Abstract
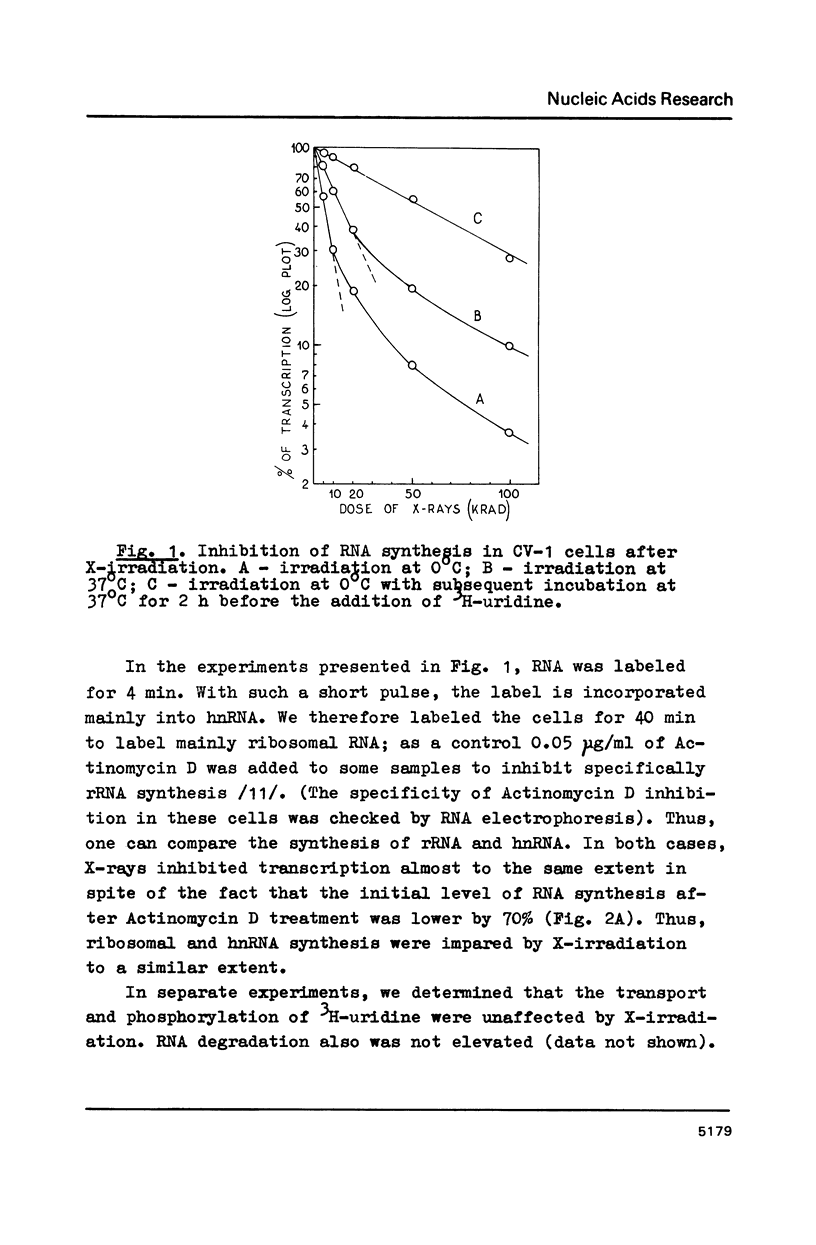
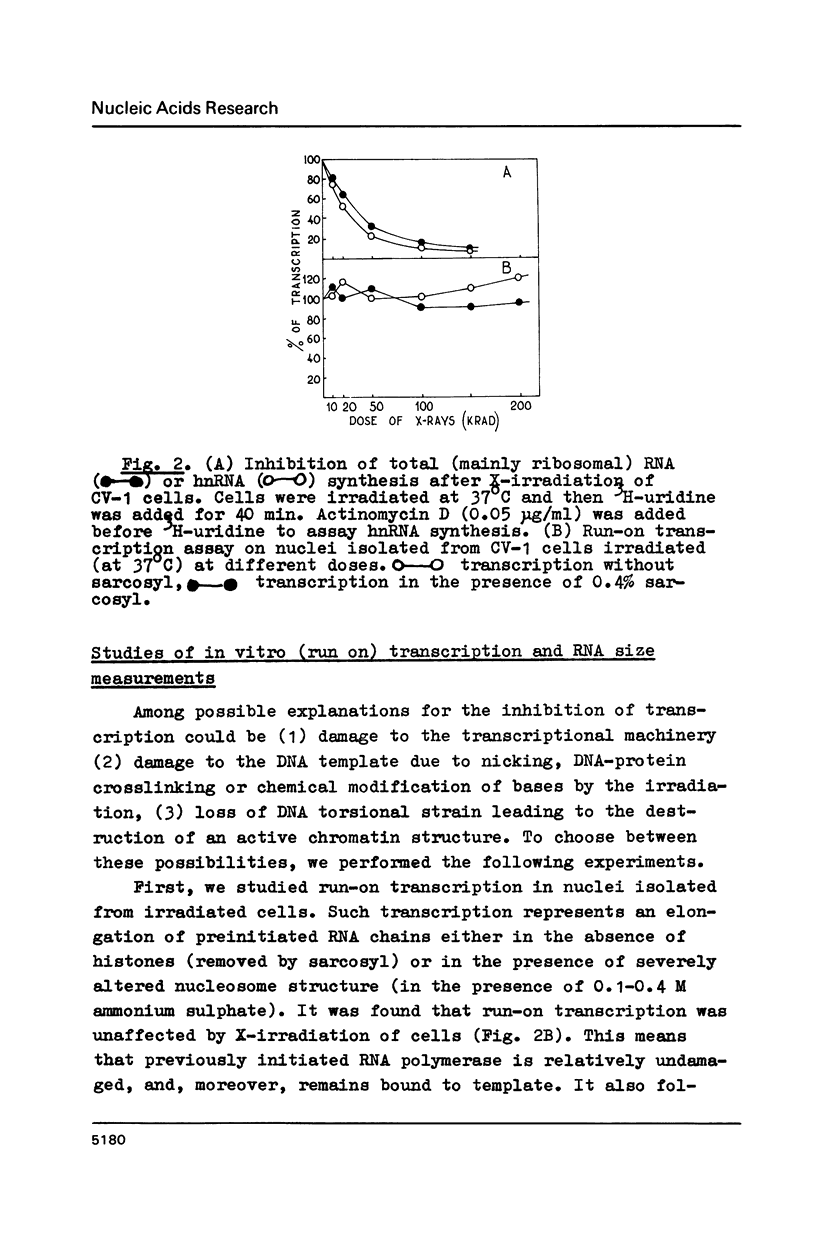
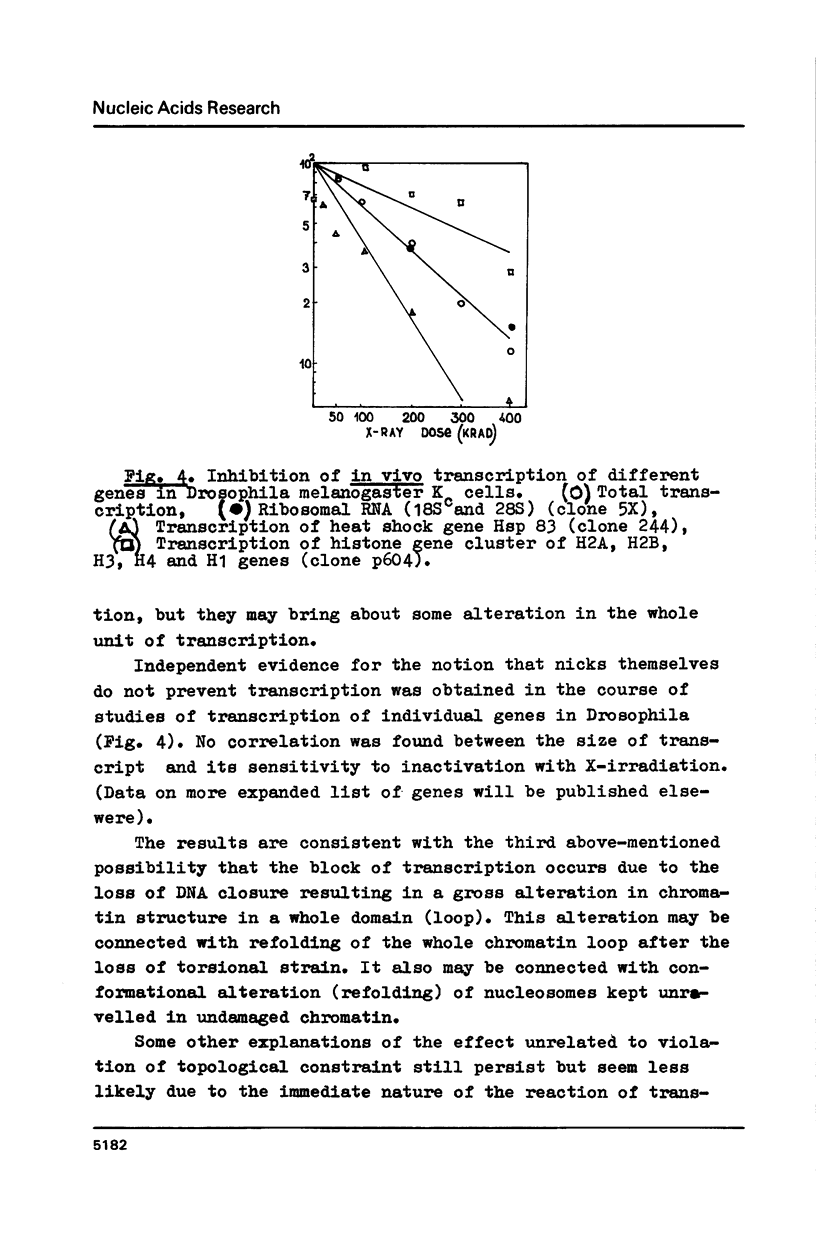
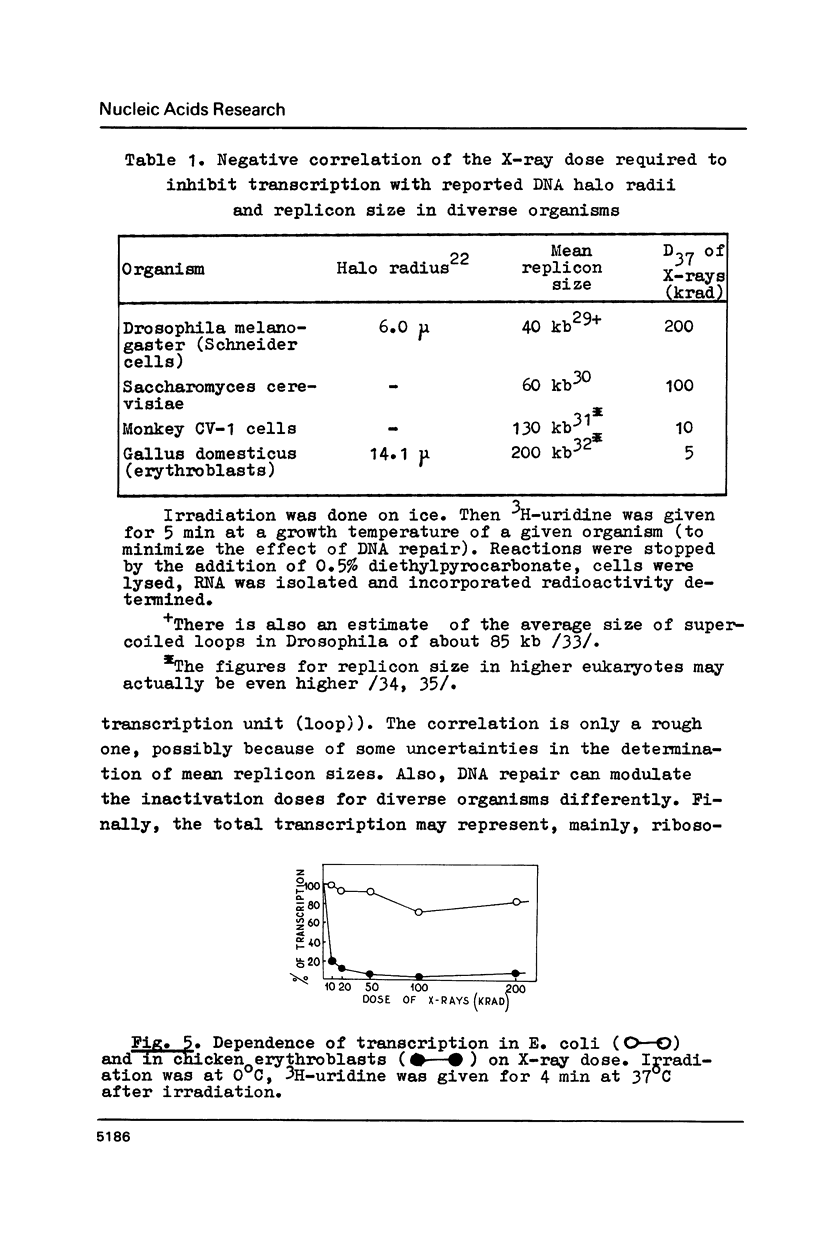
X irradiation was found to inhibit in vivo transcription in mammalian, yeast, insect and avian cells in a dose-dependent manner. Measurements of DNA nicking indicated that about one DNA single-strand break per estimated DNA loop (domain) length is sufficient to explain the effect. The inhibitory effect was partially reversed by post-irradiation incubation of cells. During such incubation DNA nicking was considerably repaired. The size of transcripts was not changed by irradiation. The in vitro (run on) activity of RNA polymerase in nuclei isolated from irradiated cells also was not altered. The dose-response curves were different in various cells, correlating with the reported unequal average domain size of supercoiled DNA (and also replicon size) in diverse organisms.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakayev V. V., Yugai A. A., Luchnik A. N. Effect of X-ray induced DNA damage on DNAase I hypersensitivity of SV40 chromatin: relation to elastic torsional strain in DNA. Nucleic Acids Res. 1985 Oct 11;13(19):7079–7093. doi: 10.1093/nar/13.19.