and disease progression. However, these mice are known to exhibit protection from cerebral ischemia and reperfusion, suggesting a potential role in modulation of vascular response to ischemic conditions.
AMPK
The AMP-activated protein kinase is involved in glucose and lipid metabolism, cell growth and autophagy, cellular polarity, and gene expression.91 AMPK modulates mitochondrial function by triggering the destruction of defective mitochondria through a process of mitochondrial-specific autophagy termed mitophagy and induction of new mitochondria through activation of mitochondrial biogenesis.91 Composed of 3 subunits, which are regulated by binding AMP, AMPK acts as a metabolic sensor, measuring the relative AMP:ATP ratio.91 Within the longevity network (Figure 3), AMPK regulates mTOR through direct phosphorylation of the TSC1/2 complex,92 modulates the IGF-1 pathway through the extracellular signal-regulated kinase (Erk) cascade,93 and controls sirtuin activity by regulating the abundance of NAD and nicotinamide phosphoribosyltransferase (Nampt).94,95 AMPK is cardioprotective during ischemia and reperfusion. Mouse models using dominant-negative AMPK have exacerbated myocardial infarction.96,97 Activation of AMPK by metformin reduces pressure overload–induced cardiac hypertrophy.98,99 Mutations in the regulatory γ2 subunit of AMPK lead to an inherited syndrome of hypertrophic cardiomyopathy and ventricular preexcitation.100–102 Being involved in energy sensing, AMPK may be a mediator of the positive effects of CR and resveratrol on both longevity and CVD. Consistent with this notion, many of the effects of resveratrol are lost in AMPK knockout mice.103 Further studies are necessary to determine the interaction of these dietary regimens, AMPK, and cardiovascular heath.
p66shc
The mammalian p66shc is a splice variant of the Shc locus, encoding proteins carrying a Src-homology 2 domain, a collagen-homology region, and a phosphotyrosine-binding domain. The splice variant p66sch contains a unique N-terminal region, which functions as a redox enzyme modulating mitochondrial ROS.104 Loss of p66shc leads a decrease in ROS and lifespan extension.105 In the cardiovascular system, loss of p66shc blocks the decline in cardiac progenitor cell senescence, decreases DNA damage, necrosis, and apoptosis, and preserves LV volume and function, thus reducing heart failure.106 Loss of p66shc also prevented Angiotensin II–induced hypertrophy and apoptotic cell death.107 Furthermore, consistent with a central role of ROS in the pathogenesis of atherosclerosis, loss of p66sch protected mice from aortic lesions when placed on a high-fat diet.108
Catalase
Catalase converts one of the major ROS, hydrogen peroxide, into water and oxygen. Targeting peroxisomal catalase to the mitochondria, leads to ≈ 20% lifespan extension, lending support to the oxidative theory of aging.109 These mice also demonstrated reduction in age-dependent LV hypertrophy and diastolic dysfunction.110 In addition, these mice were also protected from Angiotensin II–induced cardiac hypertrophy as well as pressure overload–induced and G-α-q overexpression–induced heart failure.111 Similar to p66shc knockouts, mitochondrial-targeted catalase mice emphasize the role of ROS in CVD.
