Abstract
The trend of women to become pregnant when older than in previous generations poses a paramount medical problem, for oocytes are particularly prone to chromosome missegregation, and aneuploidy increases with age. Recent data strongly suggest that as oocyte age increases sister chromatid cohesion is weakened or lost. Cohesin deterioration seems to contribute significantly to age-dependent aneuploidy, as discussed in this review.
Keywords: cohesin, aneuploidy, chromosome segregation, meiosis, oocytes
See Glossary for abbreviations used in this article.
Glossary.
- CDK1
cyclin-dependent kinase 1
- ESCO
establishment of cohesion
- Eso1
essential for S-chromatin organization
- Clr6
cryptic loci regulator 6
- GDF9
growth differentiation factor 9
- MDC1
mediator of DNA damage checkpoint
- MEF
mouse embryonic fibroblast
- PDS5
precocious dissociation of sisters 5
- PP2A
protein phosphatase 2A
- REC8
recombination (gene 8)
- SA/STAG
stromal antigen
- SCC1
sister chromatid cohesion 1
- Sgo2
shugoshin 2
- SMC
structural maintenance of chromosomes
- SYCP3
synaptonemal complex protein 3
- TEV
tobacco etch virus (protease)
- WAPL
wings apart-like
- Zp3
zona pellucida 3
As new life springs from germ cells, the stability of the germ cell genome is crucial for a species to faithfully reproduce. Nevertheless, errors occur and—at least in the oocytes of some organisms—increase with advancing age.
Gametogenesis involves mitotic divisions of early germ cells—such as primordial germ cells and gonocytes—followed by two meiotic cell divisions to generate haploid gametes. Meiosis requires highly specific chromosome structures and dynamics, necessary to reduce the chromosome content of the germ cells from 4C immediately after premeiotic S phase, through 2C in meiosis I and finally to 1C in meiosis II, to produce the haploid sperm or egg. Meiosis I and II are particularly error prone, leading to chromosome missegregation and, thus, to aneuploidy. Of the seven leading autosomal trisomies >90% are of meiotic origin. Of those, about three-quarters stem from errors in meiosis I and less than 25% occur in meiosis II [1,2,3,4]. The origin of sex chromosome trisomies such as XXY or XXX shows a similar distribution. The problem is mostly on the female side: the maternal origin of aneuploidy accounts for more than 90% of human trisomies, which is why the oocyte and its two meiotic divisions became a focal point of germ cell and aneuploidy research [1,2,3].
Oocytes and aneuploidy
Spermatocyte and oocyte meiosis are, in many respects, similar as they feature comparable chromosome structures and processes, but also differ in important aspects. Most notably, spermatogenesis begins at puberty and, according to our understanding, continues for the lifetime of the organism, with largely uninterrupted development from the spermatogonium—a germ stem cell—to the mature sperm. Oocytes, however, enter meiosis when already in the fetus, proceed until the end of prophase I and then arrest in a stage known as dictyate arrest. Thus, in a newborn girl oocytes are quiescent within primordial follicles and only begin to reach full size from puberty onwards, when within a mature follicle a cohort of oocytes is hormonally stimulated every month. These oocytes then continue meiosis I and develop until metaphase II, where one selected oocyte again arrests until fertilized. Fertilization must happen within a few days or the oocyte dies (for reviews on mammalian oogenesis, see [5,6,7,8,9,10]).
Oocytes therefore remain quiescent for up to more than four decades in humans, and a few years in mice. During this long period of arrest, the physical linkage between oocyte sister chromatids must be preserved to ensure subsequent proper chromosome segregation. With increasing age this seems to fail. The incidence of fetal trisomies increases markedly in women aged over 35 with more than one-third of aneuploid embryos at this age; therefore this problem is not a trifle. Trisomies such as Down syndrome—three copies of chromosome 21—are the leading cause for mental retardation, and have been diagnosed more frequently in recent years. For example, between 1989 and 2008, the incidence of clinically recognized trisomy 21 increased by 71% in England and Wales [11]. Much more intense prenatal screening and subsequent abortions, performed in 92% of diagnosed cases, prevented a similar rise in live births with Down syndrome, but the increase of diagnosed trisomies correlates strongly with the increasing age of pregnant women. Similar trends were reported from other countries [12]. Aneuploidy is not only a major cause for mental retardation but also for loss of pregnancies. About one-third of spontaneous abortions are aneuploid, even in young mothers. Twenty to twenty-five percent of oocytes in young mothers are estimated to be aneuploid, and this proportion increasing with age. Many of these oocytes will die or remain unfertilized, but some will survive and might give rise to an aneuploid embryo.
What goes wrong during oocyte meiosis? Several recent studies provide compelling evidence that the deterioration of sister chromatid cohesion—hereafter referred to as 'cohesion'—is a crucial reason for age-dependent aneuploidy, although certainly not the only one. Failure of the spindle assembly checkpoint (SAC) in ageing oocytes to respond properly to aberrant kinetochores and microtubule attachments, caused for example by cohesion failure, might contribute to the high frequency of aneuploidy in aged oocytes [13, 14, 15]. For example, it has been shown that expression of certain components of the SAC decreases with increasing oocyte age [16,17]. However, other studies question the role of the SAC in age-dependent aneuploidy and suggest that it functions equally well in old and young oocytes [18,19]. Whether impaired meiotic recombination adds to age-dependent aneuploidy remains questionable. Recombination in oocytes happens early in meiosis before birth—that is, before the oocytes arrest—and thus recombination errors do not increase with advancing age. However, aberrant recombination might render chromosomes more susceptible to later problems, and those might increase with age.
