Abstract
Chlorpyrifos (CPF), one of the most widely-used organophosphorus (OP) insecticides in agriculture, is degraded in the field to its oxon form, chlorpyrifos-oxon (CPO), which can represent a significant contaminant in exposures to adults and children. CPO is also responsible for the acetylcholinesterase (AChE) inhibition associated with CPF exposures; CPF is converted by liver CYP450 enzymes to CPO, which binds to and inhibits AChE and other serine active-site esterases, lipases and proteases. Young children represent a particularly susceptible population for exposure to CPF and CPO, in part because levels of the plasma enzyme, paraoxonase (PON1), which hydrolyzes CPO, are very low during early development. While a number of studies have demonstrated developmental neurotoxicity associated with CPF exposure, including effects at or below the threshold levels for AChE inhibition, it is unclear whether these effects were due directly to CPF or to its active metabolite, CPO. PON1 knockout (PON1−/−) mice, which lack PON1, represent a highly sensitive mouse model for toxicity associated with exposure to CPF or CPO. To examine the neurobehavioral consequences of CPO exposure during postnatal development, PON1−/− mice were exposed daily from PND 4 to PND 21 to CPO at 0.15, 0.18, or 0.25 mg/kg/d. A neurobehavioral test battery did not reveal significant effects of CPO on early reflex development, motor coordination, pre-pulse inhibition of startle, startle amplitude, open field behavior, or learning and memory in the contextual fear conditioning, Morris water maze, or water radial-arm maze tests. However, body weight gain and startle latency were significantly affected by exposure to 0.25 mg/kg/d CPO. Additionally, from PNDs 15–20 the mice exposed repeatedly to CPO at all three doses exhibited a dose-related transient hyperkinesis in the 20-min period following CPO administration, suggesting possible effects on catecholaminergic neurotransmission. Our previous study demonstrated wide-ranging effects of neonatal CPO exposure on gene expression in the brain and on brain AChE inhibition, and modulation of both of these effects by the PON1Q192R polymorphism. The current study indicates that the neurobehavioral consequences of these effects are more elusive, and suggests that alternative neurobehavioral tests might be warranted, such as tests of social interactions, age-dependent effects on learning and memory, or tests designed specifically to assess dopaminergic or noradrenergic function.
Keywords: Chlorpyrifos, Chlorpyrifos-oxon, Organophosphorus insecticides, Behavior, Paraoxonase
1. Introduction
The organophosphorus (OP) compound chlorpyrifos (CPF) is one of the most extensively used insecticides in the United States, with about ten million pounds applied annually to a variety of food and feed crops, to golf courses, as a nonstructural wood treatment and as a mosquitocide (Eaton et al., 2008). OP compounds were originally developed as nerve poisons and can be highly toxic, with a broad range of effects that include cancer, reproductive dysfunction, lung disease and neurobehavioral deficits (Eaton et al., 2008; Wheeler, 2002). CPF and other OP compounds act primarily through inhibition of acetylcholinesterase (AChE), leading to a buildup of acetylcholine and overstimulation at cholinergic synapses. Once absorbed into the bloodstream, metabolism of CPF occurs primarily in the liver by cytochrome P450 enzymes that oxidatively desulfurate CPF to the active metabolite chlorpyrifos-oxon (CPO), which then binds covalently to AChE and inhibits its enzymatic activity. Detoxification of CPO in the liver and blood occurs through the action of paraoxonase (PON1), which hydrolyzes CPO to form diethyl phosphate and 3,5,6-trichloropyridinol, and by carboxylesterases and other serine active-site enzymes, which bind CPO stoichiometrically (Chambers et al., 1990; Cochran et al., 1995; Shenouda et al., 2009).
PON1 levels vary by at least 15-fold among adults and 26-fold among infants (Furlong et al., 2006). The common PON1Q192R polymorphism affects the catalytic efficiency of hydrolysis of some substrates, including CPO, with PON1R192 hydrolyzing CPO more efficiently than PON1Q192 (Furlong, 2008; Li et al., 2000). Humanized transgenic mice expressing the PON1Q192 alloform (tgHuPON1Q192) are more sensitive to CPF and CPO than mice expressing the human PON1R192 alloform (tgHuPON1R192) (Cole et al., 2003, 2005), and are also more sensitive to the interactive toxicity of mixtures of OP compounds that include CPO (Cole et al., 2010; Jansen et al., 2009). PON1 knockout (PON1−/−) mice are dramatically more sensitive than wild-type (PON1+/+) mice to the toxicity of CPO and diazoxon (DZO) (Furlong, 2008; Li et al., 2000; Shih et al., 1998), and thus represent a particularly sensitive model for assessing the toxicity of these compounds. The oxon forms of CPF and diazinon can represent a significant component of exposures, due to their environmental generation in the field (Cal-EPA, 1998; Fenske et al., 2009; Vidal et al., 1998; Yuknavage et al., 1997) and their generation in the liver following exposures to CPF or diazinon.
Recent evidence suggests that exposure to pesticides is common in children, particularly for those who live in farming and ranching communities (Eskenazi et al., 1999, 2008). The primary route of exposure is through dietary ingestion, although incidental ingestion of household dust also constitutes a relevant route of exposure (Cohen Hubal et al., 2006; Egeghy et al., 2005), and CPF can be absorbed dermally (Eaton et al., 2008). Children, who often receive higher exposures to environmental chemicals (Faustman et al., 2000; Ginsberg et al., 2004a,b), represent a particularly susceptible population for pesticide exposure because they weigh less, breathe more rapidly, and often play outdoors, close to the ground, with frequent hand-to-mouth activity (Fenske et al., 2005). Additionally, plasma PON1 levels are 3–4 folds lower in young children than in adults (Furlong et al., 2006) and do not reach adult levels until two to seven years of age (Cole et al., 2003; Huen et al., 2009).
While a number of studies in humans (Eskenazi et al., 2007, 2008; Perera et al., 2006; Rauh et al., 2006; Whyatt et al., 2004; Young et al., 2005), rats (Aldridge et al., 2005; Betancourt and Carr, 2004; Betancourt et al., 2006; Chanda and Pope, 1996; Dam et al., 2000; Icenogle et al., 2004; Lassiter and Brimijoin, 2008; Levin et al., 2002; Slotkin and Seidler, 2007), mice (Venerosi et al., 2009, 2010) and cultured cells (Jameson et al., 2007; Schuh et al., 2002; Slotkin and Seidler, 2009) demonstrate developmental neurotoxicity associated with CPF exposure, it is unclear whether these effects are due to CPF or to its active metabolite, CPO. In contrast to CPF, studies on the effects of CPO have been sparse. CPO binds to and inhibits muscarinic receptors in rat striatum (Huff et al., 1994) and inhibits their phosphorylation and internalization in cultured human cells (Udarbe Zamora et al., 2008). Betancourt and Carr (2004) showed a reduction in mAChR density and NGF levels following exposure of neonatal rats to CPF, whereas rats exposed to CPO did not show these changes. In human sperm, in vitro CPO exposure is associated with greater DNA damage and chromatin alteration than was exposure to CPF (Salazar-Arredondo et al., 2008), and the PON1Q192R polymorphism has been shown to modulate pesticide effects on semen quality and DNA integrity (Perez-Herrera et al., 2008).
To examine the neurobehavioral consequences of CPO exposure during postnatal development, PON1−/− mice were exposed daily from postnatal day (PND) 4 to PND 21 to relatively low levels (0.15, 0.18, or 0.25 mg/kg/d) of CPO, followed by assessment of reflexes, motor coordination, learning and memory. This period corresponds roughly to the period of human neurodevelopment from late gestation to the first one to two years after birth (Daston et al., 2004; Rodier, 1977; Rice and Barone, 2000). PON1−/− mice are dramatically more sensitive than wild-type mice to toxicity associated with CPO exposure (Cole et al., 2005; Li et al., 2000). As such, they are relevant to the vulnerabilities of individuals with the low CPOase activity allele (Q192) of PON1, as well as the very low levels of PON1 in neonates. We report here a neurobehavioral assessment of PON1−/− mice exposed to low levels of CPO repeatedly throughout early postnatal development, as part of a larger study examining multiple endpoints of toxicity (Cole et al., 2011).
2. Materials and methods
2.1. Animals
PON1 knockout (PON1−/−) mice were generated as described (Cole et al., 2003, 2005; Shih et al., 1998), on a congenic (backcrossed B6.129) strain background. Absence of PON1 activity in heparinized saphenous-vein plasma was confirmed by measuring arylesterase or diazoxonase activity, as described (Cole et al., 2003, 2005; Stevens et al., 2008). Mice were housed in a centralized, AAALAC-accredited, Specific Pathogen Free facility at the University of Washington, and were maintained in a 12-hour light–dark cycle with unlimited access to food and water. Neurobehavioral testing occurred during the light cycle. All experiments were approved by the University of Washington Institutional Animal Care and Use Committee and carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health.
