Abstract
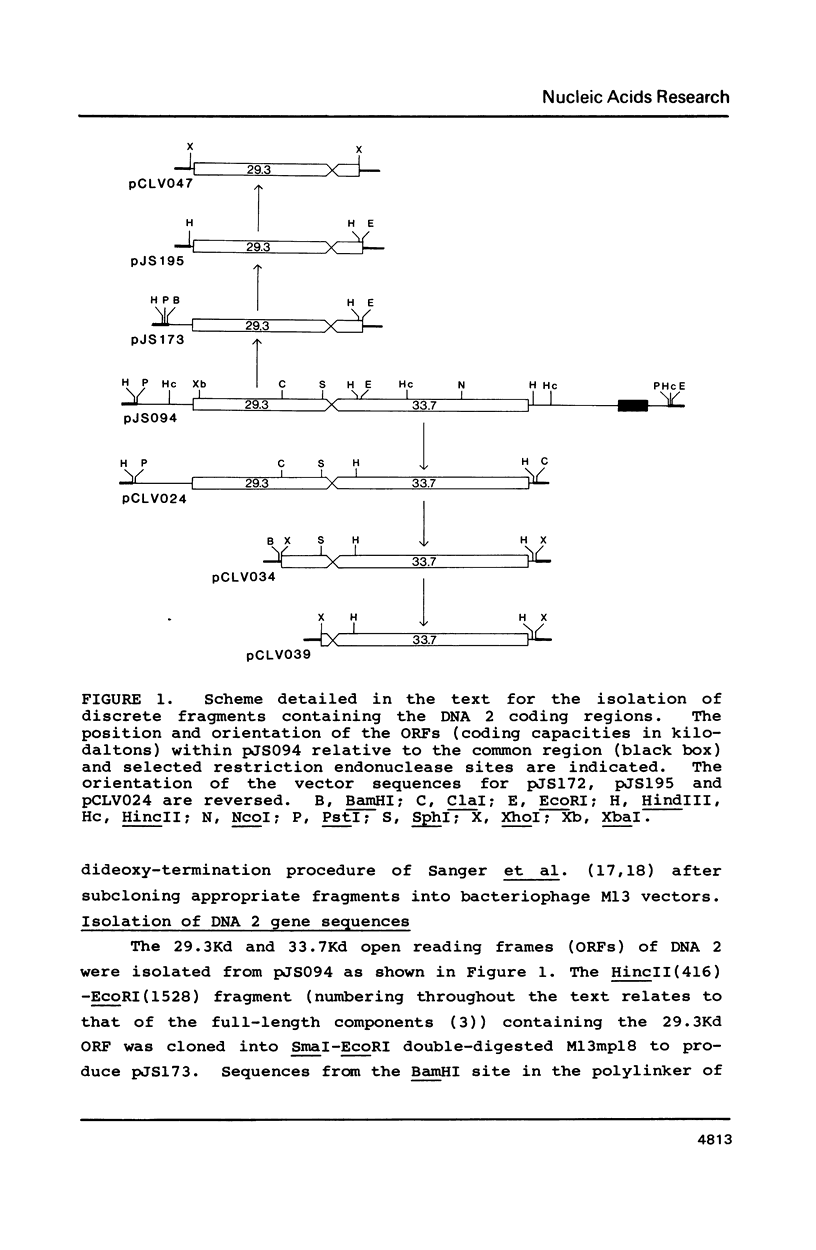
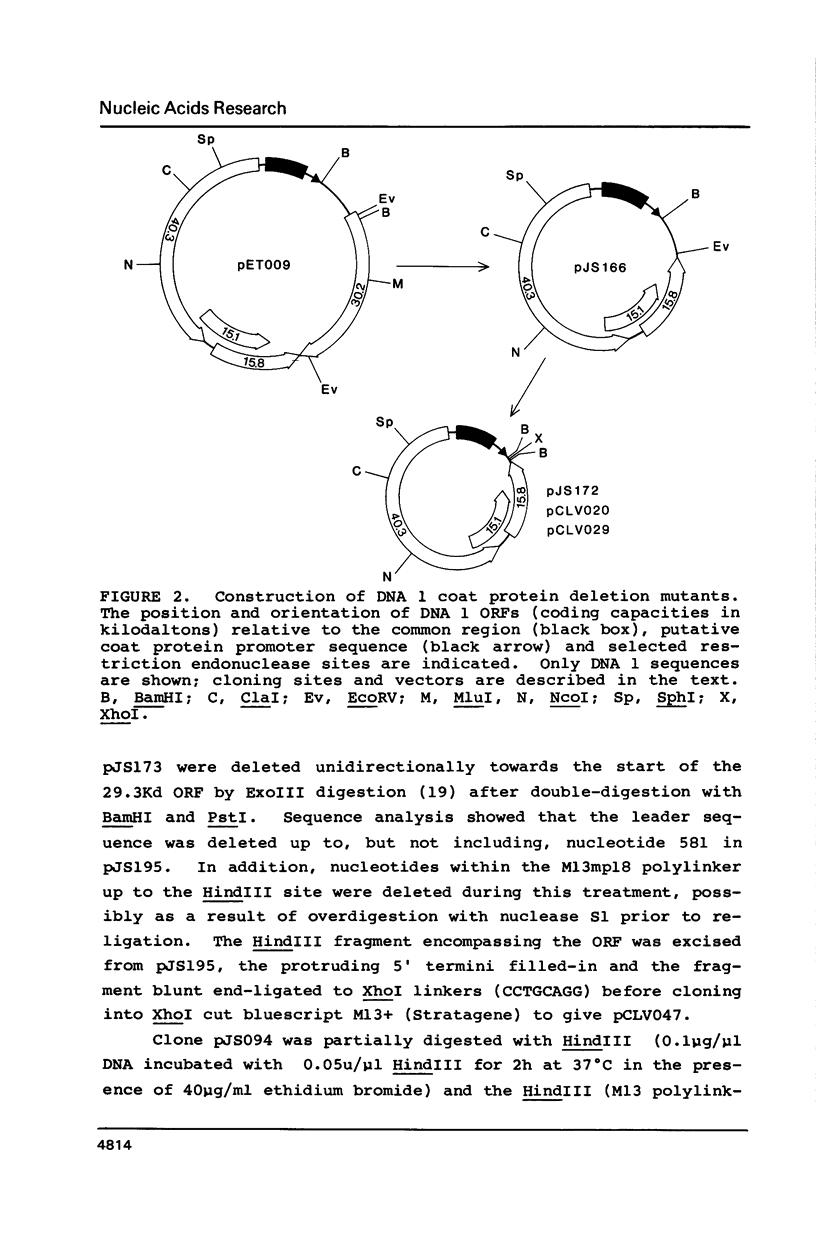
Insertion and deletion mutagenesis of both extended open reading frames (ORFs) of cassava latent virus DNA 2 destroys infectivity. Infectivity is restored by coinoculating constructs that contain single mutations within different ORFs. Although frequent intermolecular recombination produces dominant parental-type virus, mutants can be retained within the virus population indicating that they are competent for replication and suggesting that rescue can occur by complementation of trans acting gene products. By cloning specific fragments into DNA 1 coat protein deletion vectors we have delimited the DNA 2 coding regions and provide substantive evidence that both are essential for virus infection. Although a DNA 2 component is unique to whitefly-transmitted geminiviruses, the results demonstrate that