Pituitary Transcription Factor 1 and Prophet of Pituitary Transcription Factor 1
The pituitary transcription factor 1 (Pit-1) is involved in the transcriptional program for normal development and function of the pituitary gland. Patients with mutants in Pit-1 have growth hormone deficiencies.112 Prop-1, or Prophet of Pit-1, is also a pituitary transcription factor. Prop-1 mutations in patients exhibit secondary hypogonadism in addition to the deficiencies of growth hormone, prolactin, and thyroid-stimulating hormone also seen in patients with Pit-1 mutations.113 Mutations in Prop-1 are the genetic basis for the phenotypes observed in the long-lived Ames dwarf mice. Little is known about the role of these gene alterations in cardiovascular function. Cardiomyocyte size, however, was reduced in young and old Ames dwarf mice compared with wild-type. Collagen content was reduced only in the young mice, suggesting that Ames dwarf mice may receive some longevity benefit from the reduced cardiomyocyte cell size and a period of reduced collagen content in the heart during adulthood.114
Klotho
Klotho was discovered as a gene that is mutated in a mouse strain presenting multiple premature aging phenotypes and a shortened lifespan.115 In addition, overexpression of Klotho increases lifespan.116 The Klotho gene encodes a single-pass transmembrane protein that is expressed primarily in the renal tubular cells and functions in phosphate reabsorption and metabolism.117 Klotho is also observed as a circulating hormone-like factor, due to proteolytic cleavage by membrane-anchored proteases.116 In the heart, Klotho is expressed in the sinoatrial node region and is required for the sinoatrial node to function as a pacemaker under stress.25
Target of Rapamycin/Rapamycin
Target of rapamycin (TOR), is a serine-threonine protein kinase that is inhibited by the bacterial product rapamycin. Conserved from yeast to humans, TOR integrates signaling from insulin and growth factors as well as sensing intracellular amino acid levels to regulate cell size and growth, proliferation, survival, motility, protein synthesis, and transcription.118 Deletion of TOR or treatment with rapamycin has been observed to extend lifespan in yeast, worms, flies, and mammals.45,119–121 Within the longevity network, mTOR tends to play an effector role of upstream sirtuin/AMPK/IGF-1 activity (Figure 3). However, in yeast, one proposed mechanism by which TOR inhibition regulates lifespan is by enhancing multicopy suppressor of SNF1 protein 2/4 transcriptional induction of the NAD synthesis enzyme Pnc1 (yeast homolog of Nampt), thus activating sirtuin proteins.122 Conservation of this pathway in higher organisms has yet to be described. Inhibition of mTOR signaling in the heart represses cardiac hypertrophy mediated by pressure overload, potentially through blocking of mTOR control of protein translation and cell size.123,124 Furthermore, mTOR, through its involvement in the PI3K/AKT signaling pathway, plays a role in mediating hypoxia-induced angiogenesis in tumors, through regulation of hypoxia-inducible factor-1α stabilization and vascular endothelial growth factor (VEGF) expression. 125 Inhibition of PI3K/AKT/mTOR pathway through such agents as PI3K/AKT inhibitors LY294002 and wortmannin and the mTOR inhibitor rapamycin leads to decreases in VEGF secretion and angiogenesis.126 mTOR negatively regulates autophagy, and inhibition of mTOR, either through nutrient-poor conditions or rapamycin treatment, activates autophagy, allowing for the destruction of defective molecules and organelles and promoting health of cardiovascular tissues.127 The PI3K/AKT/mTOR pathway lies at the intersections of numerous signaling pathways, thus positioning this pathway as a vital mediator of aging and the cardiovascular system.
BubR1
BubR1 is a serine/threonine protein kinase and an inhibitor of the anaphase-promoting complex, regulating the mitotic spindle assembly checkpoint.128 Heterozygous mice develop normally but are tumor-prone, and mice with near-deficient levels (homozygous BubR1 hypomorphic mice) develop premature aging phenotypes and have a shortened lifespan.129,130 BubR1 hypomorphic mice exhibit arrhythmias, thought to be the primary mechanism leading to their death.19 In addition, arterial wall thickness and inner diameter were reduced in loss-of-BubR1 mice, with increased fibrosis and reduced elastic properties.131 Future studies should illuminate the role that this mitotic regulator plays in the heart, which is largely postmitotic.