2.2. Exposure of mice to chlorpyrifos-oxon
The experiments reported here were part of a larger study that examined other endpoints of toxicity in eight separate cohorts of mice with different PON1 genotypes, including PON1−/−, PON1+/+, tgHuPON1Q192, and tgHuPON1R192 mice (Cole et al., 2011). Neurobehavioral assessments were conducted in PON1−/− mice from two of these cohorts, consisting of a total of 44 PON1−/− litters (11 litters per treatment group for each of 4 treatment groups). Upon detection of a copulatory plug, females were housed five per cage until visibly pregnant, at which time they were housed individually. On PND 3, gender was determined by comparison of urogenital-anal distance and prescrotal pigmentation, and individual animals within each litter were identified by paw tattoo. On PND 4, litters were culled to six mice (3 males and 3 females). For neurobehavioral assessment of PON1−/− mice, litters were assigned randomly to one of four treatment groups: DMSO vehicle control, 0.15 mg/kg/d CPO, 0.18 mg/kg/d CPO, or 0.25 mg/kg/d CPO. All mice within a litter received the same dose of CPO. These doses (0.15, 0.18, and 0.25 mg/kg/d) were based on previous dose finding studies in PON1−/− mice that resulted in 10, 20 and 40 percent inhibition of brain AChE, respectively, on PND 4. Repeated daily exposure of PON1−/− mice to these doses from PNDs 4 to 21 resulted in inhibition of brain AChE on PND 22 by 9.7±3.7%, 16.4±2.7%, and 27.3±6.8%, respectively, in the litter-mates of the mice used for behavioral assessment (Cole et al., 2011). Mice were injected subcutaneously (SQ), as the closest approximation to a dermal exposure, which was not itself feasible due to likely grooming of the pups by the dam. Mice were injected SQ with CPO daily from PND 4 to PND 21, between the hours of 1200 and 1600, using a sterile Hamilton syringe (1 μL/g body weight). A 1 mg/ml stock solution of CPO (98% purity; Chem Service, West Chester, PA) in DMSO was used to make dilutions for injections.
One male and one female from each litter were assigned randomly to “Neurobehavioral Assessment Group A” and one male and one female were assigned to “Neurobehavioral Assessment Group B”, with the remaining two mice (one male and one female) used for measurement of brain AChE activity and/or measurement of gene expression, as described in a separate publication (Cole et al., 2011). On PND 22, mice to be used for gene expression assessment or AChE activity measurement were euthanized by CO2 asphyxiation and remaining mice were ear-tagged, transferred to the neurobehavioral testing facility and housed two per cage for the duration of testing.
2.3. Neurobehavioral assessment
Mice were monitored daily during the pre-weaning period for the appearance of developmental landmarks and tested for reflex righting, cliff avoidance, and negative geotaxis as detailed below and summarized in Supplementary Table 1. Mice in Neurobehavioral Assessment Group A underwent a behavioral test battery consisting of the rotarod (PND 23), startle/pre-pulse inhibition (PND 25 and PND 50), contextual and cued fear conditioning (PNDs 62–64) and the water radial-arm maze (PNDs 77–116). Mice in Neurobehavioral Assessment Group B underwent a behavioral test battery consisting of open field testing (PND 25) and the probe trial variant of the Morris Water Maze (PNDs 70–98), and also served as the no-shock controls for contextual and cued fear conditioning (PNDs 62–64).
2.3.1. Daily observations
Each pup was observed daily for the appearance of developmental landmarks and general health and behavior. Weights were measured daily for each mouse from PND 3 to PND 80. Starting on PND 3, the appearance of pigmentation, bi-lateral ear detachment, hair growth and eye opening were recorded for each pup. Ear detachment was recorded on the day both pinnae were fully detached. Eye opening was noted on the day that both eyes were visible, even if partially open. Pups were observed for general health and behavior both at the time of behavior testing and immediately after dosing to ensure that there were no overt signs of cholinergic toxicity, such as tremors or difficulty breathing.
2.3.2. Pre-weaning tests
Pups were tested for reflex development and neuromotor ability using tests of reflex righting, cliff avoidance and negative geotaxis (Adams, 1986; Moser, 1999). Reflex testing began after 0800 each morning and was concluded at least one hour before dosing to minimize any possible acute effects of CPO on reflexes. Beginning on PND 4, pups were tested daily for reflex righting. Pups were placed in a supine position and the time to right onto all four paws was measured. This test continued, with one trial per day, until the mice met the criterion of a 3-second latency or less on two consecutive days. On PND 6, cliff avoidance was measured using a flat plexiglass surface raised to a height of 23 cm above the lab bench. Each pup was placed with front paws and snout over the edge, and the time to turn 180° away from the cliff face was recorded. Cliff avoidance was measured daily until a latency of 6 s or less was met for two consecutive days. On PND 7, negative geotaxis was measured. Pups were placed head-downward on a 30° mesh incline and the time to turn 180° to face upward was measured. Mice were tested daily until a latency of 6 s or less was reached for two consecutive days. For all reflex tests, if mice did not complete the task within 30 s, the test was terminated and the mice were returned to their home cages. The order of reflex testing for each day consisted of cliff avoidance, followed by negative geotaxis, then reflex righting, with a rest period of at least 15 min between tests. Transient peak-dose hyperkinesis was scored daily from PND 12 until PND 21 as present or absent for each litter. The individual performing the assessments was blind to the treatment groups.
2.3.3. Post-weaning tests: startle, pre-pulse inhibition, rotarod
Auditory startle and pre-pulse inhibition of startle were tested on PND 25 and PND 50 using an automated auditory startle chamber (San Diego Instruments). During a 15-minute test session, mice were placed in the startle chamber and presented with 30 stimuli at randomized intervals. The stimuli consisted of a 120 dB tone, a 120 dB tone preceded by a 70 dB pre-pulse, or a “null” stimulus involving no tone. Each type of stimulus was presented 10 times. The order of stimulus presentation was first determined using a random number table, then all mice received the stimuli in the same order. The startle chambers used a piezoelectric sensor to measure the maximum amplitude (Vmax) of the startle response after each stimulus and the latency to the maximum startle response (Tmax). Pre-pulse inhibition of startle was calculated as the percent inhibition of the auditory startle response by the 70 dB pre-pulse, after first subtracting the startle response to the null stimulus (Crawley and Paylor, 1997; Crawley et al., 1997; Logue et al., 1997).
On PND 23 a rotarod (Coulbourn Instruments) was used to test motor coordination and cerebellar learning (Altman and Sudarshan, 1975; Moser, 1999). Mice were placed on the rotarod cylinder, which accelerated at 5 rpm/min from a baseline rate of 3 rpm, and latency to fall off of the cylinder was recorded for each of four successive trials, with a 5-minute inter-trial interval.
2.3.4. Post-weaning tests: open field behavior
An open field was used to measure activity on PND 25. Open-field testing consisted of monitoring the patterns of activity over a 15-minute period when mice were placed in an empty square enclosure 46 cm across. A Polytrack animal tracking system (San Diego Instruments) was used to measure total distance traveled and dwell time spent in the 23-cm center area versus the 11.5-cm perimeter of the open field.
2.3.5. Post-weaning tests of learning and memory
Cued and contextual fear conditioning (Cole et al., 2001; Crawley, 1999; Crawley and Paylor, 1997; Crawley et al., 1997) was evaluated on PNDs 62–64. Mice in Neurobehavioral Assessment Group B undergoing cued and contextual fear conditioning were given three tone-shock pairs over 6 min. Mice in Neurobehavioral Assessment Group A served as controls, and were treated identically as the test mice except that they did not receive any foot shocks. On the first day, test mice were trained to associate the presentation of a tone with a mild shock. Mice were taken individually into the test room and placed into one chamber of a shuttle box (Coulbourn Instruments), with the door closed. The tester faced away from the mouse, and every 6 s turned to score the presence or absence of movement. This method of scoring made assessing movements more objective, in contrast to a constant observation of the animal’s behavior throughout the test session. If mice were not moving, with the exception of movements due to respiration, they were given a score of 0. If mice had any movements beyond those of respiration, the mouse was given a score of 1. This method of scoring continued every 6 s throughout the 6-minute testing period. Two minutes into testing, the first tone was presented for 30 s. During the last second of the tone, the first shock was given. The next tone–shock pair was presented 3.5 min into testing, and the final tone–shock pair was presented 5 min into testing. Fear conditioning acquisition was analyzed using the percent of time spent freezing for each minute of the 6-minute test period. The following day, mice were assessed for contextual fear response. Mice were taken individually back into the test room, placed into the shuttle box, and movement was scored every 6 s as above. During the 6 min of testing, no tones or foot shocks were presented. Contextual fear conditioning was analyzed using the percent of time spent freezing for each of the 6 min of the test. Cued fear conditioning was tested on the third day. Mice were taken into a different testing room and placed into a novel environment (a rat cage with novel bedding). Two drops of peppermint extract were used in the bedding to increase the novelty of the environment. After 2 min, the tone was presented continuously for 5 min, and freezing behavior was scored as above. The data for cued fear conditioning are presented as the percent of freezing time for each minute of the 5-minute test session.