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Micheli G., Carri M. T., Marilley M. A relationship between replicon size and supercoiled loop domains in the eukaryotic genome. Nature. 1982 Jul 1;298(5869):100–102. doi: 10.1038/298100a0. [DOI] [PubMed] [Google Scholar]
- Chiu S. M., Oleinick N. L., Friedman L. R., Stambrook P. J. Hypersensitivity of DNA in transcriptionally active chromatin to ionizing radiation. Biochim Biophys Acta. 1982 Oct 29;699(1):15–21. doi: 10.1016/0167-4781(82)90166-x. [DOI] [PubMed] [Google Scholar]
- Chiu S. M., Oleinick N. L. The sensitivity of active and inactive chromatin to ionizing radiation-induced DNA strand breakage. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Jan;41(1):71–77. doi: 10.1080/09553008214550061. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Spectrofluorometric measurement of the binding of ethidium to superhelical DNA from cell nuclei. Eur J Biochem. 1978 Mar 15;84(2):465–477. doi: 10.1111/j.1432-1033.1978.tb12188.x. [DOI] [PubMed] [Google Scholar]
- Daniel F. B., Haas D. L., Pyle S. M. Quantitation of chemically induced DNA strand breaks in human cells via an alkaline unwinding assay. Anal Biochem. 1985 Feb 1;144(2):390–402. doi: 10.1016/0003-2697(85)90132-0. [DOI] [PubMed] [Google Scholar]
