Abstract
KCNE2, originally designated MinK-related peptide 1 (MiRP1), belongs to a five-strong family of potassium channel ancillary (β) subunits that, despite the diminutive size of the family and its members, has loomed large in the field of ion channel physiology. KCNE2 dictates K+ channel gating, conductance, α subunit composition, trafficking and pharmacology, and also modifies functional properties of monovalent cation-nonselective HCN channels. The Kcne2−/− mouse exhibits cardiac arrhythmia and hypertrophy, achlorhydria, gastric neoplasia, hypothyroidism, alopecia, stunted growth and choroid plexus epithelial dysfunction, illustrating the breadth and depth of the influence of KCNE2, mutations which are also associated with human cardiac arrhythmias. Here, the modus operandi and physiological roles of this potent regulator of membrane excitability and ion secretion are reviewed with particular emphasis on the ability of KCNE2 to shape the electrophysiological landscape of both excitable and non-excitable cells.
Keywords: cardiac arrhythmia, choroid plexus, gastric acid, hypothyroidism, KCNQ1, MiRP1, thyroid
Introduction
Potassium channels are one of the most numerous and diverse classes of membrane proteins and can be found in virtually all prokaryotic and eukaryotic cell types. They permit rapid and selective diffusion of K+ down its electrochemical gradient, typically to one of three main ends in mammalian physiology. First, voltage-dependent potassium (Kv) channels facilitate repolarization of excitable cells, generating the downstroke of the action potential by opening to allow K+ efflux in response to cellular depolarization.1 Second, “background” or “leak” K+ channels and inward rectifier K+ channels, contribute to maintaining the negatively hyperpolarized resting membrane potential of excitable cells, hence, most excitable eukaryotic cells are at rest near the potassium equilibrium potential, EK2,3 Third, certain K+ channels participate in K+ recycling, maintaining K+ gradients for purposes beyond its role as a cation contributing to membrane potential, e.g., for use as a substrate by K+ co-transporters.4,5
This diversity of function is made possible by several large K+ channel α subunit gene families,6 and also by a multitude of regulatory β subunits, that co-assemble with K+ channel α subunits to modulate aspects of their biology.7 One such β subunit family is the KCNE family, comprising five known human genes, KCNE1–5. KCNE subunits contain one TM segment, an extracellular N-terminal and intracellular C-terminal domain8 (Fig. 1A). The founding member, KCNE1, was originally termed “MinK” for ‘minimal potassium channel’ because despite its small size (129 amino acids), injection of MinK cRNA into Xenopus laevis oocytes was found to generate a slow activating K+ current.9 It was later discovered to be forming channels with an endogenous oocyte α subunit, KCNQ1 (Kv7.1), and increasing its current density such that it could be easily recorded by two-electrode voltage-clamp.10,11