Mammalian meiosis and cohesin
Cohesion is adapted to fulfil the specific needs of meiosis, which has been reviewed elsewhere [20,21,22,23,24,25,26]. The diploid set of chromosomes becomes duplicated during pre-meiotic S phase and two sister chromatids are generated per chromosome, that is 2 × 2 sister chromatids per pair of homologous chromosomes known as 'homologues'. Within each pair, the sister chromatids must be kept together by cohesion. The cohesin complex, a ring-like protein structure, embraces the two sister chromatids at their centromeres and along the chromosome arms. In prophase I, the two homologous pairs of sister chromatids associate and synapse within the meiosis-specific synaptonemal complex, a zipper-like protein–DNA structure. During this process the chromosomes condense, form characteristic axis–loop architecture and initiate meiotic DNA recombination. In prophase I, one sister chromatid of one homologue undergoes homologous recombination with one sister of the other homologue within the synaptonemal complex, thereby forming a cross-like structure called a 'chiasma'. The chiasma links the two pairs of sister chromatids even after the synaptonemal complex dissolves in late prophase I and metaphase I (Fig 1A). Chiasmata become easily visible in condensed metaphase I oocyte chromosomes, but the precursor recombination structures exist earlier during oocyte dictyate arrest—referred to as 'chiasmata' here for the sake of simplicity. Cohesin is required to maintain chiasmata until they are resolved. Resolution of chiasmata—and thus disjunction of homologues—depends on cohesin cleavage [27,28], which occurs at the metaphase I to anaphase I transition. Formation of chiasmata not only allows genetic exchange but maintains homologue association. Therefore, the existence of at least one chiasma per pair of homologues is essential to prevent their premature separation. Homologue association is important for the proper assembly and orientation of the chromosomes on the metaphase plate, where the two sister centromeres of one homologue mono-orient, that is, they attach to the same side of the spindle. This allows the spindle apparatus to separate the two pairs of sister chromatids in anaphase I, when the chiasmata are finally resolved. The two sister chromatids within each homologue remain linked because centromeric cohesion is preserved. Protection of centromeric cohesin in meiosis I is therefore another key process that ensures proper chromosome segregation and requires specific proteins such as Shugoshin and the phosphatase PP2A [29,30,31]. Centromeric cohesion is only resolved at the metaphase II to anaphase II transition, where the sister chromatids are separated and gametes containing single chromatids are generated.
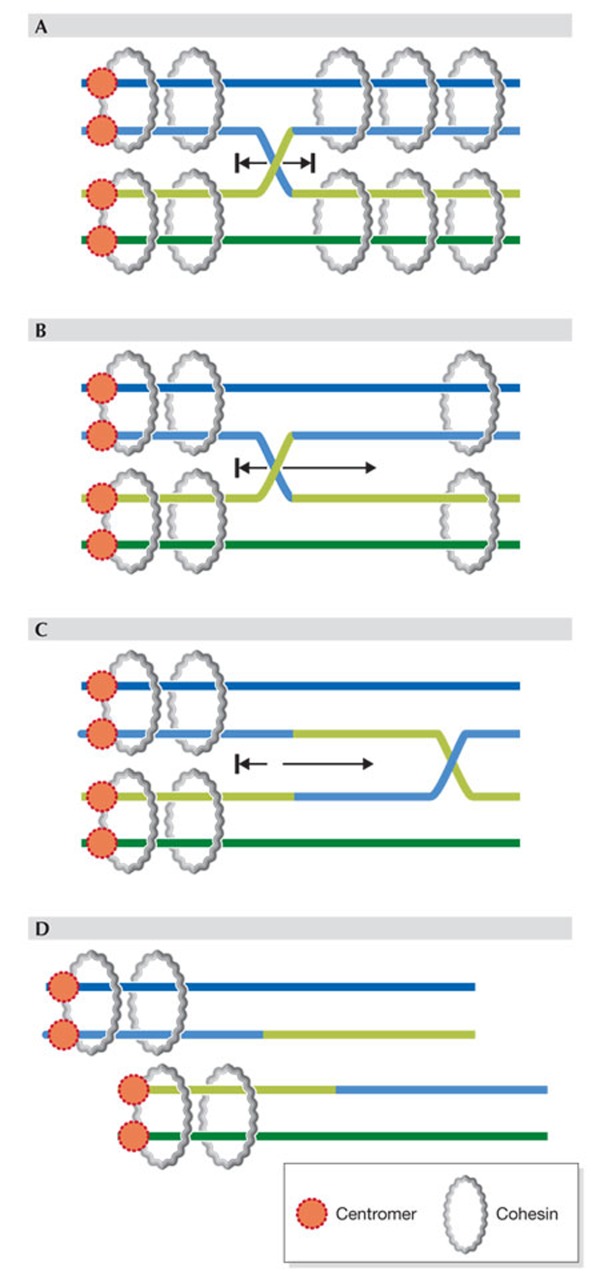
Figure 1. Cohesion, cohesin and chiasmata.
(A) Proper cohesion mediated by chromosome-wide distribution of cohesin rings prevents movement of cross-over or chiasma. (B–D) Loss of much arm cohesion allows movement of chiasma towards the centromere–distal end and eventually loss of chiasma (D), that is 'terminalization', whereby the two homologues (pairs of sister chromatids) separate prematurely.