A Cardiovascular Aging Review Series
The work discussed in the following articles in the series highlights the recent progress in understanding the mechanisms by which aging and obesity reduce cardiovascular function and how we may use this knowledge to combat cardiovascular disease. In the first part of the series, Leeuwenburgh, Marzetti, and colleagues examine involvement of mitochondrial dysfunction and deregulated autophagy on cardiovascular aging. Extensive evidence has been presented that mitochondrial function declines with age in a majority of tissues. The triggering cause and molecular mechanism of age-associated mitochondrial decline is unclear, but the functional consequences at the cellular and tissue levels have been well documented. Mitochondria generate energy through oxidative phosphorylation, of which a byproduct is an increase in ROS production, leading to free radical– imposed damage to macromolecules and cellular components. Control of mitochondria quality can occur at 3 basic stages: repair of damaged mitochondria, removal of dysfunctional mitochondria through autophagy, and generation of new mitochondria through biogenesis.132 The current knowledge of the mechanisms responsible for cardiac mitochondrial dysfunction and abnormal ROS production in advanced age will be reviewed; the role that autophagy plays in controlling mitochondria quality will be discussed in the context of cardiac hypertrophy, ischemia, heart failure, and diabetic cardiomyopathy.
In the second part of the series, Fontana, Vinciguerra, and Longo take a look at the role of growth factors on cardiovascular aging. Growth factors were at the center of initial studies pertaining to the molecular genetics of lifespan extension. Kenyon and colleagues found that loss of insulin-like growth factor 1 (IGF-1) signaling led to lifespan extension in C elegans.73 IGF-1 pathways regulate downstream phosphorylation cascades including the MAPK and mTOR pathways, leading to effects on cell growth, proliferation, and survival, in addition to lipid and glucose metabolism. The role of IGF-1 in cardiac function and aging is largely unknown, and the recent data present a complex picture of positive and negative influences of growth factors on cardiovascular health. Furthermore, clinical studies rely heavily on associations, and the limited direct mouse studies have given conflicting results. The pathways regulated by growth factors in cardiomyocytes and the effect of these pathways on cardiac aging will be discussed, including effects of IGF-1 on cardiac stem cell and myocardial regeneration. In addition, the role of adiposity of cardiac tissue is touched on.
In the third part of the series, Dai, Rabinovitch, and Ungvari revisit the role of mitochondria and cardiovascular aging. Mitochondria are the cellular sources for energy generation and are critically important for high energy–demanding tissues such as the heart. However, due to their production of ROS, they are central to the rate of aging of these tissues. The role of mitochondria dysfunction and oxidative stress in cardiovascular aging is exemplified by murine models that reduce mitochondria ROS, which have reduced age-associated changes in the heart.133 This review discusses the role and molecular mechanisms regulating oxidative stress in the aging cardiovascular system. Furthermore, discussions touch on the role of retrograde mitochondrial signaling (communication from the mitochondria to the nucleus) and mitochondrial biogenesis in cardiovascular health, including therapeutic targets to regulating mitochondria to diminish cardiovascular aging.
In the fourth part of the series, DePinho, Moslehi, and Sahin consider the implications of telomere attrition and mitochondria in the aging heart. Loss of telomeric DNA, during each successive cell division has been a central instigator in cellular aging since the identification of cellular senescence by Hayflick.134 Subsequently, extensive studies attribute telomere attrition to degeneration of stem and progenitor cell compartments and other highly dividing tissues. However, a number of recent studies have found that loss of telomeres has deleterious effects in quiescent tissues and further characterize signaling pathways that are activated in response to loss of telomeric DNA.135 Specifically, the role of telomere attrition in augmenting age-related cardiac decline are discussed, including details of a p53/mitochondrial signaling pathway.
In the fifth part of the series, Oellerich and Potente provide a current view of the role of FOXOs/sirtuins in vascular aging. FOXOs have been associated with longevity due to their role as downstream effectors of IGF-1 signaling.136 Similarly, sirtuins have been associated with lifespan and age-related diseases.49 Both FOXO factors and sirtuins have been associated with vascular development and cardiac function during aging. The upstream regulators of FOXO/sirtuin factors and downstream effector pathway involving these factors are discussed, with particular emphasis on vascular flow and hypoxic responses leading to modulators of these factors.