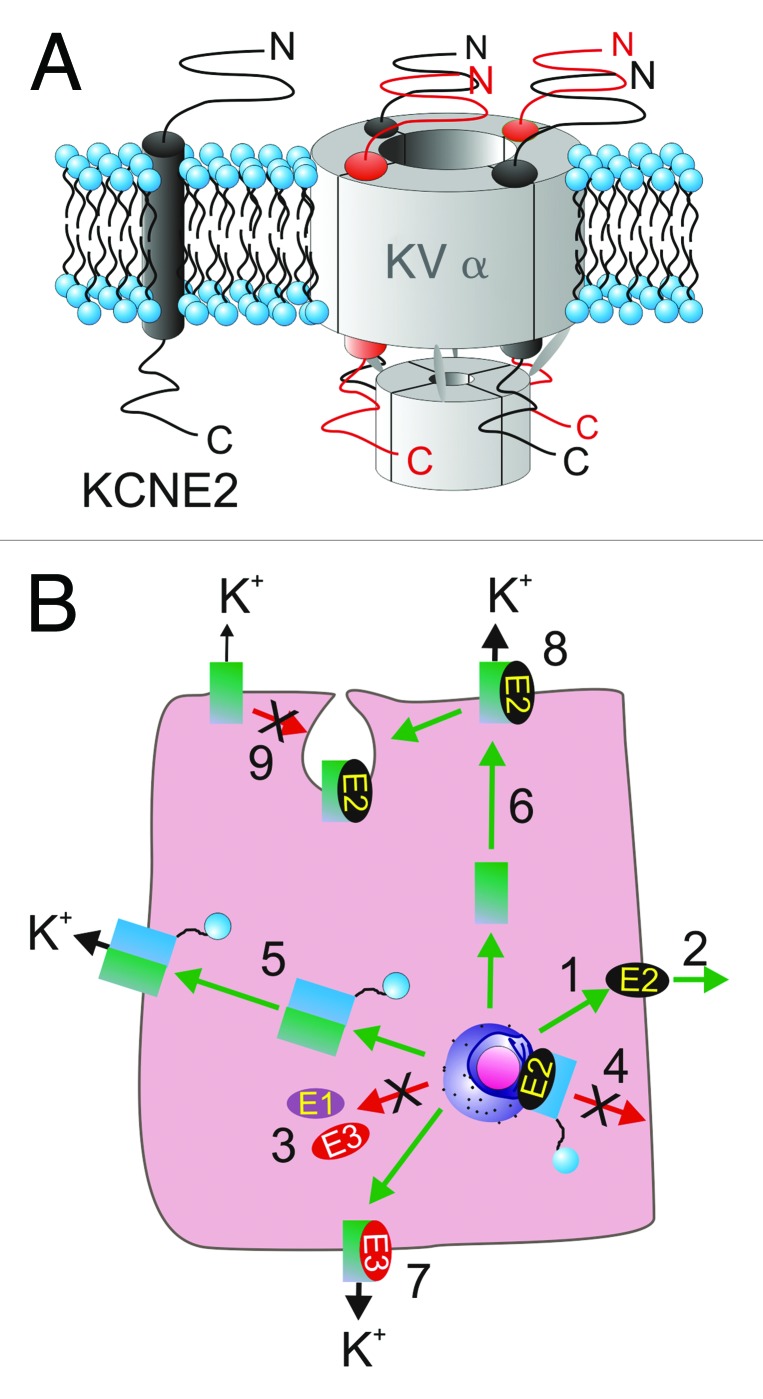
Figure 1. KCNE2 topology and stoichiometry. (A) Transmembrane topology (left) and potential stoichiometries (right) of KCNE2 in a Kv channel complex with four Kv α subunits. (B) KCNE2 can control multiple facets of the life of a K+ channel complex. (1) KCNE2 can travel to the plasma membrane in the absence of α subunits and (2) potentially be secreted outside the cell, whereas KCNE1 and KCNE3 appear to require α subunits for anterograde trafficking (3). (4) KCNE2 can prevent N-type α subunits from reaching the plasma membrane, an inhibition released by same-subfamily delayed rectifer α subunits (5). (6) KCNE2 can direct α subunit targeting in polarized cells differentially to, e.g., KCNE3 (7), and KCNE2 can alter multiple functional aspects of surface expressed α-KCNE2 complexes (8). KCNE2 can also mediate α subunit internalization from the plasma membrane (9). References in text.
Upon discovering several of the remaining members of the family, we named them MinK-related peptides (MiRPs),12 but the majority of researchers now refer to them by their KCNE nomenclature. Thus, MiRP1 = KCNE2, MiRP2 = KCNE3, etc. Even though the KCNE nomenclature is somewhat misleading as it dates back to when MinK was thought to generate currents in its own right, and hence was given an α subunit gene designation (KCNE1, similar to e.g., KCNA1 for the Kv1.1 α subunit) we will use the KCNE nomenclature here to avoid the confusion that can arise when using different nomenclature for the corresponding gene and protein. For α subunits, we will use the nomenclature most commonly used in each case, also giving alternate names at first use.
Here, we focus on KCNE2, an incredibly versatile β subunit that, by dictating multiple aspects of the biology of an array of potassium channel α subunits, exerts considerable influence on the electrophysiological landscape of several diverse cell types and tissues and facilitates a number of essential physiological processes.
Overview of the Influence of KCNE2 on K+ Channel α Subunits
The phrase “the tail wagging the dog” is used to convey a situation in which a minor element governs the major element of a system, and seems appropriate to describe the ability of the KCNE2 ancillary subunit to dictate the biological activity of numerous K+ channel α subunits in a variety of ways, described in this overview section and summarized in Figure 1B.
KCNE2 itself appears to have the capability to efficiently travel to the surface “alone” or at least in the absence of α subunits.13,14 In this respect, as in many others, it is distinct from KCNE1 and KCNE3, which at least in the cell types tested thus far, traffic far less efficiently to the plasma membrane without an α subunit escort.15,16 Surprisingly, KCNE2 is also thought to be secreted from the cell under some circumstances;14 whether this is solely an in vitro phenomenon or one with physiological relevance that occurs in vivo, remains to be understood.