- De Bernardin W., Koller T., Sogo J. M. Structure of in-vivo transcribing chromatin as studied in simian virus 40 minichromosomes. J Mol Biol. 1986 Oct 5;191(3):469–482. doi: 10.1016/0022-2836(86)90142-7. [DOI] [PubMed] [Google Scholar]
- Dreyer C., Hausen P. On the initiation of mammalian RNA polymerase at single-strand breaks in DNA. Eur J Biochem. 1976 Nov 1;70(1):63–74. doi: 10.1111/j.1432-1033.1976.tb10956.x. [DOI] [PubMed] [Google Scholar]
- GEORGIEV G. P., SAMARINA O. P., LERMAN M. I., SMIRNOV M. N., SEVERTZOV A. N. BIOSYNTHESIS OF MESSENGER AND RIBOSOMAL RIBONUCLEIC ACIDS IN THE NUCLEOLOCHROMOSOMAL APPARATUS OF ANIMAL CELLS. Nature. 1963 Dec 28;200:1291–1294. doi: 10.1038/2001291a0. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Felsenfeld G., O'Dea M. H., Gellert M. Effects of DNA supercoiling on the topological properties of nucleosomes. Proc Natl Acad Sci U S A. 1987 May;84(9):2620–2623. doi: 10.1073/pnas.84.9.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Weintraub H., McKnight S. L. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983 Mar 3;302(5903):38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- Kapp L. N., Painter R. B. X-ray inhibition of DNA synthesis at discrete times during S phase in synchronous human diploid fibroblasts. Radiat Res. 1982 Feb;89(2):424–427. [PubMed] [Google Scholar]