neither coding region is involved solely in insect transmission. The requirement for a bipartite genome for whitefly-transmitted geminiviruses is discussed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atabekov J. G., Dorokhov YuL Plant virus-specific transport function and resistance of plants to viruses. Adv Virus Res. 1984;29:313–364. doi: 10.1016/s0065-3527(08)60412-1. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Donson J., Accotto G. P., Boulton M. I., Mullineaux P. M., Davies J. W. The nucleotide sequence of a geminivirus from Digitaria sanguinalis. Virology. 1987 Nov;161(1):160–169. doi: 10.1016/0042-6822(87)90182-6. [DOI] [PubMed] [Google Scholar]
- Donson J., Gunn H. V., Woolston C. J., Pinner M. S., Boulton M. I., Mullineaux P. M., Davies J. W. Agrobacterium-mediated infectivity of cloned digitaria streak virus DNA. Virology. 1988 Jan;162(1):248–250. doi: 10.1016/0042-6822(88)90416-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Coutts R. H., Buck K. W. Demonstration of the bipartite nature of the genome of a single-stranded DNA plant virus by infection with the cloned DNA components. Nucleic Acids Res. 1983 Nov 11;11(21):7387–7396. doi: 10.1093/nar/11.21.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kikuno R., Toh H., Hayashida H., Miyata T. Sequence similarity between putative gene products of geminiviral DNAs. Nature. 1984 Apr 5;308(5959):562–562. doi: 10.1038/308562a0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G. Infectivity and complete nucleotide sequence of the genome of a South African isolate of maize streak virus. Nucleic Acids Res. 1988 Jan 11;16(1):229–249. doi: 10.1093/nar/16.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDowell S. W., Macdonald H., Hamilton W. D., Coutts R. H., Buck K. W. The nucleotide sequence