Despite being able to travel alone to the cell surface, KCNE2 can exert dominant effects early in Kv channel biosynthesis. We recently reported that KCNE2 (and KCNE1) prevent the N-type fast-inactivating subunits (Kv1.4, Kv3.3 and Kv3.4, a.k.a. KCNA4, KCNC3 and KCNC4, respectively) from anterograde trafficking beyond the Golgi (holding the majority of protein in the endoplasmic reticulum, ER).13 These KCNE2-α complexes are efficiently sequestered in the ER (e.g., KCNE2 suppresses > 95% of Kv3.4 current by this mechanism) but can be rescued by co-expression of a same-subfamily delayed rectifier Kv α subunit. Thus, Kv3.1 and Kv3.2 can each rescue Kv3.4 from KCNE2-mediated ER trapping; Kv1.4 is rescued by Kv1.1.13,93 This provides a novel mechanism by which the cell can govern the α subunit composition (and thus inactivation rate and other properties) of Kv channels. Interestingly, it appears that while Kv1.1-Kv1.4 channels retain KCNE2 even at the cell surface, Kv3.1-Kv3.4 channels leave KCNE2 behind when they exit the ER or Golgi.13,93
KCNE2 also governs the polarity of α subunit trafficking in both excitable and non-excitable cells. This will be discussed in detail in the following sections, but essentially KCNE2 ensures apical trafficking of the KCNQ1 α subunit in some polarized epithelial cell types in vivo,17,18 while in mouse ventricular cardiomyocytes KCNE2 is required for the Kv1.5 (KCNA5) α subunit to target to the intercalated discs.19
When KCNE2-α complexes reach the plasma membrane, they typically exhibit functional properties different to those of channels formed by α subunits in the absence of KCNE2. KCNE2 can alter the unitary conduction, activation, inactivation, deactivation, responses to pH, regulation by other proteins, and pharmacology of its α subunit partners—sometimes spectacularly so (for detailed review, see ref. 8 and next section). There remains disagreement in the field as to α-KCNE subunit stoichiometry, with KCNQ1-KCNE1 being the widely-studied complex in this regard. The Goldstein laboratory published three reports, each employing different counting methods (site-directed mutagenesis with macroscopic and microscopic functional analysis, and toxin binding), indicating a probable 4:2 KCNQ1-KCNE1 subunit stoichiometry, although the possibility of 4:4 was not entirely dismissed.20-22 The rigid 4:2 stoichiometry was also arrived at in a later study using a chemically releasable irreversible inhibitor.23 More recently, a variable stoichiometry with up to four KCNE1 subunits per complex depending on expression levels was supported by single molecule fluorescence bleaching;24 the variable stoichiometry had been previously postulated by others.25
Finally, as originally reported for KCNE1, KCNE2 can mediate internalization of its α subunit partners. KCNE1 has been shown to endow KCNQ1 with the capacity for clathrin-mediated endocytosis,26 and stimulation of this by protein kinase C forms the basis for long-recognized inhibitory effects of protein kinase C on the cardiac IKs current.26,27 More recently, KCNE2 was found to govern internalization of the human ether-à-go-go related gene product (hERG, also named Kv11.1 or KCNH2) α subunit.28 Thus, KCNE2 can control each facet in the life of its α subunit partners. The remainder of this review will explore the known physiological roles of KCNE2 and the ramifications of KCNE2 perturbation.
KCNE2 in Parietal Cells: Gastric Acid Secretion