- Karpov V. L., Preobrazhenskaya O. V., Mirzabekov A. D. Chromatin structure of hsp 70 genes, activated by heat shock: selective removal of histones from the coding region and their absence from the 5' region. Cell. 1984 Feb;36(2):423–431. doi: 10.1016/0092-8674(84)90235-6. [DOI] [PubMed] [Google Scholar]
- Knezetic J. A., Luse D. S. The presence of nucleosomes on a DNA template prevents initiation by RNA polymerase II in vitro. Cell. 1986 Apr 11;45(1):95–104. doi: 10.1016/0092-8674(86)90541-6. [DOI] [PubMed] [Google Scholar]
- Lorch Y., LaPointe J. W., Kornberg R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell. 1987 Apr 24;49(2):203–210. doi: 10.1016/0092-8674(87)90561-7. [DOI] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Zbarsky I. B., Georgiev G. P. Elastic torsional strain in DNA within a fraction of SV40 minichromosomes: relation to transcriptionally active chromatin. EMBO J. 1982;1(11):1353–1358. doi: 10.1002/j.1460-2075.1982.tb01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchnik A. N. Long-distance signal transfer in transcriptionally active chromatin--how does it occur? Bioessays. 1985 Dec;3(6):249–252. doi: 10.1002/bies.950030604. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Matsui T. Transcription of adenovirus 2 major late and peptide IX genes under conditions of in vitro nucleosome assembly. Mol Cell Biol. 1987 Apr;7(4):1401–1408. doi: 10.1128/mcb.7.