Learning and memory were tested in mice from Neurobehavioral Testing Group B using the Morris water maze (Morris, 1984; Voikar et al., 2001; Wenk, 1999), which began on PND 70 and was completed on PND 98. The maze consisted of a 165-gallon, circular galvanized stock tank, 122-cm in diameter and 61-cm tall, filled with room temperature water. A 10-cm square plexiglass stand was placed in the tank just below the water level to serve as the escape platform. The platform was placed in the same position every day. A Polytrack system (San Diego Instruments, San Diego, CA) was used to track the location of the mice in the maze. Stationary objects surrounding the tank were used as spatial cues. Mice were trained to acquire the task for 7 days, with 3 trials per day at 30-minute inter-trial intervals. On the first trial, mice were dropped randomly at one of the four drop locations, facing outward toward the edge of the tank, and allowed to swim for 60 s and become familiar with the tank. Mice were then guided to the escape platform, held on the platform for 30 s, then removed, dried off with a paper towel and placed under a heat lamp. On subsequent trials, mice were dropped into the tank and given 60 s to find the platform. For each trial, the latency to find the platform was recorded, as well as distance traveled and time spent in each of the 4 quadrants. Probe trials were performed at 1, 2, 3, and 4 weeks after the last training session. Mice were reintroduced to the tank with the platform removed and movements were recorded for a 2-minute period. Dwell time in each of the 4 quadrants of the maze was recorded, as well as average distance from the prior location of the platform and number of entries across the prior location of the platform.
Learning and memory in mice from Neurobehavioral Testing Group A were tested beginning on PND 77, using a water version of the 8 arm radial maze (Cole et al., 2001; Hyde et al., 1998), which requires mice to find 4 escape platforms in succession over a series of 4 daily trials. For each mouse, the start location for each trial and the platform locations to be used throughout testing were determined randomly. The start location changed with each trial. Each of the 4 escape platforms was placed at the end of an arm, just underneath the surface of the water. The platform locations remained the same over the entire testing period. Mice underwent a 12-day training period of 4 trials per day. On the first day, mice were given 2 min to find one of the 4 hidden platforms located at the end of 4 of the 8 arms. Mice were kept on the platform for 3 s, then removed, dried off, and placed under a heat lamp for the 90-second inter-trial interval. On the first day only, the arm of the platform that was found was blocked off to prevent the mouse from swimming into that same arm in the 3 subsequent trials, and allow the mouse to find the other 3 platforms. Beginning on the second day and continuing until the twelfth day of training, mice were again given a 2-minute period to locate an escape platform. If they were unable to locate the platform within 120 s, they were placed on the nearest one. After each of the 4 trials in each daily session, the discovered platform was removed, but the arms remained open. Entries into specific arms were recorded, as well as latency to find a platform. For each of the 12 days of testing, entries into specific arms were recorded and latency to find each of the 4 platforms was measured, as well as the number of working memory errors and reference memory errors. Working memory errors included entries or re-entries into platform arms for which the platform had already been found in a previous trial on that day. Reference memory errors included entries or re-entries into the non-platform arms (i.e., the arms that never contained a platform). After acquisition, retention was measured at 1, 2, 3, and 4 weeks after the training period, again with 4 trials each test day. The testing strategy during the retention sessions was identical to that during the training period.
2.3.6. Data analysis
Statistical significance was determined by analysis of variance (ANOVA) using SYSTAT software. ANOVA tests included dose, cohort, and gender as independent between-subjects variables, and age, trial or session as independent within-subjects variables for those tests involving repeated measures. A full-model ANOVA was used, including two-way and three-way interactions among dose, gender, and cohort, as well as age, trial or session for tests involving repeated measures. Repeated measures ANOVA was used for body weights and for the rotarod, contextual fear conditioning, Morris water maze, and radial-arm maze tests. Statistical significance is reported for dose and gender, as well as age, trial or session for tests involving repeated measures. Only effects observed over both cohorts are reported. For pre-weaning tests, the data for all pups within a litter were averaged. For postweaning tests, the data from 1 male and 1 female within a litter were used. Statistical significance of the dose effects of CPO on hyperkinesis for each day was determined using Fisher’s Exact Test. p-Values less than 0.05 were considered significant. All data are presented as mean±SE. Supplementary Table 2 lists the statistics for selected measures used in the study.
3. Results
3.1. Developmental landmarks, body weights and hyperkinesis
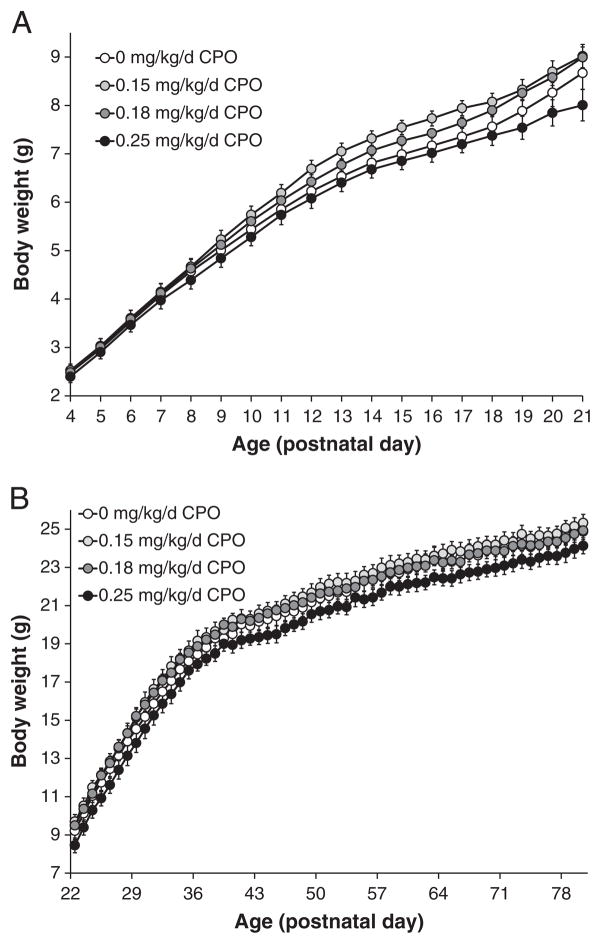
There were no overt toxic effects associated with repeated postnatal exposure to the low levels of CPO used in these studies, and mice developed apparently normally. The appearance of developmental landmarks was not different among dose groups (Suppl. Fig. 1). However, repeated exposure to CPO at 0.25 mg/kg/d was associated with reduced body weights (Fig. 1). Overall, there was a significant (p=0.029) effect of CPO dose on body weights from PNDs 4 to 21 (Fig. 1A), with a significant dose×age interaction (p<0.01), but no significant dose* gender or dose* age* gender interactions (Suppl. Table 2). On PND 21, body weights of mice exposed to 0.25 mg/kg/d CPO were decreased by 8% compared to controls (Fig. 1A). From PNDs 22 to 80, body weights continued to be affected following CPO exposure, with significant effects of dose (p=0.044) and gender (p<0.01), but no significant dose* age interaction (Fig. 1B; Suppl. Table 2). On PND 80, body weights of mice exposed to 0.25 mg/kg/d CPO were decreased by about 3% compared to controls (Fig. 1B).
Fig. 1. Body weights.
Body weights, recorded daily just prior to dosing, of PON1−/− mice (male and female) exposed to CPO at 0.15 mg/kg/d, 0.18 mg/kg/d, or 0.25 mg/kg/d, or to vehicle alone. A) Body weights during the dosing period (PNDs 4 to 21); B) body weights from PNDs 22 to 80, after completion of dosing. Values represent mean±SE.
Chronic CPO exposure also resulted in transient dose-related hyperkinesis on PND 15 through PND 20, 20-min following CPO administration (Fig. 2). Hyperkinetic activity (sudden rapid locomotor movements and/or jumping) was observed in pups dosed with CPO at 0.15 mg/kg/d, 0.18 mg/kg/d, and 0.25 mg/kg/d, beginning approximately 5 min after the injection, and lasting 10 to 15 min. No such behaviors were observed in any control mice. This transient hyperkinesis was first observed on PND 15, with the incidence increasing until PND 18 then decreasing until PND 21, when it was no longer detected (Fig. 2). There were significant effects (p<0.01; Fisher’s Exact Test) of CPO dose on the presence of the hyperkinetic activity on PNDs 16, 17, 18, and 19 (Fig. 2).
Fig. 2. Transient hyperkinesis.
The presence or absence of hyperkinesis in the 15-minute period following CPO administration was recorded for litters of PON1−/− mice exposed to CPO at 0.15 mg/kg/d, 0.18 mg/kg/d CPO, or 0.25 mg/kg/d, or to vehicle control. Hyperkinesis was not observed between PNDs 12 and 14 or on PND 21. Values represent the percentage of litters exhibiting hyperkinesis.