The oxyntic glands of the stomach contain a variety of specialized, polarized epithelial cell types with discrete functions. At the gastric pit are the surface mucus cells. In the isthmus and neck of the gland are located the mucus- and bicarbonate-secreting mucus neck cells, the gastric acid-secreting parietal cells, the histamine-secreting enterochromaffin-like (ECL) cells and the somatostatin-secreting D cells. At the base (and possibly neck) of the gland are the pepsinogen-secreting chief cells and the gastrin-secreting enteroendocrine “G” cells (Fig. 2A). The apical compartment of the parietal cells, which faces the stomach lumen, comprises a highly convoluted membrane which dynamically associates with vesicles containing the gastric H+/K+-ATPase. Increases in circulating levels of the hormone gastrin, in response to food intake, cause the release of secretagogues such as carbachol, which stimulate the H+/K+-ATPase-containing vesicles to fuse with the apical membrane proper. This permits extrusion of protons through the H+/K+-ATPase, acidifying the stomach lumen.29
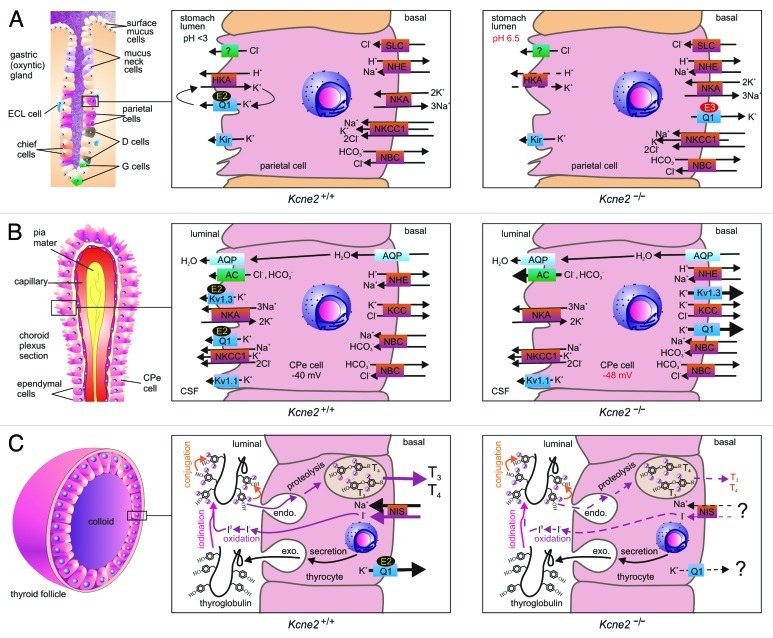
Figure 2. KCNE2 in epithelial cells. (A) KCNE2 in the gastric epithelium. Left, organization of an oxyntic gland in the stomach, with the parietal cell highlighted. Center, the gastric H+/K+-ATPase requires a luminal K+ recycling pathway for gastric acidification, formed by KCNQ1 (Q1) and KCNE2 (E2). Right, in Kcne2−/− mice, KCNQ1 is incorrectly targeted to the parietal cell basolateral membrane by upregulated KCNE3 (E3), and the mice are achlorydric because the gastric H+/K+-ATPase lacks a suitable K+ recycling pathway. (B) KCNE2 in the choroid plexus epithelium. Left, organization of the choroid plexus epithelium (CPe). Center, KCNE2 forms apical K+ channel complexes with KCNQ1 and Kv1.3, which probably contribute to setting membrane potential and regulating anion secretion into the CSF. Right, in Kcne2−/− mice, KCNQ1 and Kv1.3 are incorrectly targeted to the CPe cell basolateral membrane where they pass abnormally large outward K+ currents. Anion secretion appears to be increased, probably because the larger outward K+ current hyperpolarizes the CPe cell Em. (C) KCNE2 in the thyroid epithelium. Left, organization of a thyroid follicle, skirted by thyroid epithelial cells (thryocytes). Center, biosynthesis of thyroid hormones T3 and T4 requires I- to pass from the blood into the central colloid, where it is oxidized and organified by incorporation into thyroglobulin (iodination and conjugation). The product is endocytosed back into the thyrocyte, converted to T3 and T4 by proteolysis, then transported into the blood. KCNQ1-KCNE2 channels form on the basolateral membrane, as does the sodium iodide symporter (NIS). Right, in Kcne2−/− mice (and in Kcnq1−/− mice), thyroid hormone biosynthesis is disrupted by an incompletely understood mechanism characterized by impaired thyroid I- accumulation.
Efficient functioning of the H+/K+-ATPase requires a constant source of K+ on the luminal side, because K+ influx through the transporter is required for H+ efflux. As the intracellular K+ is higher than that outside, an ion channel would be predicted to perform this role efficiently, providing rapid, selective diffusion down an electrochemical gradient (Fig. 2A). However, this particular job is not as simple as it first appears. The environment outside the parietal cell would be extremely hostile to most ion channels, with a pH of 1–3 deep in these proton-rich canyons.
