For over 100 years, immunologists have recognized that there are sites in the body in which immune responses do not occur. These immunologically “privileged” sites have long held the promise of solving the problems of autoimmunity and graft rejection, because somehow they violate the accepted rules of immunology, allowing foreign agents and tissues to persist. In the last year, interest has focused on a molecule called Fas-ligand (FasL; also called CD95L or Apo-1L), which appears to be required for some tissues to display such a privileged status (discussed below). FasL functions to induce apoptotic cell death in most cells that express its receptor, Fas (1). Fas-bearing cells include cells of the immune system, and thus FasL functions in immunological privilege in this way: tissues that naturally express FasL kill infiltrating lymphocytes and inflammatory cells. If simply placing such a molecule into any tissue of choice would confer privilege by killing off any invading immune cells, such that the tissue would not be destroyed in a transplant rejection, then any recipient could accept such a graft. Investigators set out to bring home this Holy Grail of transplant biology.
Meanwhile, FasL was shown to play other roles in the body. As discussed in more detail below, activated cytotoxic T lymphocytes (and other cells) often express high levels of FasL and the ability of FasL to kill cells bearing Fas accounts for some destructive effects mediated by these cells. FasL not only protects tissues from immune assault, but also can damage those tissues that express Fas. Privilege and peril. Blocking the function of FasL is clearly one key to preventing tissue damage under a number of different circumstances.
Despite the promise afforded by this remarkable receptor/ligand pair, things have become less simple, and two papers previously published in the Proceedings (2, 3) add still more levels of complexity to what is already a complicated story. One of these papers (2) shows that naturally occurring alleles of FasL have dramatically different abilities to trigger apoptosis through Fas, suggesting that in different settings stronger or weaker FasL function might be favored. The other paper (3) challenges the idea that FasL can confer immunological privilege, in that expression of FasL in the pancreatic islets of FasL-trangenic mice appears to induce an inflammatory infiltrate, and these engineered islets are not protected from graft rejection in allogenic recipients.
Before FasL was characterized, it was suspected that it would be intriguing. Ligation of the Fas molecule with antibodies is a potent inducer of apoptosis in different cell types (4). Mice (5) and humans (6, 7) with a defect in Fas expression or function show a profound lymphoaccumulative disorder (the lpr phenotype), associated (in mice) with a dramatic acceleration of age-associated autoimmune phenomena. Genetic evidence suggested defects in the FasL gene result in a similar phenotype called gld (8). This was, in fact, the case (9), and suggested a role for FasL in immune homeostasis rather than surveillance, because these animals display a lymphoaccumulative disorder with accelerated autoimmune syndromes, but no obvious defects in antiviral or antitumor defenses. Thus, while FasL expression on T lymphocytes (1) and natural killer cells (10) is clearly a mechanism by which these cells can kill other cells, it seemed likely that the targets were susceptible lymphocytes, i.e., those lymphocytes that accumulate in animals with defects in either the ligand or receptor. The conclusion was that a major function of FasL/Fas interactions is to limit lymphoid expansion via lymphoid-lymphoid interactions.
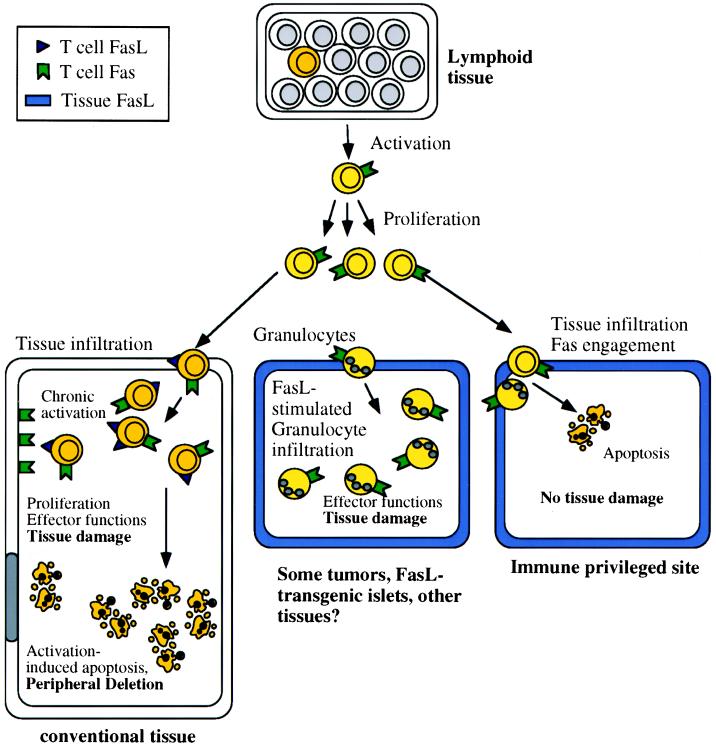
This view was strongly supported by a number of studies on activation-induced apoptosis in T cells. Engaging the T cell receptor on previously activated or transformed T cells (11–14) up-regulates expression of FasL and Fas, and the cells then undergo apoptosis as a consequence of FasL/Fas interactions. Activation-induced apoptosis also accounts for the phenomenon of peripheral deletion in vivo, in which T cells responding to a strong antigenic stimulus decrease in number over time, a process that may be important in immune homeostasis (Fig. 1 Left). Peripheral deletion is at least partially defective in animals deficient in Fas (15, 16), lending support to the view that expression of this ligand-receptor pair is important for depleting excess lymphocytes after an immune response.