The so-called 'terminalization' of chiasmata has been discussed controversially since the early days of germ cell cytogenetics. Terminalization, proposed originally by Darlington and Dark [32], was suggested as the loss of chiasmata through their movement towards the ends of chromosomes and the eventual slippage off those ends [33,34,35,36,37,38]. Terminalization of chiasmata is therefore different from chiasmata resolution, which happens quickly at the end of metaphase I to allow anaphase I to proceed. Terminalization would result in loss of linkage between homologues, failure to properly localize the homologues together on the metaphase plate and therefore missegregation and aneuploidy. The maintenance of chiasmata could require cohesion, as without cohesion they might move and terminalize (Fig 1B–D). Cohesin might physically block chiasmata movement and thus prevent terminalization. As cohesin cleavage triggers the loss of chiasmata and is required for disjunction of the homologues [28], cohesin seems necessary and sufficient for maintaining chiasmata, although the existence of additional 'chiasma binders' cannot be entirely ruled out.
The cohesin complex is comprised of two SMC proteins—SMC1 and SMC3—which heterodimerize to form a large, V-shaped structure. The open side of the cohesin complex is closed by a member of the kleisin protein family, such as RAD21 known as SCC1 or MDC1, which thereby forms a tripartite ring (also reviewed in [39,40,41,42]). A fourth protein, SA or STAG, associates with the kleisin and completes the complex. In mitotic cells there is one type each of SMC1 (SMC1α), SMC3, RAD21 and two SA proteins (SA1, SA2). Vertebrate meiocytes contain additional variants, which include the SMC1β, two kleisins (REC8, RAD21L) and an additional SA protein, SA3/STAG3. The timing and level of expression of these proteins varies throughout meiosis, but in principle 18 different cohesin complexes could be formed and there is evidence for the existence of at least six [43]. The specific roles of these complexes remain largely elusive.
Cohesin is required to prevent aneuploidy
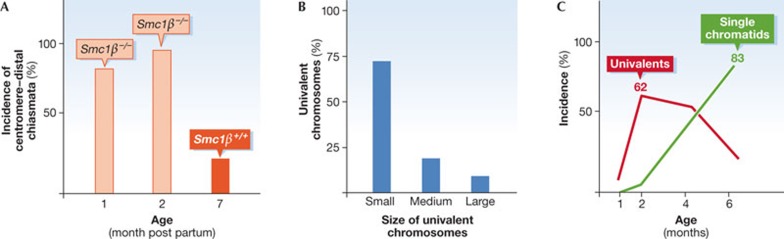
Is cohesin important for avoiding aneuploidy in mammalian oocytes? Evidence to support this came from the analysis of oocytes from Smc1β−/− mice. After isolation and maturation in vitro, they showed increased single homologues, that is pairs of sister chromatids in metaphase I when the two homologues should have remained associated through chiasmata (Fig 2; [44,45]). In Smc1β−/− mice the chiasmata were lost. Interestingly, the presence of separate homologues was increased in oocytes isolated from older mice. A detailed analysis of the chromosomal position of sites of chiasmata showed that, with increasing age, these sites if present are localized more frequently at the centromere–distal regions of chromosomes. This suggests movement of chiasmata towards the non-centromeric end and slippage of the chiasmata off the chromosomes, or in other words, terminalization. If correct, then naturally short mouse chromosomes should suffer loss of chiasmata more frequently than long chromosomes. This prediction was confirmed (Fig 2B); not only did the number of single homologues increase with oocyte age, but the incidence of single chromatids subsequently increased, implying that centromeric cohesion between the two chromatids within one pair was lost (Fig 2C). These findings suggested that cohesin SMC1β is a factor in age-dependent aneuploidy. The Smc1β−/− mouse was considered the first mouse model that could reflect some aspects of human age-dependent aneuploidy [46,47]. Strongly increased aneuploidy was seen in other mouse mutants, such as those lacking the synaptonemal complex component SYCP3 [48], but their aneuploidy did not show such age dependence.
Figure 2. Meiotic errors in in Smc1β−/− oocytes.
(A) Incidence of centromere–distal sites of chiasmata in oocytes from one-month-old and two-month-old female mice. Red columns: Smc1β−/− oocytes, yellow column: control value in Smc1β+/+ oocytes with low distal chiasmata even at seven months of age. (B) Distribution of metaphase I univalent chromosomes, which measures the loss of linkage of homologues, in Smc1β−/− oocytes depending on the length of the chromosomes, which are classified as short, medium or long. Short chromosomes suffer most loss of linkage, that is, loss of chiasmata. (C) Incidence of univalent chromosomes (red curve) and of single chromatids (green curve) with increasing age of the Smc1β−/− oocytes. Initially, homologues separate prematurely and univalents accumulate, which are later separated into single chromatids. In Smc1β+/+ oocytes there are no univalents at six months of age. For details see the work of Hodges and colleagues [44]. Smc1, structural maintenance of chromosomes.
Deficiency of the meiosis-specific kleisin REC8 caused a more striking phenotype in mouse oocytes, which were absent already shortly after birth [49,50]. Thus, no age effect could be studied in this mouse model. The meiosis-specific kleisin RAD21L does not appear to be involved in cohesin-associated age-dependent aneuploidy, although its absence causes a female age-dependent sterility phenotype, as RAD21L does not seem to function in oocyte meiotic cohesion [51].