In the sixth part of the series, Lahteenvuo and Rosenzweig evaluate the role of angiogenesis impairment and endothelial dysfunction in cardiovascular aging. Age-related impairment of angiogenesis contributes to increased end-organ damage seen in the elderly and directly links cardiovascular health with systemic tissue homeostasis.137 Angiogenesis is critical in the elderly as a mechanism for repairing tissues after damage caused by events such as ischemic stroke, myocardial infarction and ischemia, and lower-limb ischemia. Processes and pathways contributing to impairment of angiogenesis, including cellular senescence, telomere attrition, oxidative damage, NO, hypoxia, and vascular growth factors are discussed, in addition to therapeutic approaches for improving angiogenesis in the elderly.
In the final part of the series, Tartar and Bodmer discuss nonmammalian models of cardiovascular aging. Various model organisms have rudimentary heart and circulatory systems, such as those observed in zebrafish and Drosophila. Even with a rudimentary cardiovascular system, the powerful use of genetics in these systems has, and will continue to, allow for these models to be useful in discovering novel pathways regulating cardiovascular biology. Although zebrafish are rarely used for aging research, Drosophila has been used in discovery and confirmation of various aging pathways. This review discusses the history and discoveries made in various nonmammalian models both in terms of longevity and cardiovascular aging.
Aging, although an unavoidable cardiovascular risk factor, may overcome all the other risk factors collectively. Therefore, understanding fundamental mechanisms that dictate the pace of aging could lead to significant advancements into both preventative and therapeutic treatments of CVD. The articles presented in this review series shed light the interconnected roles of aging and cardiovascular research and provide a perspective on new and exciting areas to investigate by integrating these two tightly linked research areas.
Acknowledgments
We would like to apologize to all those investigators whose work could not be cited due to space limitations.
Sources of Funding
B.J.N. is supported by the BIDMC/Harvard Translational Research in Aging Training Program (T32 AG023480). D.A.S. is supported by the Glenn Foundation for Medical Research, the Juvenile Diabetes Research Foundation, and the National Institutes of Health.
Non-standard Abbreviations and Acronyms
- Akt
also known as protein kinase B
- AMPK
AMP-activated protein kinase
- BubR1
budding uninhibited by benzimidazoles related 1
- CLK-1
clock 1
- CR
calorie restriction
- CVD
cardiovascular disease
- eNOS
endothelial nitric oxide synthetase
- EP
electrophysiology
- Erk
extracellular signal-regulated kinase
- FOXO
forkhead transcription factors
- IGF-1
insulin-like growth factor-1
- LV
left ventricle
- MCLK1
mammalian clock 1
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- Nampt
nicotinamide phosphoribosyltransferase
- PI3K
phosphatidylinositol-3-kinase
- Pit-1
pituitary transcription factor 1
- PNC1
pyrazinamidase and nicotinamidase 1
- Prop-1
prophet of Pit-1
- ROS
reactive oxygen species
- SIR2
silent information regulator 2
- SIRT
sirtuin
- TOR
target of rapamycin
- TSC1/2
tuberous sclerosis complex 1/2
- UCP2
uncoupling protein 2
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
D.A.S. is a consultant and inventor on patents licensed to GSK, a company developing sirtuin-related medicines.