of cloned wheat dwarf virus DNA. EMBO J. 1985 Sep;4(9):2173–2180. doi: 10.1002/j.1460-2075.1985.tb03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P. M., Donson J., Morris-Krsinich B. A., Boulton M. I., Davies J. W. The nucleotide sequence of maize streak virus DNA. EMBO J. 1984 Dec 20;3(13):3063–3068. doi: 10.1002/j.1460-2075.1984.tb02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley J., Markham P. G., Callis R. J., Pinner M. S. The nucleotide sequence of an infectious clone of the geminivirus beet curly top virus. EMBO J. 1986 Aug;5(8):1761–1767. doi: 10.1002/j.1460-2075.1986.tb04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. The molecular biology of geminiviruses. Adv Virus Res. 1985;30:139–177. doi: 10.1016/s0065-3527(08)60450-9. [DOI] [PubMed] [Google Scholar]
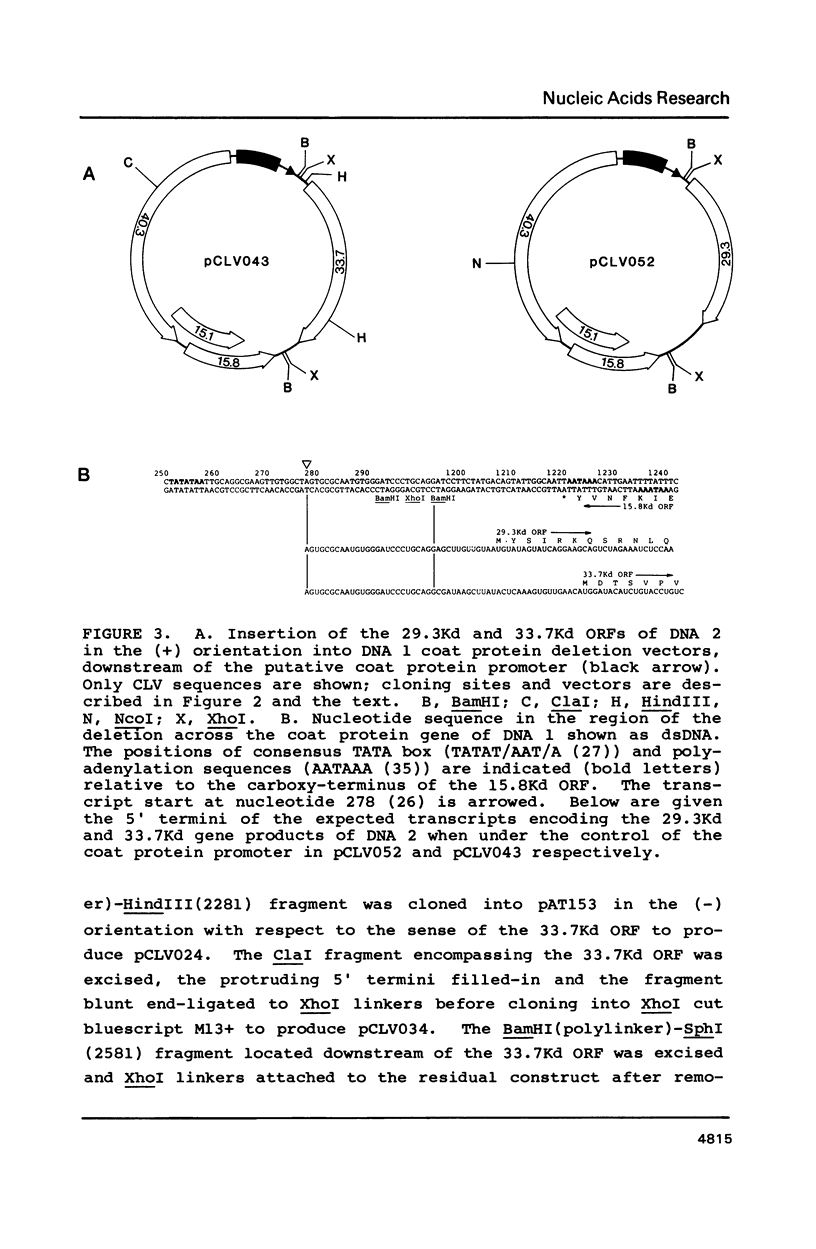
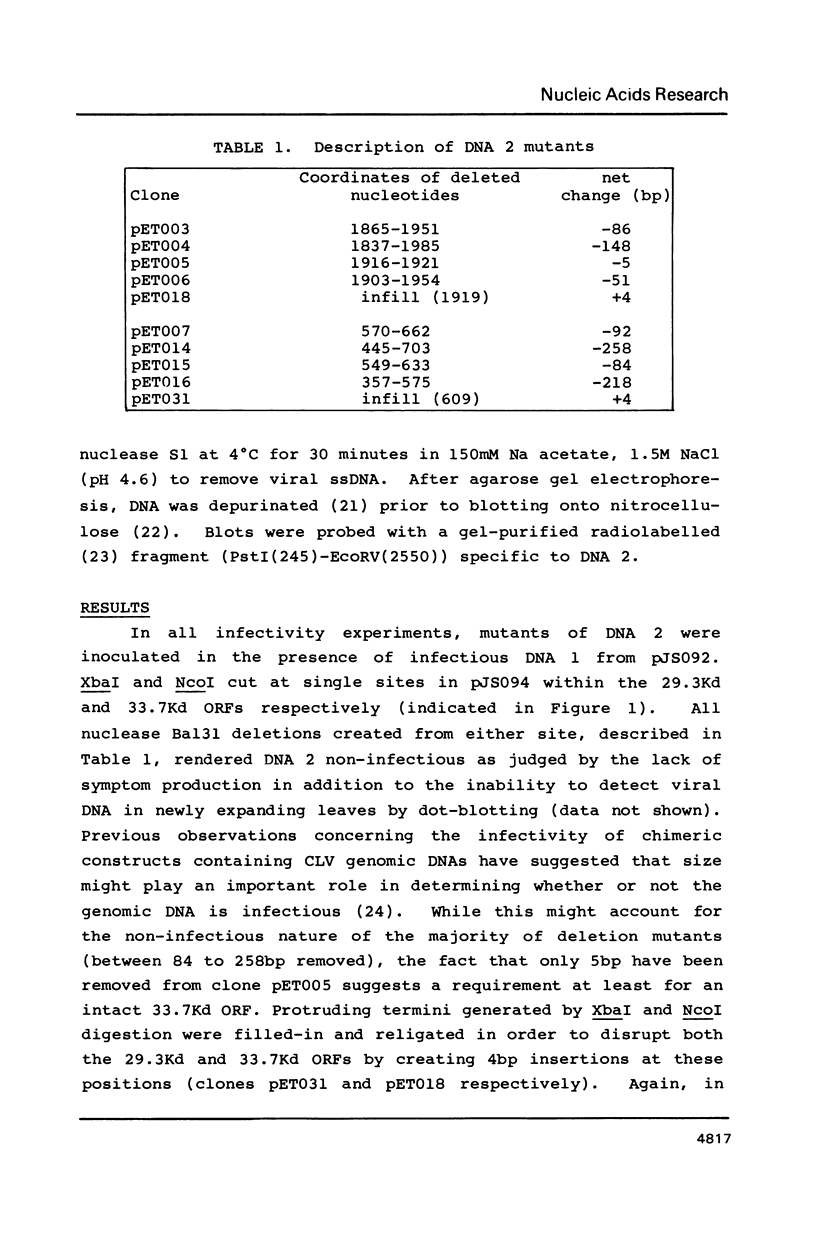