There are probably not many humans that lack SMC1β or other cohesins—mitotic cohesins are essential—and thus the question emerged about the properties and fate of cohesin during the long oocyte arrest. Is the cohesin that is formed and loaded onto chromosomes on entry into meiosis stable for years and decades? Is there continuous expression and loading of fresh cohesin onto chromosomes in quiescent oocytes? Is there refurbishing of cohesion when an oocyte is activated to resume meiosis? What happens in aged oocytes to proteins that are known to protect kleisins from degradation such as Shugoshin? Several studies published recently address these and related questions.
Two groups asked whether in rapidly ageing mouse strains or very old mice of commonly used strains, which are not deficient in any cohesin gene, sister chromatid cohesion in oocytes shows signs of fatigue. Indeed, oocytes from such old mice produce aneuploid eggs at higher frequencies than oocytes from young animals [19,52]. In addition, these studies found an age-dependent loss of REC8 from the centromeres of old oocytes, in agreement with an earlier report describing reduced meiotic cohesins REC8, STAG3 and SMC1β [53]. When the loss of REC8 is greater than 90% compared with that in young adults, the frequency of chromosome missegregation in activated oocytes increases significantly [52]. Loosening of centromeric cohesion in metaphase I and metaphase II oocytes was observed in mice up to 19 months old, in which the interkinetochore distance was increased significantly, which is indicative of weakened cohesion. This increase correlated with loss of cohesin. Furthermore, mono-orientation of the sister kinetochores in meiosis I, known to depend on cohesin, would be compromised if cohesin is largely lost. Indeed, mitosis-like bi-orientation was suggested for some of the chromosome segregation defects seen in old oocytes [52]. Shugoshin, which protects kleisins in centromeric cohesin from separase-catalysed cleavage [54,55], is present in oocytes but diminishes with increasing age [19], which could leave REC8 unprotected and thereby lead to loss of cohesion. Why shugoshin is gradually lost is unknown, as is whether this is a cause or consequence of loss of cohesin. However, there is less Sgo2 in Smc1β−/− oocytes, suggesting that cohesin depletion is linked to loss of Sgo2 [19].
Sister chromatid cohesion is supported by chromosome entanglements through catenation that is introduced during replication or by topoisomerase II. Topoisomerase II, which can introduce and remove catenation, removes these catenanes during the S and G2 phases, and does so in certain circumstances, for example if spindle tension increases [56]. Catenation was shown recently to depend on cohesin, at least in yeast, thereby preventing decatenation by Topo II and possibly even supporting catenation of closely apposed sister chromatids [57]. Thus, if cohesin deteriorates slowly, the activity to Topo II might be shifted towards decatenation, which would accelerate the loss of cohesion.
The increasing loss of cohesin in ageing wild-type oocytes suggests that there is insufficient expression and loading of new cohesin to maintain its initial levels at centromeres. Is cohesin expression required after the oocytes have entered dictyate arrest? Or is the cohesin that was produced during early prophase sufficient to provide cohesion long after birth, at least throughout most of the reproductive life of mice? Revenkova and colleagues used an inducible SMC1β deficiency to answer this question [58]. A strain carrying a floxed Smc1β locus was bred with the GDF9–Cre transgenic strain, which expresses Cre in the primordial follicles right after birth. The floxed gene was excised in four days after birth and thus SMC1β was not expressed from then on. Yet, these mice—unlike constitutive Smc1β-deficient mice—remained fully fertile for at least 14 months after birth as compared with non-excised controls. The ovaries and oocytes of the Smc1β-excised animals looked wild-type, and both the localization of their chiasmata and their homologue cohesion appeared unaffected. These findings indicate that there is no need for Smc1β gene expression after mouse oocytes have entered dictyate arrest. The cohesin produced before prophase I is sufficient—at least until the above described effects of cohesin fatigue emerge in very old animals.
Nevertheless, if cohesin were expressed during oocyte quiescence, it could perhaps be loaded onto chromosomes, although not in sufficient levels to compensate the cohesin loss seen in very old wild-type mice. Single-oocyte real-time PCR indeed showed Smc1β mRNA present in dictyate-arrested mouse oocytes at least up to six months of age, although mRNA levels were only about 10% of the high levels seen in pachytene oocytes [44].
This notable expression of Smc1β in dictyate-arrested oocytes would at least allow for reloading of some newly synthesized cohesin onto chromosomes. However, cohesin reloading was shown either not to occur or to happen very inefficiently [59]. The authors used mouse strains expressing TEV-cleavable REC8 or SCC1, and thus microinjection of TEV into Rec8TEV/TEV or Scc1TEV/TEV oocytes destroyed REC8- or SCC1-cohesin. Even in the presence of the separase inhibitor securin, the TEV-mediated loss of REC8 cohesin resulted in loss of chiasmata. In addition, sister chromatids became separated when TEV mRNA was injected into Rec8TEV/TEV germinal vesicle oocytes—an oocyte stage before nuclear envelope breakdown that happens on entering metaphase I. Scc1TEVMyc/TEVMyc oocytes, however, were not similarly affected by TEV. Furthermore, TEV expression in oocytes that were arrested in meiosis II led to disjunction of sister centromeres in Rec8TEV/TEV oocytes, but did not affect cohesion between sister centromeres in Scc1TEVMyc/TEVMyc oocytes. Thus, REC8-type cohesin is necessary and sufficient for arm and centromere cohesion in oocytes. Expression of a non-cleavable variant of REC8 prevented loss of cohesion and chiasmata if expressed early in meiosis, but did not rescue this loss if expressed in growing oocytes. This shows that newly expressed REC8-type cohesin is either insufficiently loaded in growing oocytes, or not loaded at all, and cannot compensate for a sudden loss of the same type of cohesin. However, expression of the non-cleavable REC8 in growing oocytes was achieved using Cre-mediated removal of a stop cassette and Cre was expressed relatively late from the Zp3 promoter. Therefore too little REC8 expression might have been induced too late for rescue.