3.2. Reflexes
There were no significant effects of repeated CPO exposure on neonatal reflexes, as evaluated by measuring the righting reflex (Suppl. Fig. 2) (p=0.694), cliff avoidance (not shown) (p=0.623), and negative geotaxis (not shown) (p=0.798). Mice exposed repeatedly to CPO at 0.15 mg/kg/d, 0.18 mg/kg/d, or 0.25 mg/kg/d acquired the reflexes over a similar developmental time course as the control mice exposed to vehicle alone.
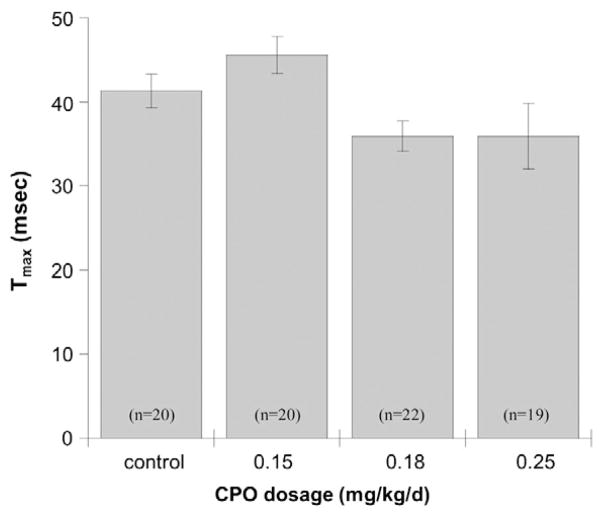
Auditory startle response and pre-pulse inhibition of the startle response were measured on PND 25 and again on PND 50. There were no statistically significant effects of CPO exposure at PND 25. However, on PND 50 there was a significant (p=0.020) reduction in the time to maximum startle amplitude (Tmax). Control mice had a 40-millisecond interval between presentation of the tone and the time of maximum startle amplitude, whereas the interval was reduced by about 5 ms in mice exposed to 0.18 mg/kg/d CPO or 0.25 mg/kg/d CPO (Fig. 3). Startle amplitude showed a tendency towards higher values in mice exposed to 0.18 mg/kg/d or 0.25 mg/kg/d CPO, but the effect was not significant (p=0.088). On PND 50 there was also a statistically significant (p=0.045) effect of dose on prepulse startle amplitude, but the effect was limited to the 0.18 mg/kg/d CPO exposure group, with no apparent effect at 0.25 mg/kg/d, and no effect on prepulse inhibition of startle (p=0.704).
Fig. 3. Auditory startle.
The latency (Tmax) to maximum startle amplitude following administration of the 120-dB auditory pulse was recorded on PND 50 for PON1−/− mice exposed to 0.15 mg/kg/d CPO, 0.18 mg/kg/d CPO, or 0.25 mg/kg/d CPO, or to vehicle alone. Values represent mean±SE.
3.3. Motor coordination and open field behavior
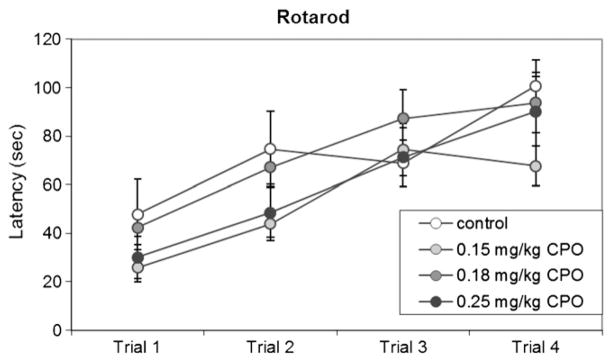
There was no significant effect of repeated CPO exposure on motor coordination or cerebellar learning in the rotarod task (Fig. 4). PON1−/− mice in all dose groups showed improvement over the 4 successive trials (p<0.01), and there was no significant effect of dose on latency to fall from the rotarod (p=0.151) (Fig. 4). In mice tested in the open field on PND 25, there were no differences among dose groups in the total distance traveled (p=0.788), time spent in the center area (p=0.499), or number of entries into the center area (p=0.940) (data not shown).
Fig. 4. Rotarod.
Latency to fall from the accelerating rotarod is shown, for each of 4 successive trials on PND 23, in PON1−/− mice exposed to 0.15 mg/kg/d CPO, 0.18 mg/kg/d CPO, or 0.25 mg/kg/d CPO, or to vehicle alone. Values are expressed as mean±SE.
3.4. Learning and memory
The impact of repeated CPO exposure on learning and memory was assessed in PON1−/− mice using contextual and cued fear conditioning, the Morris water maze, and the water radial-arm maze.
3.4.1. Contextual and cued fear conditioning
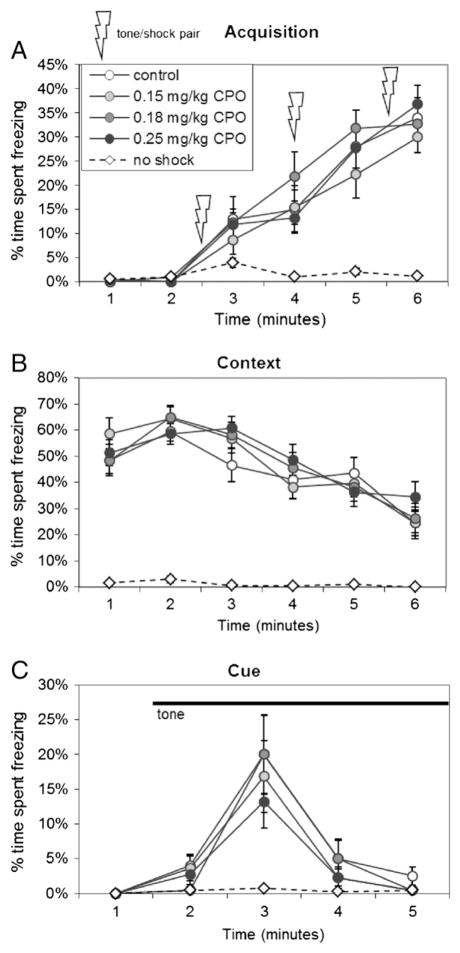
In the contextual fear conditioning test, all mice that received foot shocks paired with auditory cues during training showed increased freezing behavior over the training period (p<0.01), with no differences in freezing behavior among treatment groups (p=0.457) (Fig. 5A). Control mice that did not receive foot shocks explored the chamber actively for the duration of testing, and consequently showed very low freezing behavior (Fig. 5A). When placed back into the chamber on the following day for assessment of contextual learning, mice that had received shocks on the previous day showed substantial freezing behavior, decreasing gradually over the 6-minute testing period (p<0.01) (Fig. 5B). There were no differences in contextual learning among treatment groups (p=0.916). Two days after training, freezing response to the auditory cue was assessed by placing the mice in a novel arena for 2 min, then presenting the auditory cue (Fig. 5C). All mice actively explored the new arena for the first 2 min. Mice that had received foot shocks during training showed freezing behavior upon presentation of the auditory cue (Fig. 5C; p<0.01), and again there were no differences among treatment groups (p=0.777).
Fig. 5. Fear conditioning.
(A) Mice were trained to associate the context (the test environment) and the cue (an auditory tone) with a mild foot shock administered, along with the cue, 3 times over a 6-minute training period. The percentage of time spent freezing was recorded for each minute of testing. B) The following day, mice were returned to the testing chamber to measure the fear response associated with the context of the testing environment. C) On the following day, mice were placed in a novel environment and after 2 min were presented with the cue, which continued for 3 min. The percent of time spent freezing was recorded for each minute of testing. Values are expressed as mean percent of time spent freezing±SE for PON1−/− mice exposed to 0.15 mg/kg/d CPO (light-gray circles), 0.18 mg/kg/d CPO (dark-gray circles), or 0.25 mg/kg/d CPO (black circles), or to vehicle alone (white circles). An additional control group (white diamonds) received the tone with no shock.
3.4.2. Morris water maze
In the Morris water maze, there were no differences among treatment groups in acquisition of the task (p=0.671) (Fig. 6A). Latencies to find the submerged platform decreased throughout the training sessions (p<0.01), and were similar among the mice exposed to vehicle and mice that had been exposed repeatedly to 0.15 mg/kg/d CPO, 0.18 mg/kg/d CPO, or 0.25 mg/kg/d CPO (Fig. 6A; Suppl. Table 2). Retention was tested at 1, 2, 3, and 4 weeks after acquisition of the task, using a probe trial in which mice explored the maze for 2 min in the absence of a platform. There were no differences in retention among mice of the 4 treatment groups, as measured by percent dwell time in the target (p=0.528) and opposite (p=0.965) quadrants (Fig. 6B–E), number of target crossings (p=0.924) (data not shown), average distance from platform over the 2-minute period (p=0.751) (data not shown), and latency to target (p=0.346) (data not shown). Selected statistics are shown in Supplementary Table 2.