References
- 1.Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: benefits and challenges. Nat Rev Cardiol. 2011;8:13–28. doi: 10.1038/nrcardio.2010.162. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises, part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises, part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 5.Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 6.Fleg JL, O’Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 7.Schulman SP, Lakatta EG, Fleg JL, Lakatta L, Becker LC, Gerstenblith G. Age-related decline in left ventricular filling at rest and exercise. Am J Physiol. 1992;263:H1932–H1938. doi: 10.1152/ajpheart.1992.263.6.H1932. [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. the Framingham Heart Study. Ann Intern Med. 1988;108:7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 9.Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–1088. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 10.Izzo JL, Jr, Shykoff BE. Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med. 2001;2:29–34. [PubMed] [Google Scholar]
- 11.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Collins C, Tzima E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp Gerontol. 2011;46:185–188. doi: 10.1016/j.exger.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heffernan KS, Fahs CA, Ranadive SM, Patvardhan EA. L-arginine as a nutritional prophylaxis against vascular endothelial dysfunction with aging. J Cardiovasc Pharmacol Ther. 2010;15:17–23. doi: 10.1177/1074248409354599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000;87:540–542. doi: 10.1161/01.res.87.7.540. [DOI] [PubMed] [Google Scholar]
- 15.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H(2)O(2) Am J Physiol Heart Circ Physiol. 2009;297:H1087–H1095. doi: 10.1152/ajpheart.00356.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart: myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 17.Goldspink DF, Burniston JG, Tan LB. Cardiomyocyte death and the ageing and failing heart. Exp Physiol. 2003;88:447–458. doi: 10.1113/eph8802549. [DOI] [PubMed] [Google Scholar]
- 18.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 19.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczesny B, Bhakat KK, Mitra S, Boldogh I. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech Ageing Dev. 2004;125:755–765. doi: 10.1016/j.mad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Mah LJ, El-Osta A, Karagiannis TC. Gammah2ax as a molecular marker of aging and disease. Epigenetics. 2010;5:129–136. doi: 10.4161/epi.5.2.11080. [DOI] [PubMed] [Google Scholar]
- 22.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 23.Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- 24.Anversa P, Leri A, Kajstura J, Nadal-Ginard B. Myocyte growth and cardiac repair. J Mol Cell Cardiol. 2002;34:91–105. doi: 10.1006/jmcc.2001.1506. [DOI] [PubMed] [Google Scholar]
- 25.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y. Sinoatrial node dysfunction and early unexpected death of mice with a defect of Klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005 doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 27.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the life-span and upon the ultimate body size. J Nutr. 1935;10:63. [PubMed] [Google Scholar]
- 28.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swindell WR. Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev. 2012;11:254–270. doi: 10.1016/j.arr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–H1219. doi: 10.1152/ajpheart.00685.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen T, Nioi P, Pickett CB. The nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi M, Yamamoto M. Nrf2-keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Ishii T, Itoh K, Yamamoto M. Roles of nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5. [DOI] [PubMed] [Google Scholar]
- 38.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- 39.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein e-deficient mice. Mech Ageing Dev. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 41.Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263–271. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemi M, Keenan KP, McCoy C, Hoe CM, Soper KA, Ballam GC, van Zwieten MJ. The relative protective effects of moderate dietary restriction versus dietary modification on spontaneous cardiomyopathy in male Sprague-Dawley rats. Toxicol Pathol. 2000;28:285–296. doi: 10.1177/019262330002800208. [DOI] [PubMed] [Google Scholar]
- 43.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genom. 2005;23:343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 47.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Bostrom P, Wu J, Jedrychowski MP, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaeberlein M, McVey M, Guarente L. The sir2/3/4 complex and sir2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian sirt6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 52.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 54.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Xu W, McBurney MW, Longo VD. Sirt1 inhibition reduces igf-i/irs-2/ras/erk1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of sir2 overexpression on lifespan in C Elegans and drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lan F, Cacicedo JM, Ruderman N, Ido Y. Sirt1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in amp-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. Sirt1 promotes proliferation and prevents senescence through targeting lkb1 in primary porcine aortic endothelial cells. Circ Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