How about the function of SCC1, which is abundant in oocytes? TEV-mediated destruction of SCC1, not of REC8, in zygotes caused loss of sister chromatid cohesion in metaphase of the first embryonic division. This indicates that meiotic REC8-dependent and SCC1-independent cohesion in oocytes is replaced by REC8-independent and SCC1-dependent cohesion in the zygote. This switch and the rapid initial embryonic cell divisions require substantial somatic cohesin, which might explain the reappearance and accumulation of Smc1α mRNA in growing oocytes.
In wild-type mice and in humans rapid loss of all cohesin during dictyate arrest is unlikely. Cohesin probably disappears rather slowly. Slow, inefficient re-loading of cohesin—if it exists—might prevent loss of cohesion in young mammals, but eventually cannot compensate for cohesin deterioration. This and the above-mentioned threshold of less than 10% remaining cohesin for maintenance of cohesion indicate that cohesin dosage is important. Heterozygous human carriers of cohesin mutations might therefore be at higher risk of generating aneuploid oocytes if there is haploinsufficiency. A study by the Hunt, Hassold and Jessberger labs indicates that this might indeed be the case, as heterozygous cohesin mouse mutants have increased chromosomal abnormalities in metaphase I and II (unpublished results).
How is cohesin lost in ageing oocytes?
Evidence for cohesin decay as a leading cause for age-dependent aneuploidy is accumulating, but how is cohesin lost? Conversely, how can cohesin complexes provide sister chromatid cohesion for many years, in humans perhaps for a remarkable 30 or 40 years? Cleavage or spontaneous hydrolysis of one single peptide bond at any site within the large cohesin ring could open it and therefore eliminate the topological linkage of two sister chromatids.
Separase cleaves the kleisin subunit of cohesin to allow chromosome segregation [60]. This protease must be tightly controlled to prevent early loss of cohesion and premature segregation. Over many years, even very low separase activity in dormant oocytes might cause significant loss of cohesin. The effects of increasing separase activity were analysed using two independent approaches in mouse oocytes, either knockdown of securin or expression of a separase mutant that is non-phosphorylatable and thus cannot be inhibited by CDK1 [61]. In six-week-old oocytes, both pathways of separase inhibition, that is, securin expression and separase phosphorylation, must be prevented to cause premature loss of cohesion. Oocytes from 19-month-old mice showed significantly more prematurely separated bivalents and chromatids after separase activation although low doses of the activators were used. Thus, cohesion is reduced in old oocytes and increased separase activity particularly affects them. Whether leaky separase activation starting after birth until old age would accelerate cohesion loss in mouse oocytes, and whether low levels of separase activity contribute to loss of cohesion in ageing human oocytes remains to be shown.
Other, more unspecific processes might contribute to slow cohesin deterioration such as oxidative damage or spontaneous hydrolysis of peptide bonds, but little is known about these mechanisms. Similarly, it is unknown whether and how many distinct cohesin complexes contribute to arm and centromeric cohesion in oocytes. The details of the initial loading of the various cohesins and their complexes onto spermatocyte or oocyte chromosomes remain to be determined.
Another mechanism for removal of cohesion is through anti-establishment factors known as releasins and perhaps by cohesin deacetylases (Fig 3; reviewed in [39]). SMC3 requires acetylation at two adjacent lysine residues in its amino-terminal domain to establish and maintain cohesion after the cohesin complex has been loaded onto chromatids during replication [62,63,64]. Acetylation of SMC3 in mitotic cells or extracts happens only if SMC3 is chromatin-bound, and is required to establish cohesion. Two acetyl transferases, ESCO1 and ESCO2, exist in mammals [65], which acetylate the two conserved lysines in SMC3. The distinct functions of ESCO1 and ESCO2 are not yet understood in great detail. ESCO2 is essential in mice as its disruption causes prenatal death probably by affecting cohesion at pericentric heterochromatin [66], whereas ESCO2 mutations in humans cause the non-lethal Roberts syndrome [67,68]. Disparities in chromosome architecture and centromere location between mice and men, and putative alternative splicing in human cells, have been alluded to as possible explanations for this difference.
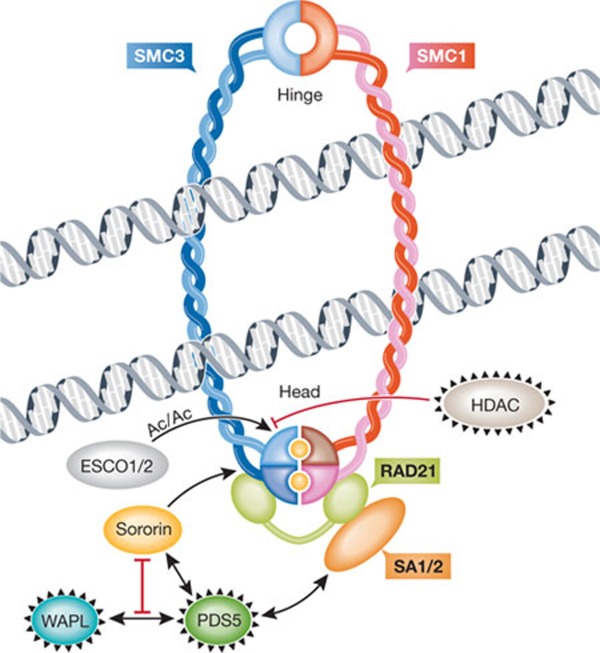
Figure 3. Cohesion-promoting and cohesion-weakening factors.