A number of candidate ion channels were found to be expressed at the parietal cell apical membrane: KCNQ1, Kir4.1 and Kir2.1.4,30,31 The latter two would seem ideal: inward rectifier K+ channel α subunits only contain two TM segments, lacking the intrinsic voltage sensor domain that would necessitate cellular depolarization for their activation, and hence possess the capability to be constitutively active at negative voltages (parietal cells are thought to spend most of the time between -20 and -40 mV). Their current is only attenuated at more depolarized voltages owing to intracellular block of their elongated pore by Mg2+ and polyamines such as spermine and spermidine.32 Furthermore, Kir2.1 is acid-activated.30
The KCNQ1 α subunit is, at first glance, a less obvious candidate: it belongs to the 6-TM segment family of Kv α subunits, bears a voltage sensor, and homomeric KCNQ1 channels inactivate if held at depolarizing voltages. However, KCNE2 provides the missing piece to this puzzle, and the heteromeric KCNQ1-KCNE2 channel has been established as the molecular correlate of the apical, parietal cell K+ recycling current required for gastric acid secretion. Both components of this channel are essential: targeted deletion of either Kcnq1 or Kcne2 causes achlorhydria and increased gastric mass arising from mucus neck cell hyperplasia.33-36 KCNE3 is also detectable in the stomach, and appears to be expressed at relatively low levels in parietal cells, but is more readily detected in other gastric cells, possibly mucus neck cells and/or guard cells.18,37 However, Kcne3−/− mice do not exhibit gastric hyperplasia, achlorhydria, or any other obvious gastric phenotype, indicating KCNE3 is not required for gastric acid secretion.18 In contrast, the importance of KCNE2 to gastric health is highlighted by the extreme phenotype of aging Kcne2−/− mice: a progression from hyperplasia to metaplasia, 6-fold higher stomach mass than that of age-matched Kcne2+/+ littermates, complete penetrance of a condition termed gastritis cystica profunda, and even incidences of gastric neoplasia.18
KCNE2 and KCNE3 each convert the KCNQ1 tetramer from a voltage-dependent delayed rectifier to a constitutively active, relatively voltage-independent channel that provides an open but K+-selective pore at negative voltages.38,39 Work on the KCNQ1-KCNE3 channel indicates that KCNE3 probably both interacts with the KCNQ1 pore directly, and also locks open the KCNQ1 voltage-sensing S4 domain, which in turn holds open the pore, and that these actions require residues in the transmembrane and extracellular, juxtamembrane regions of KCNE3.40-44 However, unlike KCNE3, KCNE2 also provides KCNQ1 with a property essential to its task in parietal cells—enhanced activation with low extracellular pH; in contrast, KCNQ1-KCNE3 channels are pH-insensitive, and homomeric KCNQ1 is inhibited by low pH.45 Furthermore, in Kcne2-deleted mice, KCNQ1 traffics incorrectly, to the parietal cell basolateral membrane. This is because KCNE3, the expression of which is upregulated in Kcne2−/− mouse parietal cells, traffics KCNQ1 basolaterally in the absence of KCNE2 (Fig. 2A); in double-knockout Kcne2−/−Kcne3−/− mouse parietal cells, KCNQ1 targets to the apical side.18 Interestingly, Kcne2−/−Kcne3−/− mice have a more severe gastric phenotype than Kcne2−/− mice, indicating that returning KCNQ1, in its homomeric form, to the apical side is not sufficient to restore gastric acid secretion—it requires KCNE2 to perform its function there.18 Thus, KCNE2 shapes parietal cell physiology by ensuring a constitutive, acid-upregulated K+ flux at the apical side (Fig. 2A).
More recently, other parietal cell K+ channels have been found to act in an opposite fashion to KCNQ1-KCNE2, providing a brake to acid secretion. KCa3.1 is expressed in the parietal cell basolateral membrane and blunts carbachol-stimulated gastric acidification.46 Kir4.1 is located in the apical membrane of parietal cells, co-localizing with the gastric H+/K+-ATPase.31 Kir4.1−/− mice show increased gastric acid secretion, and Kir4.1 appears to contribute to control of acid secretion, perhaps by providing a balance between K+ loss through KCNQ1-KCNE2 vs. K+ reabsorption via the H+/K+-ATPase; Kir4.1 may also regulate secretory membrane recycling.47
KCNE2 in Choroid Plexus Epithelial Cells: Cerebrospinal Fluid Secretion
The tissue in which KCNE2 signal is most readily detectable by immunohistochemistry is one in which its physiological role was only recently reported. In the choroid plexus epithelium (CPe), KCNE2 protein is robustly expressed at the apical membrane.17 The CPe is a polarized nonexcitable monoepithelial cell layer that lines the lateral and fourth ventricles of the brain, and is the major site of cerebrospinal fluid (CSF) production, secretion and regulation.48,49 Accordingly, the flux of ions from the basolateral (blood) side to the apical (CSF) side of the CPe must be exquisitely regulated, as it is highly influential upon physiological aspects of the CNS including neuronal function and intracranial pressure50-53 (Fig. 2B).
One of the roles of the CPe is to maintain CSF [K+] at 3 mM, below that of the serum, which is typically 4–5 mM. However, this job is thought to be performed primarily by K+/Cl- co-transporters actively removing K+ from the CSF through the CPe to the blood. Apical K+ channels, in contrast, are thought to regulate CPe cell membrane potential to control anion flux to and from the CPe and CSF; they may also prevent hypokalorrachia (abnormally low CSF [K+]) by permitting K+ efflux from the CPe50,52 (Fig. 2B).