Fig. 6. Morris water maze.
A) Morris maze — acquisition. Training in the Morris water maze consisted of 3 trials per day for 8 days to locate a hidden platform. Mean latencies for finding the platform over the 3 daily trials are shown during training (task acquisition). If mice did not find the platform within 60 s, they were placed on the platform, and the latency was recorded as 60 s. Data represent the mean latencies±SE for PON1−/− mice exposed to 0.15 mg/kg/d CPO, 0.18 mg/kg/d CPO, or 0.25 mg/kg/d CPO, or to vehicle alone. B–E) Morris maze — retention. Retention was assessed at 1 week (B), 2 weeks (C), 3 weeks (D) and 4 weeks (E) following the last day of training, and consisted of one trial of 2-minute duration. Data represent the time spent in the target quadrant (where the platform was located during training) versus time spent in the opposite quadrant. Values are expressed as mean±SE.
3.4.3. Water radial-arm maze
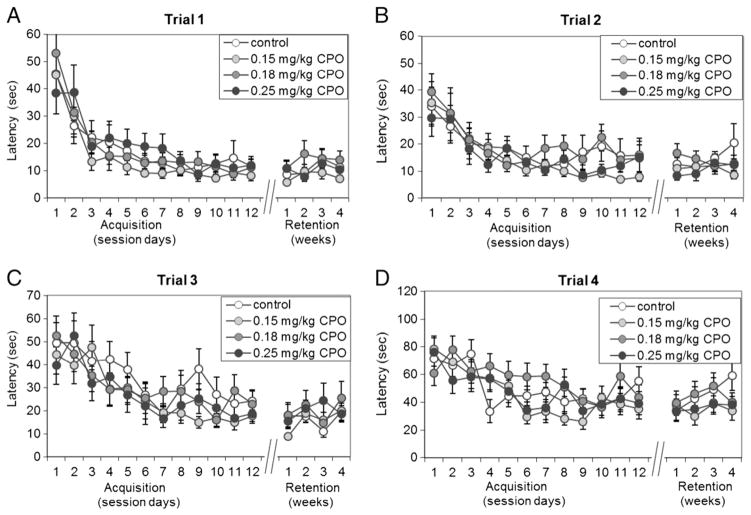
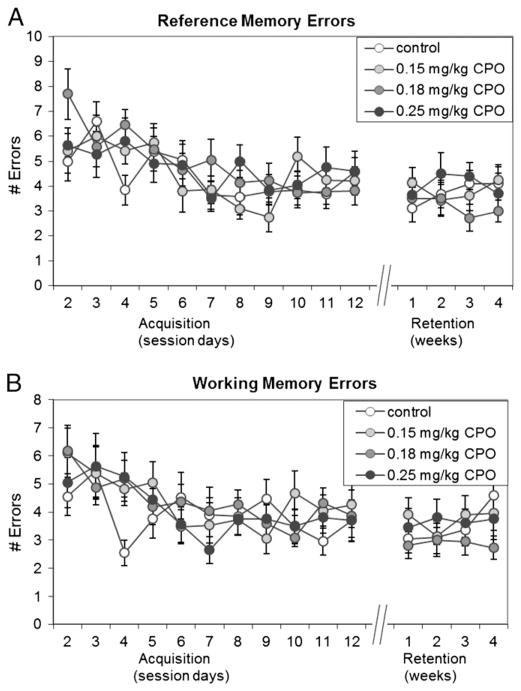
A water version of the 8-arm radial-arm maze was used to test spatial learning and memory in the mice from Group A. Mice were trained over 12 sessions, with 1 session per day, to locate 4 hidden platforms in succession, 1 platform for each of 4 trials. Retention was then tested at 1, 2, 3, and 4 weeks after acquisition of the task. Because the platforms were always located in the same 4 arms, the test allowed for measurement of reference memory as well as working memory. Additionally, the increasing difficulty of the task over the 4 successive trials allowed assessment of working memory and reference memory over a range of memory loads, by examining each of the 4 trials separately. Working memory errors included entries into arms that had previously contained a platform in that session, and reentries into any arms, including the start arms, during the same test session. Reference memory errors included entries and reentries into arms that never contained a platform and reentries into start arms. While there were significant (p<0.01) reductions in latency to find the platform over time for each of the 4 different trials (Fig. 7A–D), as well as significant (p<0.01) reductions in the numbers of reference memory errors (Fig. 8A) and working memory errors (Fig. 8B; Suppl. Table 2), there were no significant differences among treatment groups in latencies to find the platforms in trial 1 (Fig. 7A; p=0.323), trial 2 (Fig. 7B; p=0.102), trial 3 (Fig. 7C; p=0.334), or trial 4 (Fig. 7D; p=0.121) over the 12 training sessions or in the 4 retention sessions. Additionally, there were no significant differences among treatment groups in the total number of reference memory errors (Fig. 8A; p=0.583) or working memory errors (Fig. 8B; p=0.790) over the 12 training sessions or the 4 retention sessions. As expected, latencies to find the platforms in trials 3 and 4 (Fig. 7C–D) were longer than in trials 1 or 2 (Fig. 7A–B), reflecting the increased difficulty of the tasks in those trials.
Fig. 7. Radial-arm maze— latency.
the latency to find the 4 hidden platforms was measured for each of 12 daily sessions, with each session consisting of 4 sequential trials of increasing difficulty. Acquisition of the 4 tasks (finding the platform in each of the 4 successive trials) in sessions 1 through 12 was followed by retention testing at 1 week, 2 weeks, 3 weeks and 4 weeks following the completion of training. Values represent the latencies (mean±SE) to find the hidden platforms in (A) Trial 1, (B) Trial 2, (C) Trial 3 and (D) Trial 4 for the 12 daily acquisition sessions and for the 4 weeks of retention testing.
Fig. 8. Radial-arm maze— errors.
The total number of A) reference memory errors and B) working memory errors were recorded for the daily acquisition sessions 2 through 12, and for the four retention sessions at 1 week, 2 weeks, 3 weeks and 4 weeks. Session 1 was not included in the analysis because it was not possible to make reference memory errors or working memory errors in this session due to blockade of the arms after acquisition of each platform. Reference memory errors included entries into arms that never contained a platform, and re-entries into start arms. Working memory errors included re-entries into arms that previously contained a platform in that session plus re-entries into any other arms visited previously in that session. Values are expressed as the mean number of errors±SE.
4. Discussion and conclusions
We report here the results of a neurobehavioral assessment performed on PON1−/− mice exposed to CPO daily from PNDs 4 to 21. The neurobehavioral test battery did not reveal significant effects of neonatal CPO exposure on early reflex development, motor coordination, open field behavior, or learning or memory in the Morris water maze, radial-arm maze, or contextual fear conditioning tests. However, there were significant effects of repeated daily exposure to CPO on startle latency, hyperkinesis, and body weight, which merit further investigation. These results are discussed within the context of a larger study involving additional physiological endpoints of CPO toxicity in PON1−/−, PON1+/+, tgHuPON1Q192 and tgHuPON1R192 mice (Cole et al., 2011).
4.1. Neurobehavioral effects of CPO: developmental landmarks and reflex development
PON1−/− mice exposed to 0.25 mg/kg/d CPO exhibited slightly lower (~8% lower) body weight by PND 21, compared to vehicle-injected controls, and the lower body weights persisted beyond the dosing period until at least PND 80, when the body weights of mice exposed to 0.25 mg/kg/d CPO were ~3% lower than vehicle-treated mice. At higher doses (0.35 or 0.50 mg/kg/d), repeated exposure to CPO was also associated with reduced body weight in PON1−/− mice and in humanized transgenic mice expressing the tgHuPONQ192 alloform, but not in PON1+/+ mice or mice expressing the tgHuPON1R192 alloform (Cole et al., 2011). Despite the slight reduction in body weight, there were no significant effects of repeated daily exposure to CPO at dosages up to 0.25 mg/kg/d on the appearance of developmental landmarks or reflexes such as cliff avoidance, negative geotaxis, or the righting reflex. However, PON1−/− mice exposed to 0.18 mg/kg/d or 0.25 mg/kg/d CPO had a slightly reduced latency to maximum startle amplitude when presented with a 120 dB tone, albeit with little effect on startle amplitude or pre-pulse inhibition of startle. This effect was seen on PND 50, but not on PND 25, suggesting that repeated developmental CPO exposure was associated with subtle effects on the primary acoustic startle circuit that did not surface until about a month after the last exposure. The shorter startle latency seen in CPO-exposed mice in the current study would suggest that repeated CPO exposure strengthens synaptic connections within the acoustic startle circuit, which involves the auditory nerve, ventral cochlear nucleus, pontine reticular formation and spinal motor neurons (Swerdlow et al., 1999; Koch and Schnitzler, 1997). This would be consistent with our previous gene expression data indicating that the Nervous System Development (GO:0007399), Axon Guidance (GO:0007411), Synapse Organization (GO:0050808) and Regulation of Synaptic Plasticity (GO:0048167) gene sets were among the most highly affected gene sets following neonatal exposure to CPO (Cole et al., 2011). Intriguingly, other studies have shown that a small degree of cholinergic enhancement can have positive trophic effects on neuronal development (Timofeeva et al., 2008; Hohmann, 2003; Lauder and Schambra, 1999; Meck and Williams, 1997). The lack of effects on pre-pulse inhibition of the startle reflex indicates that sensorimotor gating by the forebrain, which regulates plasticity of the acoustic startle circuit (Swerdlow et al., 1999; Koch and Schnitzler, 1997), is unaffected by repeated low-level CPO exposure.