Figure 1.
Some immunological effects of FasL. Chronically activated T lymphocytes express both Fas and FasL, and in conventional tissues (Left) this can result in apoptotic death of the T cells (peripheral deletion) and induction of apoptosis in other Fas-expressing cells. Immunologically privileged tissues (Right) constitutively express FasL, and infiltrating T cells and granulocytes rapidly undergo apoptosis. Thus, the tissue is protected from any damage that might result from an immune response. In some tissues, however (Center), FasL induces a granulocytic infiltration, which can damage the tissue. The conditions that favor one or the other of these contrasting effects of tissue FasL are unknown.
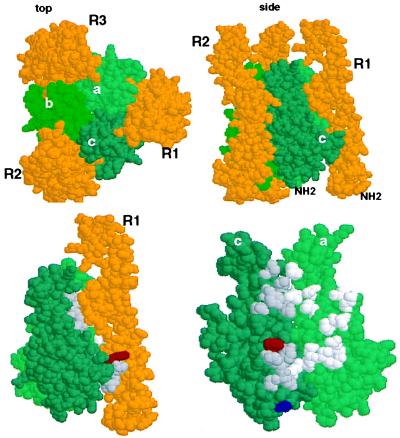
Substantial evidence indicates that FasL is a trimer with global structural features in common with related ligands in the tumor necrosis factor (TNF) superfamily. The interaction of FasL with its receptor is not fully elucidated, but we can make reasonable guesses about its nature based on the crystal structure of the ligand-receptor complex of the related ligand lymphotoxin-α (LTα) and one of its receptors, TNFR60 (17). As shown in Fig. 2, we expect that FasL functions as a trimer, clustering three Fas molecules. The face formed by two FasL subunits interacts with one chain of Fas (Fig. 2B). This model becomes especially interesting in light of the finding by Kayagaki et al. (2), who found that a polymorphism in FasL dramatically affects its ability to induce apoptosis in target cells. The two residues affected by this polymorphism are identified in the structure in Fig. 2B. One of these, residue E/G218, is predicted to interact directly with residues on Fas (based on its counterpart in LTα). These probably include charged residues, D and K, based on proximity analysis, and the presence or absence of a negative charge on residue 218 therefore might affect its interactions. In contrast, the other residue, T/A184, is not predicted to interact with residues on Fas, although it is possible that the N-terminal structure of Fas (which is not as restricted by disulfide bonds as in the TNFR60 molecule upon which this model is based) might be available for interaction with residues (including 184) on the ligand. However, the likelihood that E/G218 directly interacts with the receptor suggests that the difference in the function of the two forms of FasL reflect differences in affinity of interaction. A possible reason such a polymorphism might exist is discussed below.
Figure 2.
The E218G allelic substitution lies in the receptor binding region of Fas Ligand. (Upper) Space-filling depiction of LTα TNFR60 ligand–receptor complex from the crystal structure derived by Banner et al. (17) (viewed with RasMol). The three receptor (R) chains (gold) surround the LTα subunits (green) that form the trimeric ligand. The upper left panel (top) is viewed from the perspective of the receptor-expressing cell with the receptor’s N terminus extending away from the reader; in the right panel (side) the N terminus of the elongated receptor protrudes away from the cell surface. (Lower) Location of the T184A and E218G polymorphisms of FasL in the structure of LTα. Residues Phe-110 (red) and Ser-70 (blue) of LTα are equivalent to FasL 218 and 184 as identified by sequence alignment of TNF, LTα, and FasL (Pam250 matrix) and constrained by positions of conserved residues in the D-E and B-C β-strands of LTα. [β-strand nomenclature is that defined by Eck (37)]. Left side shows the ligand-receptor complex (side view) and right side depicts the binding site (with R1 removed) rotated 90° clockwise, exposing the contact residues. Amino acids that contact receptor with a surface area >20 Å2 (17) in the “a” and “c” LTα subunits (“c” subunit, dark gray; “a” subunit, light gray).