As in the parietal cell, KCNE2 forms apical K+ channels with KCNQ1 in the CPe; in addition, KCNE2 forms apical CPe K+ channels with Kv1.3 (KCNA3).17 Kv1.3 is a Kv α subunit most noted for its role in the T cells of the immune system, and is consequently highly studied as a therapeutic target for immunosuppressant drugs that may be able to prevent, for example, transplant organ rejection, and a host of autoimmune diseases including multiple sclerosis.54-56 Kv1.3-KCNE2 channels retain their voltage dependence, but exhibit lower macroscopic current density than their homomeric Kv1.3 counterparts; the mechanism for this current reduction being thus far unclear.17
Targeted deletion of Kcne2 in mice results in upregulation of KCNQ1 and Kv1.3 outward currents in the CPe, and an increase in the outward rectification of K+ current overall, which tallies well with the reported effects of KCNE2 on each of these α subunits. Strikingly, in the absence of KCNE2, KCNQ1 and Kv1.3 traffic incorrectly, to the basolateral instead of the apical membrane, recapitulating what was observed in Kcne2−/− mouse parietal cells. In Kcne2−/− mice, the CPe cells also exhibit a 9 mV membrane potential hyperpolarization, and 14% increase in CSF [Cl-] that probably results from this hyperpolarization.17,18
Thus, KCNE2 shapes CPe cell K+ flux by maintaining two apical K+ fluxes, one constitutively active (KCNQ1-KCNE2), one voltage-dependent (Kv1.3-KCNE2) (Fig. 2B).
KCNE2 in Thyrocytes: Thyroid Hormone Biosynthesis
Thyrocytes, or thyroid epithelial cells, line the colloid-containing follicles of the thyroid gland. The task of the follicles is to produce and secrete into the bloodstream thyroid hormones, a major endocrine regulator of a broad range of physiological functions. The sodium/iodide symporter (NIS), located basolaterally in thyrocytes, actively transports iodide, an essential component of thyroid hormone, from the blood into the thyrocyte.57 Iodide is then oxidized by Duox at the apical membrane-colloid interface, and covalently bound to thyroglobulin, a process termed “organification.”58 The iodide-bound thyroglobulin is then transported back across the apical membrane, through the secretory pathway of the thyrocyte, and secreted into the blood stream as thyroxin (T4) and triiodothyronine (T3). T4 is converted into T3, the more active form, in cells by deiodinases (Fig. 2C).
KCNE2 is expressed in thyrocytes, together with KCNQ1, but in this case the two appear to be located at the basolateral side.59 While several KCNE subunits are expressed in thyrocytes,60 currents with the characteristics of KCNQ1-KCNE2 channels are detectable in the FRTL5 rat thyrocyte cell line, i.e., linear current voltage relationship combined with XE991 sensitivity. In Kcne2-deleted mice, a defect in thyroid iodide accumulation, and consequent hypothyroidism, is observed. This, in turn, results in retarded growth and skeletal development, alopecia and cardiac hypertrophy. These symptoms occur at a particularly early age in pups from Kcne2−/− dams, owing to hypothyroidism of the dam during gestation and lactation.59
The mechanism for defective iodide accumulation arising from Kcne2 deletion has not yet been resolved, but a likely explanation is disruption of NIS-mediated iodide uptake, with another possibility being defective iodide organification (Fig. 2C). That this disruption reflects impaired function of the KCNQ1-KCNE2 channel is strongly supported by pharmacological and functional evidence,59 and the finding that Kcnq1−/− mice also exhibit hypothyroidism.60 We do not yet know why KCNQ1-KCNE2 function, and presumably K+ flux through this channel, might be important for NIS function (and/or the organification process)—is it related to membrane potential, or establishment of gradient of a particular ion or ions per se? Furthermore, it has not been ascertained whether Kcne2 deletion impairs thyrocyte KCNQ1 trafficking, nor whether it increases (as in the CPe) or decreases thyrocyte KCNQ1 outward current (Fig. 2C).
Interestingly, KCNE2 expression in FRTL5 cells is highly regulated by cyclic adenosine monophosphate, a major downstream effector of thyroid hormone. Also, KCNQ1-KCNE2 current is upregulated by thyroid hormone.59 These data suggest that in vivo, KCNQ1-KCNE2 channel expression is increased in response to increased need for thyroid hormone production, perhaps to facilitate increased transport capability of NIS, by an as yet unknown mechanism. This would be consistent with the finding that virgin Kcne2−/− mice are euthyroid at 3–6 mo of age, whereas gestating, lactating or aging Kcne2−/− mice are hypothyroid—suggesting a reduced capacity for the Kcne2−/− thyroid gland to cope during times of an increased need for thyroid hormone, or when the thyroid is aging and other aspects of thyroid function may be impaired. Kcne2−/− pups born to Kcne2−/− dams, or any pups fed by Kcne2−/− dams, exhibit the severest hypothyroidism-related symptoms, but this is partly due to impaired milk ejection in the dams.59
We do not yet know what factors determine the apical localization of KCNQ1-KCNE2 in the CPe and parietal cells, vs. the basolateral localization in thyrocytes, or whether in the latter KCNE2 influences KCNQ1 localization as it does in the other systems, but it is not uncommon for channels or transporters to exhibit cell-type dependent localization, e.g., NKCC1 is located basolaterally in parietal cells and outer medullary cells but apically in the CPe.61-63 Current knowledge is that KCNE2 is required for KCNQ1 to function as a basolateral K+ channel required for thyroid iodide accumulation.