Other studies in humans and rodents have shown effects of CPF exposure on body weights and reflexes. In two studies of urban-dwelling women and children living in New York City prior to the EPA ban on household use of CPF, prenatal exposure to CPF was associated with significant reduction in birth weight and length (Perera et al., 2006; Whyatt et al., 2004). However, in a cohort of children with lower levels of cord-blood CPF studied after the curtailment of household applications, there was no evidence of exposure-related fetal growth alterations (Berkowitz et al., 2004). Exposure of rats to CPF during gestation and the early postnatal period has been recently linked to delayed effects in the form of excessive weight gain during adolescence (Lassiter and Brimijoin, 2008), an opposite effect to what was seen in the current study in mice. In a group of women and children living in the Salinas Valley, California, maternal biomarkers for OP compounds were associated with impaired reflexes in young infants (Young et al., 2005). The follow-up of these children has revealed a complex constellation of findings (Eskenazi et al., 2007). A study by Dam et al. (2000) examined the behavioral effects of postnatal CPF exposure in developing rats, finding gender-specific behavioral deficits. Female rats displayed significant deficits in reflex righting and negative geotaxis when exposed to CPF at 1 mg/kg/d, whereas male rats did not show any significant deficits. Gestational exposure studies with rats have also shown treatment-related effects of the parent organophosphorothiorates (e.g., CPF) on the righting reflex and cliff avoidance behavior (Chanda and Pope, 1996). The current study suggests that these reported effects of CPF exposure on reflexes were due directly to CPF, since CPO exposure was not associated with impaired cliff avoidance or righting reflex in the current study.
4.2. Neurobehavioral effects of CPO: motor coordination, locomotor activity, open field
There were no significant effects of repeated CPO exposure on motor coordination or cerebellar learning on the rotarod, or on open field behavior. In contrast to our results with mice, male rats tested in the open field have been reported to exhibit behavioral abnormalities, with suppressed locomotor activity and rearing (Dam et al., 2000). However, from PNDs 15 to 20, mice exposed repeatedly to CPO at 0.15 mg/kg/d, 0.18 mg/kg/d or 0.25 mg/kg/d exhibited a transient hyperkinesis in the 20-minute period following CPO administration. This behavior could possibly be related to cholinergic enhancement, although the 20-minute period following exposure is not likely to correspond to the time of maximum cholinesterase inhibition; in neonatal rats exposed to CPO, the maximum inhibition of cholinesterase occurs 60 min after exposure (Betancourt and Carr, 2004). It is unclear why the transient hyperkinesis was not observed until PND 15 and disappeared by PND 21 — its appearance and disappearance during development may be related to compensatory changes in the sensitivity of specific subsets of neurons involved in the regulation of movement. The similarity of this hyperkinetic behavior to behaviors induced by perturbation of the catecholaminergic system (Barroso-Chinea and Bezard, 2010; Kim and Palmiter, 2003; Nicholas, 2007) suggests possible transient effects of repeated CPO exposure on dopaminergic or noradrenergic neurotransmission in the CNS. It would be of interest to use neurobehavioral tests that are specifically designed to assess dopaminergic or noradrenergic function, social interactions, or possibly age-dependent effects on learning and memory. For example, in recent studies by Venerosi et al. (2009, 2010), neurobehavioral development was studied in mice by measuring the ultrasonic vocalizations of CPF-treated and control pups after separation from the mother and siblings. CPF exposure significantly decreased the frequency and duration of ultrasonic calls, providing evidence of a depressive effect on behavior in the first two weeks of life that is essential for communicating emotional distress and physical location to the mother (Hofer et al., 2002).
4.3. Neurobehavioral effects of CPO: learning and memory
There were no significant effects of repeated CPO exposure on learning or memory in the three tests chosen for this study. The lack of effects of repeated neonatal CPO exposure on learning or memory was somewhat surprising, but not entirely inconsistent with other studies examining OP effects on cognition, which have shown either impaired or improved cognition. When biological measures of maternal exposure were used in the Salinas Valley cohort of women and children, mental scores at 2 years of age were reduced as a function of CPF exposure; however, when the children’s biomarkers of exposure were used in the analysis, scores tended to be enhanced on both mental and psychomotor tests (Eskenazi et al., 2007). The authors suggested that children with higher levels of CPF exposure may be more active explorers of their environment, conferring them with the mixed blessing of greater brain stimulation and increased environmental pesticide exposure. In the New York City cohort, cord-blood CPF levels were associated with reduced mental and psychomotor scores at 3 years of age, and with an increase in the number of children diagnosed with attention problems, Attention Deficit Hyperactivity Disorder, or pervasive developmental delay (Rauh et al., 2006).
While the human epidemiological studies are suggestive of neurodevelopmental effects in children at subclinical exposure levels, the results are not clear-cut, and suggest a complex pattern of effects. Rodent studies have been important in more clearly defining the impact of CPF on development, and have been used to identify additional mechanisms of toxicity besides AChE inhibition (Pope, 1999; Schuh et al., 2002). Exposure of neonatal rats to CPF at subtoxic doses was associated with decreased cell density in the cerebellum and other brain regions (Whitney et al., 1995). Levin et al. (2002) demonstrated effects of gestational CPF exposure on hyperactivity in rats of both sexes, and gender-specific effects on learning and memory. When exposure took place earlier in gestation, neurobehavioral effects persisted into adulthood, and were characterized by hyperactivity and adverse changes in learning and memory (Icenogle et al., 2004). In the current study, neonatal CPO exposure did not appear to have an impact on learning or memory in mice.
4.4. Neurobehavioral effects of neonatal CPO exposure in the context of the larger study
The relatively few neurobehavioral effects associated with CPO exposure may reflect the relative insensitivity of neurobehavioral tests as endpoints of toxicity (Crabbe et al., 1999; Enserink, 1999; Wahlsten et al., 2006), especially for low-level exposures that would be expected to produce subtle effects difficult to measure in rodents but significant for neural function in humans. The general lack of effects is not due to lack of sensitivity of our particular laboratory’s testing methods, as other studies performed in our rodent neurobehavioral testing laboratory have revealed differences in the rotarod and open-field (Choi et al., 2010), fear-potentiated startle response (Fadok et al., 2009), and the Morris water maze (Pan et al. unpublished data).
The dosages of CPO selected for assessment of neurobehavioral effects in the current study were relatively low (0.15 mg/kg/d, 0.18 mg/kg/d, and 0.25 mg/kg/d CPO), and were all well below the threshold for acute inhibition of brain AChE in adult mice. However, on PND 4, when levels of PON1 and carboxylesterase are low, these doses were associated with inhibition of AChE; the EC50 for acute inhibition of brain AChE by CPO was 0.63 mg/kg in PON1+/+ mice and 0.23 mg/kg in PON1−/− mice (Cole et al., 2011). Furthermore, repeated postnatal exposure to CPO at the dosages used for neurobehavioral assessment (0.15 mg/kg/d, 0.18 mg/kg/d, or 0.25 mg/kg/d) was associated with dose- and genotype-related decreases in brain AChE activity on PND 22 (Cole et al., 2011). For example, in PON1−/− littermates of the mice used for behavioral assessment, brain AChE on PND 22 was reduced by 9.7±3.7%, 16.4±2.7%, and 27.3±6.8%, at those three doses. Thus, the effects that were observed in the current study occurred in the context of lower brain AChE activity in the treated mice, which may underlie or at least influence these effects. Further, it is possible that this reduction in brain AChE activity was associated with neurotrophic effects that offset any cognitive impairments related to the CPO exposures. Betancourt and Carr (2004) showed that neonatal rats exposed repeatedly to CPF at 1.5 or 3.0 mg/kg/d showed persistent inhibition of AChE, in addition to reduced levels of the muscarinic acetylcholine receptor (mAChR) and neurotrophins, whereas repeated CPO exposure at 0.25 or 0.35 mg/kg/d resulted in only transient inhibition of AChE, with no effects on mAChR or neurotrophins. Intriguingly, they saw signs of cholinergic hyperstimulation (tremors) at the 0.35 mg/kg/d dose of CPO that corresponded to the time of maximum AChE and disappeared 2-hours after dosing (Betancourt and Carr, 2004).
