Abstract
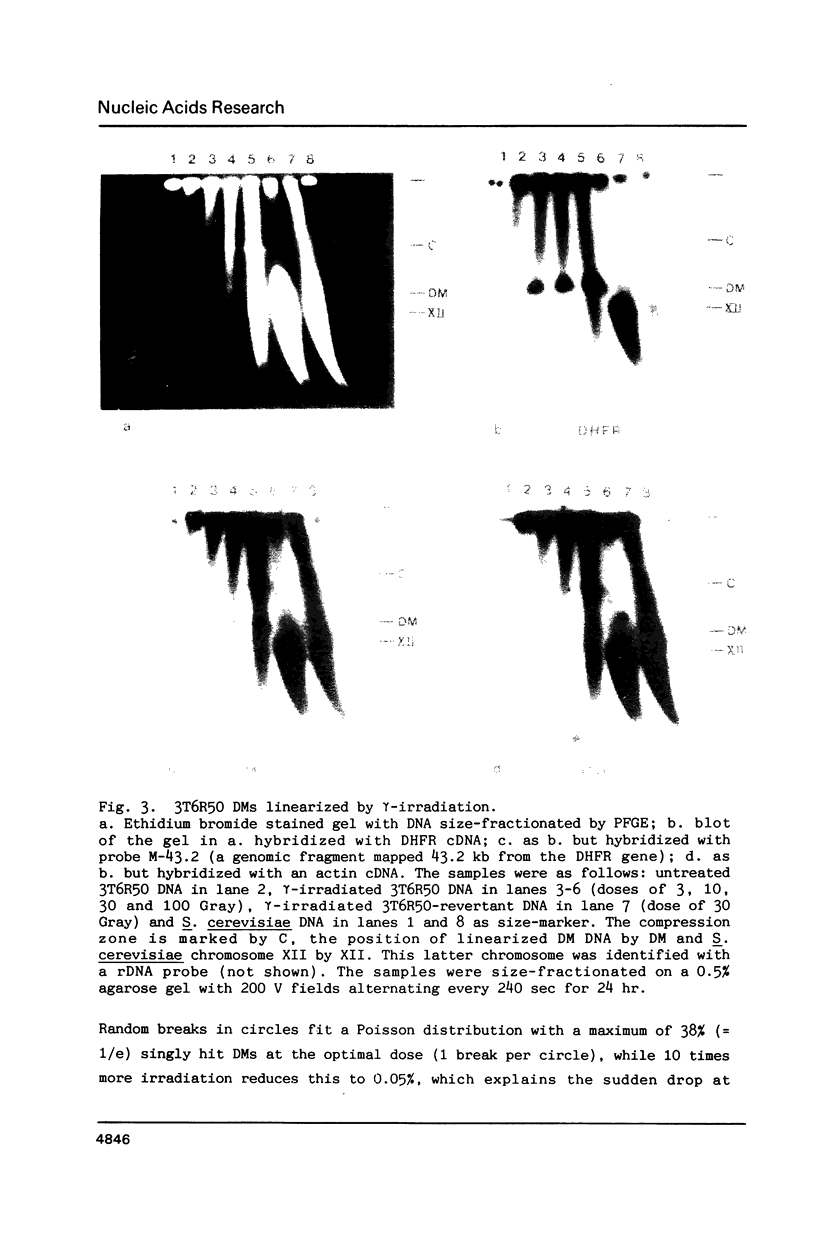
In pulsed field gradient gel electrophoresis (PFGE) the intact deproteinized circular DNA of Mycoplasma (800 kb) and Escherichia coli (4700 kb) remains trapped in the slot. We show here that gamma-irradiation of the DNA in agarose plugs is a convenient method to partially convert these circles into full-length linears, migrating with the expected mobility in PFGE. We have used this method to study the structure of Double Minute chromosomes (DMs) from the methotrexate (MTX)-resistant mouse cell line 3T6R50. Intact deproteinized DM DNA is immobile in these gels, but is converted into a single band of about 2500 kb by either gamma-irradiation, DNaseI in the presence of Mn2+, or restriction enzymes. We conclude that the DM DNA in 3T6R50 cells consists of a homogeneous population of 2500-kb circles.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Kooter J. M., Michels P. A., Moberts R. M., Borst P. Pulsed field gradient electrophoresis of DNA digested in agarose allows the sizing of the large duplication unit of a surface antigen gene in trypanosomes. Gene. 1986;42(3):313–322. doi: 10.1016/0378-1119(86)90235-0. [DOI] [PubMed] [Google Scholar]
- Blok J., Loman H. The effects of gamma-radiation in DNA. Curr Top Radiat Res Q. 1973 Dec;9(2):165–245. [PubMed] [Google Scholar]
- Bode H. R., Morowitz H. J. Size and structure of the Mycoplasma hominis H39 chromosome. J Mol Biol. 1967 Jan 28;23(2):191–199. doi: 10.1016/s0022-2836(67)80026-3. [DOI] [PubMed] [Google Scholar]
- Borst P., Van der Bliek A. M., Van der Velde-Koerts T., Hes E. Structure of amplified DNA, analyzed by pulsed field gradient gel electrophoresis. J Cell Biochem. 1987 Aug;34(4):247–258. doi: 10.1002/jcb.240340404. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Beverley S. M., Schimke R. T. Relationship of amplified dihydrofolate reductase genes to double minute chromosomes in unstably resistant mouse fibroblast cell lines. Mol Cell Biol. 1981 Dec;1(12):1077–1083. doi: 10.1128/mcb.1.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Gaudray P., De Rose M. L., Emery J. F., Meinkoth J. L., Nakkim E., Subler M., Von Hoff D. D., Wahl G. M. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987 May;7(5):1740–1750. doi: 10.1128/mcb.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G., Zöller L., Matz B., Delius H., Speck P. T., Flügel R. M. Analysis of Mycoplasma hyorhinis genome by use of restriction endonucleases and by electron microscopy. J Bacteriol. 1982 May;150(2):788–794. doi: 10.1128/jb.150.2.788-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodemont H. J., Soriano P., Quax W. J., Ramaekers F., Lenstra J. A., Groenen M. A., Bernardi G., Bloemendal H. The genes coding for the cytoskeletal proteins actin and vimentin in warm-blooded vertebrates. EMBO J. 1982;1(2):167–171. doi: 10.1002/j.1460-2075.1982.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. J., Borst P. Mapping of VSG genes on large expression-site chromosomes of Trypanosoma brucei separated by pulsed-field gradient electrophoresis. Gene. 1986;43(3):213–220. doi: 10.1016/0378-1119(86)90209-x. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Maurer B. J., Lai E., Hamkalo B. A., Hood L., Attardi G. Novel submicroscopic extrachromosomal elements containing amplified genes in human cells. Nature. 1987 Jun 4;327(6121):434–437. doi: 10.1038/327434a0. [DOI] [PubMed] [Google Scholar]
- Melgar E., Goldthwait D. A. Deoxyribonucleic acid nucleases. II. The effects of metals on the mechanism of action of deoxyribonuclease I. J Biol Chem. 1968 Sep 10;243(17):4409–4416. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Matsumoto T., Niwa O., Klco S., Fan J. B., Yanagida M., Cantor C. R. An electrophoretic karyotype for Schizosaccharomyces pombe by pulsed field gel electrophoresis. Nucleic Acids Res. 1987 Jun 11;15(11):4481–4489. doi: 10.1093/nar/15.11.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]