KCNE2 in Cardiac Myocytes: Ventricular Repolarization
An earlier report contended KCNE2 expression was weak in canine cardiac ventricles, and predominantly expressed in the His-Purkinje system.64 However, other reports have contradicted these findings, demonstrating more robust KCNE2 expression in human, canine, guinea-pig and rat ventricles.28,65-67 In particular, a very recent study showed higher expression of KCNE2 protein in human, rat and guinea pig ventricle compared with atrium, and downregulation of ventricular KCNE2 in failing human heart compared with non-failing human heart.28 This study, which included use of ectopic expression of KCNE2 as a positive control for myocyte expression, suggested how western bands in some previous studies may have been misinterpreted, possibly contributing to contradictory reports in the past.
KCNE2 forms complexes with several different Kv channels in mammalian ventricle depending on species (Fig. 3), with an array of functional effects, and has the capacity to potentially regulate many more channels according to in vitro data, including the HCN1, 2 and 4 monovalent cation-nonspecific pacemaker channels.68-70 Given this, KCNE2 can be considered a potent regulator of multiple aspects of cardiac myocyte excitability.
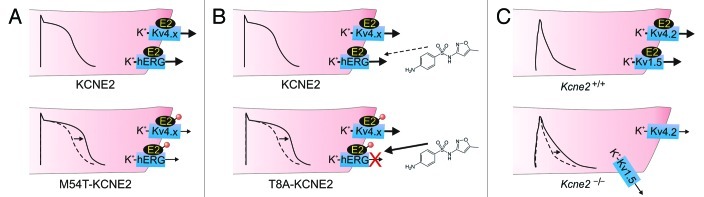
Figure 3. KCNE2 in ventricular myocytes. (A) KCNE2 is thought to regulate hERG and Kv4.x channels in the ventricular cardiomyocytes of large mammals. Inherited mutations (red circle) in human KCNE2 that cause loss of function of hERG-KCNE2 channels (and also in some cases Kv4.x-KCNE2 complexes) are associated with delayed ventricular repolarization manifesting clinically as Long QT syndrome. This probably arises from prolonged ventricular action potentials (arrow) arising from reduced K+ flux, especially in phase 3. One example is M54T-KCNE2, a mutation that speeds hERG-KCNE2 channel deactivation. (B) Some human KCNE2 polymorphisms, including T8A, have no appreciable effect on channel function at baseline, but increase the susceptibility of hERG-KCNE2 channels to inhibition by therapeutic agents, in this case sulfamethoxazole, a component of the antibiotic Bactrim. This inhibition can lengthen the QT interval and predispose to drug-induced torsades de pointes, a dangerous ventricular arrhythmia. (C) In mice, KCNE2 regulates Kv4.2 and Kv1.5 in ventricular myocytes. Kcne2 deletion reduces Kv4.2 and Kv1.5 currents and increases mouse ventricular myocyte action potential duration (arrow). Kv1.5 targeting to the intercalated discs is also impaired in Kcne2−/− mouse ventricles.