- 59.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing ucp2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J. The direct involvement of sirt1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh HS, McBurney M, Robbins PD. Sirt1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and depTOR. J Biol Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawashima T, Inuzuka Y, Okuda J, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Kawamoto A, Narazaki M, Matsuda T, Adachi S, Takemura G, Kita T, Kimura T, Shioi T. Constitutive sirt1 overexpression impairs mitochondria and reduces cardiac function in mice. J Mol Cell Cardiol. 2011;51:1026–1036. doi: 10.1016/j.yjmcc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 66.Li L, Gao P, Zhang H, Chen H, Zheng W, Lv X, Xu T, Wei Y, Liu D, Liang C. Sirt1 inhibits angiotensin II-induced vascular smooth muscle cell hypertrophy. Acta Biochim Biophys Sin (Shanghai) 2011;43:103–109. doi: 10.1093/abbs/gmq104. [DOI] [PubMed] [Google Scholar]
- 67.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase sirt1 promotes membrane localization and activation of akt and pdk1 during tumorigenesis and cardiac hypertrophy. Sci Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 68.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. Sirt1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guarani V, Deflorian G, Franco CA, et al. Acetylation-dependent regulation of endothelial notch signalling by the sirt1 deacetylase. Nature. 2011;473:234–238. doi: 10.1038/nature09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, Ouchi Y. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2054–2062. doi: 10.1161/ATVBAHA.110.216739. [DOI] [PubMed] [Google Scholar]
- 71.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C Elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 74.Guttridge DC. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:443–450. doi: 10.1097/01.mco.0000134364.61406.26. [DOI] [PubMed] [Google Scholar]
- 75.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF-1) and type 1 IGF receptor (IGF1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 76.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. Igf-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 78.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 79.Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. IGF-I alleviates diabetes-induced rhoa activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–R802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 80.Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- 81.Prele CM, Reichelt ME, Mutsaers SE, Davies M, Delbridge LM, Headrick JP, Rosenthal N, Bogoyevitch MA, Grounds MD. Insulin-like growth factor-1 overexpression in cardiomyocytes diminishes ex vivo heart functional recovery after acute ischemia. Cardiovasc Pathol. 2012;21:17–27. doi: 10.1016/j.carpath.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 82.Li Q, Yang X, Sreejayan N, Ren J. Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: role of oxidative stress. Rejuvenation Res. 2007;10:501–512. doi: 10.1089/rej.2007.0552. [DOI] [PubMed] [Google Scholar]
- 83.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 84.Sedding DG. Foxo transcription factors in oxidative stress response and ageing: a new fork on the way to longevity? Biol Chem. 2008;389:279–283. doi: 10.1515/BC.2008.033. [DOI] [PubMed] [Google Scholar]
- 85.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in foxo1 (fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 86.Hosaka T, Biggs WH, III, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (foxo) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by foxo transcription factors. Circ Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 88.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The foxo3a transcription factor regulates cardiac myocyte size downstream of akt signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levavasseur F, Miyadera H, Sirois J, Tremblay ML, Kita K, Shoubridge E, Hekimi S. Ubiquinone is necessary for mouse embryonic development but is not essential for mitochondrial respiration. J Biol Chem. 2001;276:46160–46164. doi: 10.1074/jbc.M108980200. [DOI] [PubMed] [Google Scholar]
- 91.Hardie DG. Amp-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brugarolas J, Kaelin WG., Jr Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 93.Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, Choi YK, Ha J. Effects of stimulation of amp-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:19102–19110. doi: 10.1074/jbc.M011579200. [DOI] [PubMed] [Google Scholar]