Anti-cohesion factors WAPL, HDAC and PDS5 are circled in black, and pro-cohesion factors are sororin and ESCO1/2. The hinge and head (amino/carboxy-terminal) domains of SMC proteins are indicated. Sister chromatids are shown being held together by a cohesin ring, that is, after cohesin has been loaded onto sister chromatids. The loading factors SCC2 and SCC4 are therefore not shown. Yellow circles within the cohesin head domains represent ATP, which needs to be bound and hydrolysed for stable cohesion. The relationship between ATP binding/hydrolysis and the action of pro- or anti-cohesion factors is unknown. The ring can open at the hinge domains, as postulated in the dual gate hypothesis [39], but is not depicted here as its regulation remains largely elusive. Other models of cohesin association with sister chromatids have been proposed, such as a 'handcuff' model comprising two cohesin rings that interact with each other [79]. AC, acetylation; ATP, adenosine triphosphate; ESCO, establishment of cohesion; HDAC, histone deacetylase; PDS5, precocious dissociation of sisters 5; SA, stromal antigen; SCC2/SCC4, sister chromatid cohesion 2/4; SMC1/3, structural maintenance of chromosomes; WAPL, wings apart-like.
Mammalian histone deacetylase HDAC8 has been suggested to deacetylate SMC3 [39] and might be required for cohesin dissociation from chromosomes [69]. Whether deacetylation is a cause or consequence of cohesin dissociation, and the mechanism by which this dissociation would be promoted in mammals by deacetylation, has not been described. It is not clear whether HDAC8 is the only deacetylase that functions in this process. The Schizosaccharomyces pombe acetylase Eso1, which is antagonized by deacetylase Clr6, ensures not only stable cohesion but also mono-orientation of the kinetochores in meiosis I [70]. Failure to mono-orient the two kinetochores of one homologue might lead to missegregation and therefore aneuploidy.
The roles of these acetyl transferases and their counteracting deacetylase(s) in oocytes have not been reported, but a shift in balance between these activities could contribute to loss of cohesion, perhaps even in an age-dependent manner. WAPL is a releasin that interacts with the kleisin and with SA1 in human mitotic cells [71]. It associates with PDS5, of which two variants exist in mammals—PDS5A and PDS5B [71,72,73]. WAPL and PDS5 together provide anti-cohesion-establishment and releasin activities. These two functions might occur through the same biochemical mechanism. The expression and function of releasins in mammalian oocytes remains to be explored.
Releasins are antagonized by the protein Sororin, which promotes the maintenance of cohesion [74,75]. Sororin and WAPL compete for binding to PDS5 and, in the absence of WAPL, Sororin is no longer required. SMC3 acetylation is necessary for Sororin association, but might have additional functions. Sororin seems to associate primarily with acetylated SMC3—but perhaps not exclusively—in a replication-dependent manner, as evidenced by experiments using inhibitors in HeLa cells and Xenopus laevis extracts [76]. MEFs from mice lacking ESCO2 have an approximately 50% reduction in SMC3 acetylation and similarly impaired recruitment of Sororin, most strongly affected at the pericentric heterochromatin [66]. On entering mitotic M phase, Sororin becomes phosphorylated and dissociates from chromatin. This is thought to allow the removal of cohesin in mitotic prophase, which depends on phosphorylation. CDK1 has been recently implicated in Sororin phosphorylation [77], which allows interaction with and recruitment of Polo-like kinase to cohesin to phosphorylate SA2 and trigger the mitotic prophase I cohesin removal pathway [78]. Whether this is coordinated with separase inhibition by CDK1 in prophase I remains to be seen.
Exploring aneuploidy: the significance of chromosomal imbalance.
This review series—published in this issue of EMBO reports—also includes:
A balancing act: focus on aneuploidy Nonia Pariente
Losing balance: the origin and impact of aneuploidy in cancer Andrew J Holland and Don W Cleveland
Chromosomal instability and aneuploidy in cancer: from yeast to man Sarah J Pfau and Angelika Amon
Cancer chromosomal instability: therapeutic and diagnostic challenges Nicholas McGranaham, Rebecca A Burrell, David Endesfelder, Marco R Novelli and Charles Swanton
Although a detailed picture of the processes that maintain cohesion in mitotic cells is starting to emerge (Fig 3), little is known about the contribution of this intricate network of antagonistic factors (Fig 3) to the maintenance of cohesion in dictyate-arrested oocytes or in meiosis in general (Sidebar A). It is not far-fetched to assume, however, that disturbances in the balance between pro- and anti-cohesion forces will significantly affect long-term cohesion in oocytes and thus the occurrence of aneuploidy. Furthermore, additional factors that regulate the association of cohesin with chromatin will probably be identified, such as proteins that affect the dimerization of the hinge domain.
Sidebar A | In need of answers.
Does leaky separase expression in young oocytes contribute to age-dependent aneuploidy?
Why are cohesin-protecting proteins lost with increasing oocyte age?
What is the contribution of distinct cohesin complexes to cohesion in oocytes?
How and when is cohesin initially loaded onto meiotic chromosomes?
Is there low-level of cohesin reloading onto chromosomes after prophase I?