Differences in AChE inhibition associated with repeated postnatal CPO exposure (at 0.35 or 0.50 mg/kg/d) were also paralleled by changes in cerebellar gene expression that were modulated by PON1 status (Cole et al., 2011). PON1−/−, PON1+/+, tgHuPON1Q192 and tgHuPON1R192 mice exposed to 0.35 or 0.50 mg/kg/d CPO all showed significant differences in gene expression compared with vehicle-treated controls, as well as significant differences in gene expression among genotypes (Cole et al., 2011). A number of other studies have shown that developmental exposure of mice (Moreira et al., 2010), rats (Aldridge et al., 2005; Betancourt et al., 2006; Slotkin and Seidler, 2007; Stapleton and Chan, 2009), or cultured cells in vitro (Mense et al., 2006; Slotkin and Seidler, 2009) to CPF were associated with changes in the expression of multiple genes involved in nervous system development and function. The lack of effects of CPO on learning and memory, motor coordination and reflexes in the current study suggests that the changes in cerebellar gene expression are not associated with particularly strong neurobehavioral consequences, at least in mice.
These results do not rule out effects of CPO exposure on other neurobehavioral test paradigms or effects at different ages. Tests other than those chosen for inclusion in the current study might reveal differences that were missed here. For example, while performance of zinc transporter-3 knockout mice in the Morris water maze was unaffected when examined in 3-month-old mice, the effects did manifest at 6-months of age (Adlard et al., 2010), and effects could also be uncovered by performing the platform reversal variant of the test instead of the probe-trial variant (Martel et al., 2011). Morris water maze testing in the current study was performed at about 3 months of age (PNDs 70–98); it would be interesting to see if CPO effects manifest at older ages.
The relatively few neurobehavioral effects associated with CPO exposure in this study suggest that the neurobehavioral and biochemical consequences of CPF exposure reported in rats, mice and humans may be independent of its conversion to CPO. However, the similarity of effects of CPO and CPF on gene expression in the brain following neonatal exposure of rats and mice (Aldridge et al., 2005; Cole et al., 2011; Slotkin and Seidler, 2007) argues against this possibility. Some of the differences in neurodevelopmental effects of CPO and CPF are likely due to their different sites of detoxication. CPF is converted to CPO in the liver, and may also exert CPO-independent effects. After a CPF exposure, the parent organophosphorothioate may also be converted to CPO in the brain, allowing the newly-formed CPO to act locally in that organ. In contrast, after direct exposure to low levels of CPO, much of the CPO entering the bloodstream may be hydrolyzed by PON1 or bound stoichiometrically to other serum esterases before it reaches the brain. Compared to CPF, CPO has at least a 1000-fold lower biomolecular rate constant for inhibition of brain AChE (Huff et al., 1994), and when administered to mice in vivo, CPO is 50 to 100 times more potent than CPF at inhibition of brain AChE, based on the dosages required to produce 50% inhibition in adult mice (Cole et al., 2005), which are higher than the dosages used here. The significantly higher toxicity of CPO compared to CPF is relevant for actual exposures, because the oxon form can comprise from 2% to more than 17% of OP residues in the field (Cal-EPA, 1998; Vidal et al., 1998; Yuknavage et al., 1997; Eaton et al., 2008). Unfortunately, developmental toxicity studies that address CPO directly have been lacking. Estimated exposure rates for CPF in the general human population were estimated by Eaton et al. (2008) to be around 0.01 μg/kg/day, with farmworkers 20- to 40-fold higher (0.20–0.40 μg/kg/d), and the US EPA chronic RfD is 0.3 μg/kg/d with a 100-fold uncertainty factor (Eaton et al., 2008). With CPF concentrations in air ranging from 2 to 285 μg/m3 or higher, deposition samplers measuring CPF levels as high as 20 ng/cm2 (Eaton et al., 2008), and CPO comprising as much as 2–17% of exposures, it is conceivable that a child living in an agricultural community could be exposed to microgram quantities of CPO in aggregate, similar levels to those used in the current study.
4.5. Conclusion
In our previous study (Cole et al., 2011), neonatal CPO exposure was associated with wide-ranging effects on gene expression in the brain and on AChE inhibition, and PON1 status modulated these effects (Cole et al., 2011). These findings are consistent with our previous studies demonstrating that PON1 status plays a critical role in determining sensitivity to CPO and other OP insecticides (Cole et al., 2003, 2005, 2010; Jansen et al., 2009; Li et al., 2000). Remarkably, the neurobehavioral tests used in the current study did not show dramatic effects of repeated postnatal CPO exposure at the same dosages where effects were observed on cerebellar gene expression and AChE inhibition. For assessing the neurobehavioral consequences of OP exposure, alternative neurobehavioral tests would be warranted that are specifically designed to assess dopaminergic or noradrenergic function, social interactions, or possibly age-dependent effects on learning and memory. Given the variability in PON1 levels among humans and differences in the neurodevelopmental toxicity of CPO and CPF, it would be of great value to determine the extent to which PON1 status modulates the neurodevelopmental consequences of exposure to CPF itself.
Supplementary Material
Highlights.
We examined the neurobehavioral consequences of postnatal exposure to chlorpyrifos oxon (CPO).
PON1 knockout mice were used as a sensitive model of organophosphate toxicity.
CPO exposure was associated with transient hyperkinesis and modest effects on behavior.
The larger encompassing study identifies multiple effects of neonatal CPO exposure modulated by PON1.
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences [P42 ES04696, U19 ES11387, P30 ES07033, and ES09601] and the United States Environmental Protection Agency [R826886]. The transgenic and knockout mouse lines were kindly provided by Dr. Aldons J. Lusis, Diana Shih, and Aaron Tward at the University of California, Los Angeles. The authors thank Steve Ellis for statistical analyses and Betsy Walter, Christina Pettan-Brewer, Nathan Yee, Tara Arndt and Allison Forbes for their technical assistance.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.ntt.2012.02.003.
Conflict of interest statement
No competing interests.
Sources of support: ES-09601/EPA-R826886, U19 ES11387, ES09883, ES07033, ES04696, T32-AG00057.
References
- Adams J. Methods in behavioral teratology. In: Riley EPAV, CV, editors. Handbook of behavioral teratology. New York: Plenum Press; 1986. pp. 67–97. [Google Scholar]
- Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci. 2010;30:1631–6. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–31. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Bezard E. Basal ganglia circuits underlying the pathophysiology of levodopa-induced dyskinesia. Front Neuroanat. 2010;4:pii, 131. doi: 10.3389/fnana.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112:388–91. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Carr RL. The effect of chlorpyrifos and chlorpyrifos-oxon on brain cholinesterase, muscarinic receptor binding, and neurotrophin levels in rats following early postnatal exposure. Toxicol Sci. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci. 2006;92:500–6. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- Cal-EPA. Project nos C96-040 and C96-041:28. 1998. Report for the application and ambient air monitoring of chlorpyrifos (and the oxon analogue) in Tulare County during Spring/Summer 1996. [Google Scholar]
- Chambers H, Brown B, Chambers JE. Noncatalytic detoxication of 6 organophosphorus compounds by rat-liver homogenates. Pestic Biochem Physiol. 1990;36:308–15. [Google Scholar]
- Chanda SM, Pope CN. Neurochemical and neurobehavioral effects of repeated gestational exposure to chlorpyrifos in maternal and developing rats. Pharmacol Biochem Behav. 1996;53:771–6. doi: 10.1016/0091-3057(95)02105-1. [DOI] [PubMed] [Google Scholar]
- Choi WS, Abel G, Klintworth H, Flavell RA, Xia Z. JNK3 mediates paraquat- and rotenone-induced dopaminergic neuron death. J Neuropathol Exp Neurol. 2010;69:511–20. doi: 10.1097/NEN.0b013e3181db8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran RC, Kishiyama J, Aldous C, Carr WC, Jr, Pfeifer KF. Chlorpyrifos: hazard assessment based on a review of the effects of short-term and long-term exposure in animals and humans. Food Chem Toxicol. 1995;33:165–72. doi: 10.1016/0278-6915(94)00124-7. [DOI] [PubMed] [Google Scholar]
- Cohen Hubal EA, Egeghy PP, Leovic KW, Akland GG. Measuring potential dermal transfer of a pesticide to children in a child care center. Environ Health Perspect. 2006;114:264–9. doi: 10.1289/ehp.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–65. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–64. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- Cole TB, Walter BJ, Shih DM, Tward AD, Lusis AJ, Timchalk C, et al. Toxicity of chlorpyrifos and chlorpyrifos oxon in a transgenic mouse model of the human paraoxonase (PON1) Q192R polymorphism. Pharmacogenet Genomics. 2005;15:589–98. doi: 10.1097/01.fpc.0000167327.08034.d2. [DOI] [PubMed] [Google Scholar]
- Cole TB, Jansen K, Park S, Li WF, Furlong CE, Costa LG. The toxicity of mixtures of specific organophosphate compounds is modulated by paraoxonase 1 status. Adv Exp Med Biol. 2010;660:47–60. doi: 10.1007/978-1-60761-350-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Beyer RP, Bammler TK, Park SS, Farin FM, Costa LG, et al. Repeated developmental exposure of mice to chlorpyrifos oxon is associated with paraoxonase 1 (PON1)-modulated effects on cerebellar gene expression. Toxicol Sci. 2011 Jun 14; doi: 10.1093/toxsci/kfr157. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res. 2000;121:179–87. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Daston G, Faustman E, Ginsberg G, Fenner-Crisp P, Olin S, Sonawane B, et al. A framework for assessing risks to children from exposure to environmental agents. Environ Health Perspect. 2004;112:238–56. doi: 10.1289/ehp.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Ungerer A. Scopolamine-induced deficits in a two-trial object recognition task in mice. Neuroreport. 1997;8:1173–8. doi: 10.1097/00001756-199703240-00023. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knockout mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–6. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Quackenboss JJ, Catlin S, Ryan PB. Determinants of temporal variability in NHEXAS-Maryland environmental concentrations, exposures, and biomarkers. J Expo Anal Environ Epidemiol. 2005;15:388–97. doi: 10.1038/sj.jea.7500415. [DOI] [PubMed] [Google Scholar]
- Enserink M. Behavioral genetics. Fickle mice highlight test problems Science. 1999;284:1599–600. doi: 10.1126/science.284.5420.1599a. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107(Suppl 3):409–19. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, et al. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102:228–36. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–97. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ Health Perspect. 2000;108(Suppl 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Curl CL, Shirai JH, Kissel JC. Biologic monitoring to characterize or-ganophosphorus pesticide exposure among children and workers: an analysis of recent studies in Washington state. Environ Health Perspect. 2005;113:1651–7. doi: 10.1289/ehp.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske R, Yost M, Galvin K, Tchong M, Negrete M, Palmendez P, et al. Washington state department of health: pesticide air monitoring (drift) study. http://www.doh.wa.gov/ehp/Pest/drift.htm2009.