- 94.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and sirt1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fulco M, Sartorelli V. Comparing and contrasting the roles of Ampk and sirt1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. Amp-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through ampk- and cox-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu YN, Xiao H, Ma XW, Jiang SY, Xu M, Zhang YY. Metformin attenuates pressure overload-induced cardiac hypertrophy via ampk activation. Acta Pharmacol Sin. 2011;32:879–887. doi: 10.1038/aps.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang CX, Pan SN, Meng RS, Peng CQ, Xiong ZJ, Chen BL, Chen GQ, Yao FJ, Chen YL, Ma YD, Dong YG. Metformin attenuates ventricular hypertrophy by activating the amp-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin Exp Pharmacol Physiol. 2011;38:55–62. doi: 10.1111/j.1440-1681.2010.05461.x. [DOI] [PubMed] [Google Scholar]
- 100.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the gamma(2) subunit of amp-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 101.Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, Roberts R. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 102.Li J, Coven DL, Miller EJ, Hu X, Young ME, Carling D, Sinusas AJ, Young LH. Activation of ampk alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol Heart Circ Physiol. 2006;291:H1927–H1934. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- 103.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. Amp-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci PG. Not all shc’s roads lead to ras. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 105.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 106.Cosentino F, Francia P, Camici GG, Pelicci PG, Luscher TF, Volpe M. Final common molecular pathways of aging and cardiovascular disease: role of the p66shc protein. Arterioscler Thromb Vasc Biol. 2008;28:622–628. doi: 10.1161/ATVBAHA.107.156059. [DOI] [PubMed] [Google Scholar]
- 107.Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, Quaini F, Padura IM, Lanfrancone L, Pelicci P, Emanueli C. Genetic deletion of the p66shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005;46:433–440. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- 108.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 110.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, II, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kerr J, Wood W, Ridgway EC. Basic science and clinical research advances in the pituitary transcription factors: pit-1 and prop-1. Curr Opin Endocrinol Diabetes Obes. 2008;15:359–363. doi: 10.1097/MED.0b013e3283060a56. [DOI] [PubMed] [Google Scholar]
- 113.Pfaffle RW, Blankenstein O, Wuller S, Kentrup H. Combined pituitary hormone deficiency: role of pit-1 and prop-1. Acta Paediatr Suppl. 1999;88:33–41. doi: 10.1111/j.1651-2227.1999.tb14401.x. [DOI] [PubMed] [Google Scholar]
- 114.Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci. 2010;6:475–490. doi: 10.7150/ijbs.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 116.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuro-o M. A potential link between phosphate and aging: lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270–275. doi: 10.1016/j.mad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 119.Kaeberlein M, Powers RW, III, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 120.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C Elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 122.Medvedik O, Lamming DW, Kim KD, Sinclair DA. Msn2 and msn4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 124.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 125.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/pten/akt/frap pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 126.Karar J, Maity A. Pi3k/akt/mtor pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Halapas A, Armakolas A, Koutsilieris M. Autophagy: a target for therapeutic interventions in myocardial pathophysiology. Expert Opin Ther Targets. 2008;12:1509–1522. doi: 10.1517/14728220802555554. [DOI] [PubMed] [Google Scholar]
- 128.Bolanos-Garcia VM, Blundell TL. Bub1 and bubr1: multifaceted kinases of the cell cycle. Trends Biochem Sci. 2011;36:141–150. doi: 10.1016/j.tibs.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Q, Liu T, Fang Y, Xie S, Huang X, Mahmood R, Ramaswamy G, Sakamoto KM, Darzynkiewicz Z, Xu M, Dai W. Bubr1 deficiency results in abnormal megakaryopoiesis. Blood. 2004;103:1278–1285. doi: 10.1182/blood-2003-06-2158. [DOI] [PubMed] [Google Scholar]
- 130.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. Bubr1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 131.Matsumoto T, Baker DJ, d’Uscio LV, Mozammel G, Katusic ZS, van Deursen JM. Aging-associated vascular phenotype in mutant mice with low levels of bubr1. Stroke. 2007;38:1050–1056. doi: 10.1161/01.STR.0000257967.86132.01. [DOI] [PubMed] [Google Scholar]
- 132.Chu CT. A pivotal role for pink1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum Mol Genet. 2010;19:R28–R37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 135.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greer EL, Brunet A. Foxo transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 137.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowledge Environ. 2004;2004:pe7. doi: 10.1126/sageke.2004.7.pe7. [DOI] [PubMed] [Google Scholar]