Our comfortable notions of the primary role of FasL in immune regulation were upset (albeit happily) by the realization that functional FasL plays an essential role in the phenomenon of immune privilege mentioned above (Fig. 1 Right). After viral inoculation into a classically “privileged” tissue, the anterior chamber of the eye, lymphocytes, and granulocytes that are recruited undergo apoptosis via exposure to resident FasL on the epithelial surfaces such that no tissue injury occurs (18, 19). This apoptosis does not occur in the eyes of animals with defective FasL (the gld defect), and the resulting uncontrolled inflammation destroys the tissue. FasL is thus necessary for the maintenance of the privileged status of the eye.
A striking protective effect of FasL expressed in the testes was observed by transplantation of allogenic testis to the kidney capsule of recipient mice (20). If the donor animal was defective in FasL or the recipient in Fas, the foreign tissue was rapidly rejected by a vigorous immune response, but when both ligand and receptor were functional the graft was maintained. This remarkable observation (and that concerning the anterior chamber of the eye) raised expectations that allogeneic tissues could be protected by ectopic expression of FasL, with obvious consequences for transplantation. Soon thereafter, it was shown that syngeneic myoblasts expressing ectopic FasL effectively protected allogeneic pancreatic islets coimplanted under the kidney capsule of animals made diabetic by streptozotocin treatment (21). These grafts, which were quickly rejected if myoblasts did not express FasL, maintained function for an extended period of time. Consistent with this was the observation that murine or allogeneic rat islets showed delayed rejection when coimplanted with FasL-expressing testes tissue in rats (22).
One of the most successful forms of transplantation in humans is that of corneas, with less than one-third rejection after 5 years, without tissue matching or immunosuppression. Recently, it was shown that human corneas express functional FasL (23), raising the possibility that this molecule acts to protect these grafts in humans. Examination of corneal transplants in mice supported this idea; while approximately 45% of allogeneic cornea transplants survived for an extended period (as described by others), no graft survival was seen if the cornea expressed defective FasL (gld) or the recipients had a defect in Fas expression (lpr). As with the testes, protection of allogeneic grafts was dependent upon the presence of functional FasL.
One other recent example of FasL-dependent immune privilege was described in a different context. A number of different murine and human tumors, including many nonlymphoid tumors, have been observed to constitutively express functional FasL (24–26). For example, a FasL-expressing melanoma was capable of inducing potent antitumor immunity, providing that the host was defective in Fas expression (25). This suggested that the mechanism responsible for protecting tissues from autoimmune destruction during inflammatory responses, or during graft rejection, also could function to protect cells from that tissue from immune surveillance after transformation.
These observations strongly implicate nonlymphoid FasL in the control of immune responses, via induction of apoptosis in infiltrating lymphocytes and granulocytes. However, we also know that FasL can induce tissue damage. In graft-vs.-host disease, the ability of the graft effector cells to express functional FasL contributes to the destructive assault (27, 28). Anti-Fas antibody induces apoptosis in hepatocytes in vivo (29), and this has led to the idea that FasL-induced apoptosis of these cells contributes to some forms of hepatitis.
Recently, another interesting twist on FasL-induced apoptosis was reported in Hashimoto thyroiditis (30). Thyrocytes express functional FasL constitutively (as a mechanism of immune privilege?), but normally do not express Fas. However, in Hashimoto thyoiditis patients, the thyrocytes do express Fas, and these cells undergo apoptosis. In vitro, normal thyrocytes express Fas after exposure to interleukin-1 (IL-1), and the ensuing apoptosis is blocked by antibodies that disrupt Fas/FasL interactions. The possible protective nature of FasL on thyrocytes becomes the mechanism of their destruction. Why the system is “wired” in this way is unclear, but it is likely that this effect contributes to the disease process.
All of these functions of FasL, whether involved in protection or promotion of tissue destruction, are consistent with the idea that FasL engages Fas to induce apoptosis, and all can be explained on this basis. However, a new perspective on these studies came with the observations of Seino et al. (31), who reported that FasL on tumor cells can induce a granulocyte-mediated rejection reaction (Fig. 1 Center). Tumors expressing FasL, implanted subcutaneously or intraperitoneally, induced a granulocyte infiltrate that was dependent upon functional Fas on the bone marrow-derived population, and the rejection was followed by a T cell-dependent anti-tumor immunity that persisted. These observations are inconsistent with an immunosuppressive effect of FasL on tumors discussed above, despite the fact that in some cases the same tumor lines were used in the contrasting studies.