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane P. W., Callan H. G. DNA replication in the chromosomes of the chicken, Gallus domesticus. J Cell Sci. 1973 Nov;13(3):821–839. doi: 10.1242/jcs.13.3.821. [DOI] [PubMed] [Google Scholar]
- Pruitt S. C., Reeder R. H. Effect of intercalating agents on RNA polymerase I promoter selection in Xenopus laevis. Mol Cell Biol. 1984 Dec;4(12):2851–2857. doi: 10.1128/mcb.4.12.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Bohmann D., Zentgraf H., Weiher H., Keller W. A transcription enhancer acts in vitro over distances of hundreds of base-pairs on both circular and linear templates but not on chromatin-reconstituted DNA. J Mol Biol. 1984 Dec 15;180(3):577–600. doi: 10.1016/0022-2836(84)90028-7. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- Yurov Y. B. Do clusters of replication units in the mammalian cells exist? Exp Cell Res. 1979 Oct 15;123(2):369–374. doi: 10.1016/0014-4827(79)90479-8. [DOI] [PubMed] [Google Scholar]
- Yurov Y. B., Liapunova N. A. The units of DNA replication in the mammalian chromosomes: evidence for a large size of replication units. Chromosoma. 1977 Apr 19;60(3):253–267. doi: 10.1007/BF00329774. [DOI] [PubMed] [Google Scholar]