- Furlong CE. The Bert La Du memorial lecture: paraoxonases: an historical perspective. In: Mackness MMB, Aviram M, Paragh G, editors. The paraoxonases: their role in disease, development and xenobiotic metabolism. Dordrecht, The Netherlands: Springer; 2008. pp. 3–31. [Google Scholar]
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farm-worker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics. 2006;16:183–90. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Hattis D, Miller R, Sonawane B. Pediatric pharmacokinetic data: implications for environmental risk assessment for children. Pediatrics. 2004a;113:973–83. [PubMed] [Google Scholar]
- Ginsberg G, Slikker W, Jr, Bruckner J, Sonawane B. Incorporating children’s toxicokinetics into a risk framework. Environ Health Perspect. 2004b;112:272–83. doi: 10.1289/ehp.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Shair HN, Brunelli SA. Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci. 2002;Chapter 8(Unit 8):14. doi: 10.1002/0471142301.ns0814s17. [DOI] [PubMed] [Google Scholar]
- Hohmann CF. A morphogenetic role for acetylcholine in mouse cerebral neocortex. Neurosci Biobehav Rev. 2003;27:351–63. doi: 10.1016/s0149-7634(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Huen K, Harley K, Brooks J, Hubbard A, Bradman A, Eskenazi B, et al. Developmental changes in PON1 enzyme activity in young children and effects of PON1 polymorphisms. Environ Health Perspect. 2009;117:1632–8. doi: 10.1289/ehp.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther. 1994;269:329–35. [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–44. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, et al. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon, and diazinon. Environ Health Perspect. 2007;115:65–70. doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KL, Cole TB, Park SS, Furlong CE, Costa LG. Paraoxonase 1 (PON1) modulates the toxicity of mixed organophosphorus compounds. Toxicol Appl Pharmacol. 2009;236:142–53. doi: 10.1016/j.taap.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Palmiter RD. Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice. Proc Natl Acad Sci U S A. 2003;100:1346–51. doi: 10.1073/pnas.252753799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30:125–30. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107(Suppl 1):65–9. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, et al. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–41. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, et al. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10:767–79. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–86. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Martel G, Hevi C, Kane-Goldsmith N, Shumyatsky GP. Zinc transporter ZnT3 is involved in memory dependent on the hippocampus and perirhinal cortex. Behav Brain Res. 2011;223:233–8. doi: 10.1016/j.bbr.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–5. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Lan C, Zhou M, Bentsman G, Volsky DJ, et al. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol Sci. 2006;93:125–35. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- Moreira EG, Yu X, Robinson JF, Griffith W, Hong SW, Beyer RP, et al. Toxicogenomic profiling in maternal and fetal rodent brains following gestational exposure to chlorpyrifos. Toxicol Appl Pharmacol. 2010;245:310–25. doi: 10.1016/j.taap.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser VC. Neurobehavioral screening in rodents. Curr Protoc Toxicol. 1999;11:11.2.1–11.2.16. doi: 10.1002/0471140856.tx1102s06. [DOI] [PubMed] [Google Scholar]
- Nicholas AP. Levodopa-induced hyperactivity in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Mov Disord. 2007;22:99–104. doi: 10.1002/mds.21235. [DOI] [PubMed] [Google Scholar]
- Perera F, Viswanathan S, Whyatt R, Tang D, Miller RL, Rauh V. Children’s environmental health research—highlights from the Columbia center for children’s environmental health. Ann N Y Acad Sci. 2006;1076:15–28. doi: 10.1196/annals.1371.018. [DOI] [PubMed] [Google Scholar]
- Perez-Herrera N, Polanco-Minaya H, Salazar-Arredondo E, Solis-Heredia MJ, Hernandez-Ochoa I, Rojas-Garcia E, et al. PON1Q192R genetic polymorphism modifies organophosphorous pesticide effects on semen quality and DNA integrity in agricultural workers from southern Mexico. Toxicol Appl Pharmacol. 2008;230:261–8. doi: 10.1016/j.taap.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit Rev. 1999;2:161–81. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Correlations between prenatally-induced alterations in CNS cell populations and postnatal function. Teratology. 1977;16:235–46. doi: 10.1002/tera.1420160220. [DOI] [PubMed] [Google Scholar]
- Salazar-Arredondo E, de Jesus Solis-Heredia M, Rojas-Garcia E, Hernandez-Ochoa I, Quintanilla-Vega B. Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reprod Toxicol. 2008;25:455–60. doi: 10.1016/j.reprotox.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 2002;182:176–85. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- Shenouda J, Green P, Sultatos L. An evaluation of the inhibition of human butyrylcholinesterase and acetylcholinesterase by the organophosphate chlorpyrifos oxon. Toxicol Appl Pharmacol. 2009;241:135–42. doi: 10.1016/j.taap.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–7. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–74. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T, Seidler F. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res Bull. 2009;78:211–25. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton AR, Chan VT. Subtoxic chlorpyrifos treatment resulted in differential expression of genes implicated in neurological functions and development. Arch Toxicol. 2009;83:319–33. doi: 10.1007/s00204-008-0346-2. [DOI] [PubMed] [Google Scholar]
- Stevens RC, Suzuki SM, Cole TB, Park SS, Richter RJ, Furlong CE. Engineered recombinant human paraoxonase 1 (rHuPON1) purified from Escherichia coli protects against organophosphate poisoning. Proc Natl Acad Sci U S A. 2008;105:12780–4. doi: 10.1073/pnas.0805865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci. 1999;877:202–16. doi: 10.1111/j.1749-6632.1999.tb09269.x. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, et al. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res Bull. 2008;77:404–11. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udarbe Zamora EM, Liu J, Pope CN. Effects of chlorpyrifos oxon on M2 muscarinic receptor internalization in different cell types. J Toxicol Environ Health A. 2008;71:1440–7. doi: 10.1080/15287390802328887. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Scattoni ML, Calamandrei G. Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environ Health. 2009;8:12. doi: 10.1186/1476-069X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social–emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology (Berl) 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Vidal JLM, Gonzalez FJE, Galera MM, Cano MLC. Diminution of chlorpyrifos and chlorpyrifos oxon in tomatoes and green beans grown in greenhouses. J Agric Food Chem. 1998;46:1440–4. [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–81. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A. 2006;103:16364–9. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL. Assessment of spatial memory. Curr Protoc Toxicol. 1999:11:11.3.1–11.3.18. doi: 10.1002/0471140856.tx1103s00. [DOI] [PubMed] [Google Scholar]
- Wheeler WB. Role of research and regulation in 50 years of pest management in agriculture. Prepared for the 50th anniversary of the journal of agricultural and food chemistry. J Agric Food Chem. 2002;50:4151–5. doi: 10.1021/jf0256438. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–32. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, et al. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Yuknavage KL, Fenske RA, Kalman DA, Keifer MC, Furlong CE. Simulated dermal contamination with capillary samples and field cholinesterase biomonitoring. J Toxicol Environ Health. 1997;51:35–55. doi: 10.1080/00984109708984010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.