What is the role of cohesin releasins, acetylases and deactetylases in age-dependent aneuploidy?
Does opening of the cohesin ring at the hinge domains contribute to loss of cohesion in ageing oocytes?
Thus, although cohesins were identified as key to avoid aneuploidy and cohesin deterioration has been characterized as a main cause of age-dependent aneuploidy, many additional factors and mechanisms—some directly related to cohesin biology and biochemistry—need to be explored to achieve a satisfactory understanding of this phenomenon, which is of utmost importance for human health.

Rolf Jessberger
Acknowledgments
Work in the author's laboratory is supported by a grant from the Deutsche Forschungsgemeinschaft through SPP1384. I thank Attila Toth and Francois McNicoll for critical reading and discussion.
Footnotes
The author declares that he has no conflict of interest.
References
- Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2: 280–291 [DOI] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P (2007) The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 16: R203–R208 [DOI] [PubMed] [Google Scholar]
- Homer H (2007) Ageing, aneuploidy and meiosis: eggs in a race against time. Yearbook of Obstetrics and Gynaecology 12: 139–158 [Google Scholar]
- Chiang T, Schultz RM, Lampson MA (2012) Meiotic origins of maternal age-related aneuploidy. Biol Reprod 86: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden R, Lee B (2010) Portrait of an oocyte: our obscure origin. J Clin Invest 120: 973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair T (2010) Mammalian oocyte development: checkpoints for competence. Reprod Fertil Dev 22: 13–20 [DOI] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC (2009) Awakening the oocyte: controlling primordial follicle development. Reproduction 137: 1–11 [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Svetlikova M, Wimalasena J, Ayala ME, Dominguez R (2005) Oogenesis in adult mammals, including humans: a review. Endocrine 26: 301–316 [DOI] [PubMed] [Google Scholar]
- Wassarman PM (2002) Channels of communication in the ovary. Nat Cell Biol 4: s7–s9 [DOI] [PubMed] [Google Scholar]
- Jamnongjit M, Hammes SR (2005) Oocyte maturation: the coming of age of a germ cell. Semin Reprod Med 23: 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza E, Alberman E, Morris JK (2010) Down's syndrome: screening and antenatal diagnosis regionally in England and Wales 1989–2008. J Med Screen 17: 170–175 [DOI] [PubMed] [Google Scholar]
- Melve KK, Lie RT, Skjaerven R, Van Der Hagen CB, Gradek GA, Jonsrud C, Braathen GJ, Irgens LM (2008) Registration of Down syndrome in the Medical Birth Registry of Norway: validity and time trends. Acta Obstet Gynecol Scand 87: 824–830 [DOI] [PubMed] [Google Scholar]
- Homer H (2011) New insights into the genetic regulation of homologue disjunction in mammalian oocytes. Cytogenet Genome Res 133: 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U (2008) Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res 651: 14–29 [DOI] [PubMed] [Google Scholar]
- Jones KT (2011) Anaphase-promoting complex control in female mouse meiosis. Results Probl Cell Differ 53: 343–363 [DOI] [PubMed] [Google Scholar]
- Steuerwald N, Cohen J, Herrera RJ, Sandalinas M, Brenner CA (2001) Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol Hum Reprod 7: 49–55 [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Goodwin P, Klein NA, Soules MR (1996) Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 11: 2217–2222 [DOI] [PubMed] [Google Scholar]
- Duncan FE, Chiang T, Schultz RM, Lampson MA (2009) Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 81: 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister LM et al. (2010) Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 20: 1511–1521 [DOI] [PubMed] [Google Scholar]
- Cromie GA, Smith GR (2007) Branching out: meiotic recombination and its regulation. Trends Cell Biol 17: 448–455 [DOI] [PubMed] [Google Scholar]
- Kleckner N (2006) Chiasma formation: chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma 115: 175–194 [DOI] [PubMed] [Google Scholar]
- Youds JL, Boulton SJ (2011) The choice in meiosis—defining the factors that influence crossover or non-crossover formation. J Cell Sci 124: 501–513 [DOI] [PubMed] [Google Scholar]
- Yanowitz J (2010) Meiosis: making a break for it. Curr Opin Cell Biol 22: 744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Lange J, Keeney S (2010) Genome destabilization by homologous recombination in the germ line. Nat Rev Mol Cell Biol 11: 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11: 124–136 [DOI] [PubMed] [Google Scholar]
- Lichten M, de Massy B (2011) The impressionistic landscape of meiotic recombination. Cell 147: 267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo SB, Clyne RK, Fuchs J, Loidl J, Uhlmann F, Nasmyth K (2000) Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103: 387–398 [DOI] [PubMed] [Google Scholar]
- Kudo NR et al. (2006) Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 126: 135–146 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517 [DOI] [PubMed] [Google Scholar]
- Riedel CG et al. (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61 [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Tanaka K, Sakuno T, Watanabe Y (2010) Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat Cell Biol 12: 500–506 [DOI] [PubMed] [Google Scholar]
- Darlington CD, Dark SOS (1932) The origin and behaviour of chiasmata. II. Cytologia 3: 169–185 [Google Scholar]
- Imai HT, Moriwaki K (1982) A re-examination of chiasma terminalization and chiasma frequency in male mice. Chromosoma 85: 439–452 [DOI] [PubMed] [Google Scholar]
- Mark HF, Zimmering S (1977) Centromeric effect on the degree of nonrandom disjunction in the female Drosophila melanogaster. Genetics 86: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagiello G, Fang JS (1979) Analyses of diplotene chiasma frequencies in mouse oocytes and spermatocytes in relation to ageing and sexual dimorphism. Cytogenet Cell Genet 23: 53–60 [DOI] [PubMed] [Google Scholar]
- Maguire MP (1962) Pachytene and diakinesis behavior of the isochromosomes 6 of maize. Science 138: 445–446 [DOI] [PubMed] [Google Scholar]
- Godward MBE (1961) Meiosis in Spirogyra crassa. Heredity 16: 53–62 [Google Scholar]