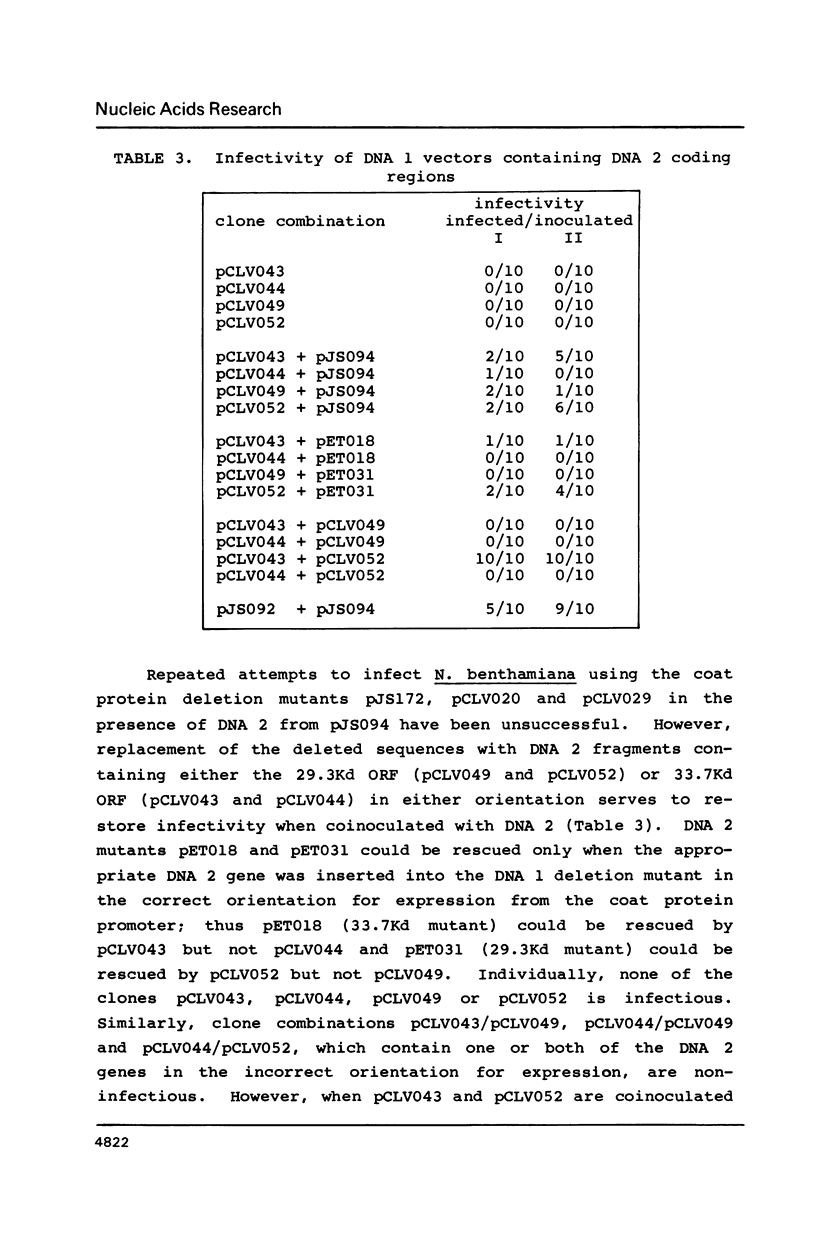
- Stanley J., Townsend R. Infectious mutants of cassava latent virus generated in vivo from intact recombinant DNA clones containing single copies of the genome. Nucleic Acids Res. 1986 Aug 11;14(15):5981–5998. doi: 10.1093/nar/14.15.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Stanley J., Curson S. J., Short M. N. Major polyadenylated transcripts of cassava latent virus and location of the gene encoding coat protein. EMBO J. 1985 Jan;4(1):33–37. doi: 10.1002/j.1460-2075.1985.tb02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Watts J., Stanley J. Synthesis of viral DNA forms in Nicotiana plumbaginifolia protoplasts inoculated with cassava latent virus (CLV); evidence for the independent replication of one component of the CLV genome. Nucleic Acids Res. 1986 Feb 11;14(3):1253–1265. doi: 10.1093/nar/14.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]