Now, a new study further challenges the immunoprotective effect of FasL for graft rejection. Allison et al. (3) report that expression of functional FasL in the pancreatic islets of transgenic mice failed to protect these islets from allogenic transplant rejection when placed under the kidney capsule of recipient mice. As with the tumor cells discussed above, the presence of FasL induced a granulocytic infiltrate in the transgenic animals themselves, which damaged (but did not destroy) the islets. Because this observation was incompatible with the results of others (20, 21), the authors re-examined the fate of allogeneic testes grafts. In these studies they failed to observe differences in the rejection of grafts from normal versus FasL-defective gld mice. However, previous studies have shown that age of the testes graft is an important variable in this effect, such that grafts from young mice can resist rejection whereas testicular tissue from adult mice may be rejected (32). Thus, slight age differences might account for the acceptance or rejection of testes grafts in the different studies. It will be interesting to examine the influence of age on FasL expression (and its relation to immune privilege) in this tissue.
We are nevertheless still left with what appear to be irreconcilable differences in the results from different laboratories. These differences cannot be readily attributed to the polymorphism in FasL described by Kayagaki et al. (2), because, for example, Bellgrau et al. (20) used testes bearing either allele (BALB/c or C57BL/6) and observed protection from rejection in both cases. Cornea grafts with the less potent allelic form of FasL were accepted at a reasonable frequency (23), and this less active FasL expressed in myoblasts was used by Lau et al. (21) to protect islets from rejection. All of the studies on immunologic privilege in the eye were performed in animals bearing the less potent FasL. Nevertheless, a more careful comparison of the efficacy of the two allelic forms of FasL in these systems should be informative.
While the polymorphism in FasL does not appear to explain the discrepancy in the above results, these different effects of FasL might help to explain the polymorphism. Taken together, the studies suggest that FasL can prevent immune responses by inducing death of lymphocytes, cause damage by killing nonlymphoid Fas+ cells of the tissues, or induce potent granulocytic inflammatory responses, depending on the circumstances. Thus, any benefit of FasL expression is offset by the damage it can cause. If the ability to endow a tissue with immunologic privilege correlates with the induction of granulocytic inflammatory responses in the two forms of FasL reported by Kayagaki et al. (2) (that is, if the same ligand-receptor interaction is involved in both types of effects, as expected), then a “weaker” FasL avoids damaging inflammatory responses at the expense of less immune privilege. Clearly, one or the other alleles will be favored in different settings. From this point of view, it may be interesting that several autoimmune-prone mouse stains appear to carry the “weaker” form of FasL.
Returning to the issue of whether FasL protects grafts from rejection or not, one possible explanation for the differences in the effects of FasL observed in these different studies might involve the site of transplant. For example, while corneas grafted to the eyes of recipients often are accepted, heterotopic cornea grafts to the skin are rejected (33). However, this cannot simply be due to differences in the effects of Fas ligation on different cell types (e.g., skin), because the proinflammatory effect of FasL appears to depend upon the presence of Fas on bone marrow-derived cells, not stroma (31). (Of course, this does not rule out the possibility that bone marrow-derived cells in the skin are important for this effect.) Ligation of Fas can induce secretion of IL-8 (34), which might contribute to the ensuing inflammation. Interestingly, the Fas-mediated intracellular signaling events leading to IL-8 secretion versus apoptosis appear to be different, suggesting that other factors might favor one outcome of Fas-ligation over the other. Thus, at some sites (or in some animal colonies?) additional signals to lymphoid and myeloid cells might result in FasL-induced cytokine/chemokine release rather than apoptosis. In that setting, FasL will be proinflammatory. On the other hand, FasL in sites such as the eye can induce a remarkably rapid apoptosis in normal splenocytes (18, 19), which might suggest that other additional factors contribute to increased susceptibility to Fas-mediated apoptosis. One candidate for such a “sensitizer” is interferon γ (35). Thus, FasL may either promote or inhibit inflammation (depending on whether it induces chemokines or apoptosis), and the choice between these outcomes may be determined by the presence or absence of other factors.
While this argument can result in repetitive motion injury due to excessive hand waving, it is testable and makes some sense. The possible requirement for a sensitizing factor necessary to promote immune privilege by FasL might account for the strikingly different observations on FasL protection of islet grafts. If syngeneic myoblasts but not islet cells provide such a second signal, then myoblasts expressing FasL will protect from graft rejection while FasL-transgenic islet cells will not. FasL-bearing tumors that promote IL-8 production rather than apoptosis will express different surface or soluble mediators than those that induce cell death in targets. Identification of the responsible secondary factors will be important for the manipulation of FasL effects.