- Novitzki E (1951) Non-random disjunction in Drosophila. Genetics 36: 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K (2011) Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol 13: 1170–1177 [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43: 525–558 [DOI] [PubMed] [Google Scholar]
- Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE (2008) Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 24: 105–129 [DOI] [PubMed] [Google Scholar]
- Hirano T (2006) At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol 7: 311–322 [DOI] [PubMed] [Google Scholar]
- Jessberger R (2011) Cohesin complexes get more complex: The novel kleisin RAD21L. Cell Cycle 10: 2053–2054 [DOI] [PubMed] [Google Scholar]
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA (2005) SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 37: 1351–1355 [DOI] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R (2004) Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 6: 555–562 [DOI] [PubMed] [Google Scholar]
- Gilliland WD, Hawley RS (2005) Cohesin and the maternal age effect. Cell 123: 371–373 [DOI] [PubMed] [Google Scholar]
- Bickel SE (2005) Aging (not so) gracefully. Nat Genet 37: 1303–1304 [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu J-G, Hoja M-R, Wilbertz J, Nordqvist K, Hoog C (2002) Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 296: 1115–1118 [DOI] [PubMed] [Google Scholar]
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC (2004) Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 40: 184–194 [DOI] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8: 949–961 [DOI] [PubMed] [Google Scholar]
- Herran Y et al. (2011) The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 30: 3091–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA (2010) Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 20: 1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Keefe DL (2008) Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online 16: 103–112 [DOI] [PubMed] [Google Scholar]
- Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10: 42–52 [DOI] [PubMed] [Google Scholar]
- Llano E et al. (2008) Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev 22: 2400–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J, Sen N, Martinez VL, De Carandini ME, Schvartzman JB, Diffley JF, Aragón L (2011) Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 331: 1328–1332 [DOI] [PubMed] [Google Scholar]
- Farcas AM, Uluocak P, Helmhart W, Nasmyth K (2011) Cohesin's concatenation of sister DNAs maintains their intertwining. Mol Cell 44: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Herrmann K, Adelfalk C, Jessberger R (2010) Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol 20: 1529–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K (2010) Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 24: 2505–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Waizenegger IC, Peters JM (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293: 1320–1323 [DOI] [PubMed] [Google Scholar]
- Chiang T, Schultz RM, Lampson MA (2011) Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes. Biol Reprod 85: 1279–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolef Ben-Shahar T, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F (2008) Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321: 563–566 [DOI] [PubMed] [Google Scholar]
- Zhang J et al. (2008) Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 31: 143–151 [DOI] [PubMed] [Google Scholar]
- Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, Koshland DE (2008) A molecular determinant for the establishment of sister chromatid cohesion. Science 321: 566–569 [DOI] [PubMed] [Google Scholar]
- Hou F, Zou H (2005) Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell 16: 3908–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan G, Kreidl E, Wutz G, Egner A, Peters JM, Eichele G (2011) Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. EMBO J 31: 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega H et al. (2005) Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet 37: 468–470 [DOI] [PubMed] [Google Scholar]
- Schule B, Oviedo A, Johnston K, Pai S, Francke U (2005) Inactivating mutations in ESCO2 cause SC phocomelia and Roberts syndrome: no phenotype–genotype correlation. Am J Hum Genet 77: 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Boukari H, Banerjee I, Sbalzarini IF, Horvath P, Helenius A (2011) Histone deacetylase 8 is required for centrosome cohesion and influenza A virus entry. PLoS Pathog 7: e1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami A, Sakuno T, Yamagishi Y, Ishiguro T, Tsukahara T, Shirahige K, Tanaka K, Watanabe Y (2011) Acetylation regulates monopolar attachment at multiple levels during meiosis I in fission yeast. EMBO Rep 12: 1189–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Gillespie PJ, Hirano T (2006) Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol 16: 2406–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM (2006) Wapl controls the dynamic association of cohesin with chromatin. Cell 127: 955–967 [DOI] [PubMed] [Google Scholar]
- Losada A, Yokochi T, Hirano T (2005) Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci 118: 2133–2141 [DOI] [PubMed] [Google Scholar]
- Rankin S, Ayad NG, Kirschner MW (2005) Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell 18: 185–200 [DOI] [PubMed] [Google Scholar]
- Schmitz J, Watrin E, Lénárt P, Mechtler K, Peters JM (2007) Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol 17: 630–636 [DOI] [PubMed] [Google Scholar]
- Nishiyama T et al. (2010) Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143: 737–749 [DOI] [PubMed] [Google Scholar]
- Dreier MR, Bekier ME 2nd, Taylor WR (2011) Regulation of sororin by Cdk1-mediated phosphorylation. J Cell Sci 124: 2976–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Panigrahi AK, Mao Q, Pati D (2011) Interaction of Sororin protein with polo-like kinase 1 mediates resolution of chromosomal arm cohesion. J Biol Chem 286: 41826–41837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Kuznetsov SG, Sharan SK, Li K, Rao PH, Pati D (2008) A handcuff model for the cohesin complex. J Cell Biol 183: 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]