There is another explanation for the differences in the outcome of islet cell transplants that may involve the treatment of the recipients. In the studies by Lau et al. (21) animals had been treated with streptozotocin to induce diabetes, a treatment that is known to be immunosuppressive in some situations (36). The role of this treatment in these studies may have to be assessed more carefully.
We have gotten used to referring to the Fas molecule as a “death receptor,” and to thinking of the function of FasL entirely in terms of inducing apoptosis. Until recently, TNF and lymphotoxin were thought of in the same way (and still carry their sinister monikers). However, there is a side to FasL that involves promotion of inflammatory responses, and an understanding of where and when this function dominates its effects is critically important. We will not easily give up our dreams of using FasL, perhaps with necessary partner molecules, to control the rejection of grafts or limit autoimmune destruction. But it’s not going to be as easy as we might have thought.
References
- 1.Suda T, Takahashi T, Golstein P, Nagata S. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 2.Kayagaki N, Yamaguchi N, Nagao F, Matsuo S, Maeda H, Okumura K, Yagita H. Proc Natl Acad Sci USA. 1997;94:3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison J, Georgiou H M, Strasser A, Vaux D L. Proc Natl Acad Sci USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagata S, Suda T. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 6.Rieux-Laucat F, Deist F L, Hivroz C, Roberts I A, Debatin K M, Fischer A, Villartay J P d. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 7.Fisher G H, Rosenberg F J, Straus S E, Dale J K, Middleton L A, Lin A Y, Strober W, Lenardo M J, Puck J M. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, Katagiri T. J Exp Med. 1990;171:519–531. doi: 10.1084/jem.171.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 10.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. J Immunol. 1996;157:2909–2915. [PubMed] [Google Scholar]
- 11.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D H. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P H. Nature (London) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 13.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, Green D R. Nature (London) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 14.Ju S T, Panka D J, Cui H, Ettinger R, el Khatib M, Sherr D H, Stanger B Z, Marshak Rothstein A. Nature (London) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 15.Singer G G, Abbas A K. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 16.Mogil R J, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos A N, Green D R. Int Immunol. 1995;7:1451–1458. doi: 10.1093/intimm/7.9.1451. [DOI] [PubMed] [Google Scholar]
- 17.Banner D W, D’Arcy A, Janes W, Gentz R, Schoenfeld H J, Broger C, Loetscher H, Lesslauer W. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 18.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Science. 1996;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 19.Griffith T S, Yu X, Herndon J M, Green D R, Ferguson T A. Immunity. 1995;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 20.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 21.Lau H T, Yu M, Fontana A, Stoeckert C J., Jr Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 22.Selawry H P, Cameron D F. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 23.Stuart P M, Griffith T S, Usui N, Pepose J, Yu X, Ferguson T A. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J C, Tschopp J. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 26.Niehans, G. A., Brunner, T., Frizzelle, S. P., Liston, J. C., Salerno, C. T., Knapp, D. J., Green, D. R. & Kratzke, R. A. (1997) Cancer Res. in press. [PubMed]
- 27.Baker M B, Altman N H, Podack E R, Levy R B. J Exp Med. 1996;183:2645–2656. doi: 10.1084/jem.183.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun M Y, Lowin B, French L, Acha-Orbea H, Tschopp J. J Exp Med. 1996;183:657–661. doi: 10.1084/jem.183.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Nature (London) 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 30.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzo A. Science. 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 31.Seino K I, Kayagaki N, Okumura K, Yagita H. Nat Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 32.Statter M B, Foglia R P, Parks D E, Donahoe P K. J Urol. 1988;139:204–210. doi: 10.1016/s0022-5347(17)42354-8. [DOI] [PubMed] [Google Scholar]
- 33.Niederkorn J Y, Callanan D, Ross J R. Transplantation. 1990;50:281–286. doi: 10.1097/00007890-199008000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Abreu-Martin M T, Vidrich A, Lynch D H, Targan S R. J Immunol. 1995;155:4147–4154. [PubMed] [Google Scholar]
- 35.Yonehara S, Ashii A, Yonehara M. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkart V, Zielasek J, Kantwerk-Funke G, Hibbe T, Schwab E, Kolb H. Int J Immunopharmacol. 1992;14:1037–1044. doi: 10.1016/0192-0561(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 37.Eck M J, Ultsch M, Rinderknecht E, de Vos A M, Sprang S R. J Biol Chem. 1992;267:2119–2122. [PubMed] [Google Scholar]