KCNE2 forms complexes with hERG when the two are co-expressed in Xenopus laevis oocytes or in Chinese hamster ovary (CHO) cells and other mammalian immortal cell lines.12,14,71-74 In adult canine ventricular tissue, KCNE2 and cERG are co-localized in cholesterol and sphingolipid-enriched membrane fractions.75 hERG (and ERG from other mammals) is unusual in that it is part of the S4 superfamily of voltage-gated ion channels that includes the Kv α subunits, but it exhibits inward rectification similar to the Kir subunits. However, the mechanism in the two channel types is distinct: in hERG, activation is followed by extremely rapid inactivation that reduces peak current at depolarized potentials,76 whereas in the Kir channels inward rectification occurs because of block by intracellular moieties at depolarized membrane potentials.32 Because of this unusual gating, and its relatively slow deactivation, hERG passes large currents during phase 3 of the ventricular myocyte action potential, when the cell is repolarizing.77,78 Hence, IKr, the current generated by hERG, is the primary repolarization current in human heart, at least at baseline conditions; whereas at higher heart-rates or when IKr is compromised by drug block, for example, the slowly-activating IKs current becomes increasingly important.79
KCNE2 right-shifts the voltage-dependence of hERG activation, accelerates hERG deactivation, and reduces its unitary conductance (and macroscopic current density) ~40%,12 although variable functional effects have been reported80 and a more recent study indicates KCNE2 also regulates hERG endocytosis.28 While KCNE2 is not required for the notoriously high sensitivity of hERG to drug-induced block, KCNE2 alters some aspects of hERG pharmacology. In heterologous expression studies in CHO cells, KCNE2 endowed hERG with the distinct biphasic E-4031 block kinetics characteristic of native IKr but in contrast to the monophasic block of homomeric hERG.12 KCNE2 also increases the susceptibility of hERG to inhibition by propranolol,81 and decreases (at least for the mature-glycosylated, T8 variant of KCNE2) susceptibility of hERG to inhibition by sulfamethoxazole.82,83
Inherited gene variants in human KCNE2 that impair the function of hERG-KCNE2 channels are associated with both congenital and drug-induced long QT syndrome (LQTS), reflecting the role of KCNE2 in ventricular repolarization12 (Fig. 3A and B). LQTS is a delay in ventricular repolarization that manifests as a prolonged QT interval on the surface electrocardiogram, and can predispose to the life-threatening arrhythmia, ventricular fibrillation.84 One KCNE2 sequence variant, T10M, was found in an individual with auditory induced syncope,85 a condition almost exclusively linked to hERG mutations.86
Relatively common polymorphisms and also rare gene variants in KCNE2 also associate with cases of drug-induced LQTS, in which IKr block by the drug reduced the density of this essential repolarization current. In two of these cases, the polymorphism in question was found, using cellular electrophysiology, to actually increase the sensitivity of hERG-KCNE2 to block by the arrhythmia-causing drug (KCNE2-T8A with sulfamethoxazole; KCNE2-Q9E with clarithromycin) (Fig. 3B). In the other cases, arrhythmia was likely precipitated by a combination of a loss-of-function KCNE2 mutation, and a drug dose that blocked even wild-type hERG-KCNE2 channels.12,83
While the weight of evidence, including studies of human, canine, guinea-pig and rat heart, suggests a role for KCNE2 in regulating IKr in vivo,12,28,65,67,85 this association is not universally agreed upon,80 and the cardiac function of KCNE2 is certainly not as simple as this. Kcne2 deletion delays ventricular repolarization in adult mice,19 in which mERG and IKr are essentially absent from ventricular myocytes.87 KCNE2 regulates the rapidly inactivating Kv4.2 (KCND2) α subunit in vitro (the most prominent effects being slowing of inactivation and increased current density),88 and its current correlate, Ito, in vivo in mouse ventricles.19 KCNE2 also forms complexes with Kv1.5 (KCNA5) in mouse ventricles, and is required for enrichment of Kv1.5 expression in the intercalated discs.19 Kcne2 deletion in mice reduces ventricular Kv1.5 current (IKur) density 2-fold, by an unknown mechanism that may be related to inability of Kv1.5 to target to the intercalated discs in the absence of KCNE219 (Fig. 3C). KCNE2 also forms complexes with Kv2.1 (KCNB1) in rat heart. KCNE2 reduces Kv2.1 current density and slows its activation, and LQTS-associated KCNE2 mutations exaggerate these effects,67 but a role for Kv2.1 in human heart has yet to be established, and the KCNE2-Kv2.1 association was not observed in mouse ventricles.19
Finally, KCNE2 alters function of the hyperpolarization activated, cyclic nucleotide-gated, monovalent cation nonspecific channels (HCN1, 2 and 4), also known as pacemaker channels, in vitro and may also do so in mammalian heart.65,69,70 KCNE2 reportedly increases unitary conductance of HCN1, 2 and 4; increases HCN2 open time, decreases HCN4 open time; and somewhat increases surface expression of HCN2 and HCN4.68 HCN2 expresses both a time- and voltage-dependent, hyperpolarization-activated current (Ih) and an instantaneous current (Iinst(HCN2)); KCNE2 was found to decrease (Ih) but increase Iinst(HCN2) when co-expressed with mouse HCN2.89 In addition, KCNE2 increases sensitivity of HCN2 instantaneous current to enhancement by tanshinone IIA, one of the main active components from the Chinese herb Danshen, which has heart rate-reducing and anti-ischemic properties.90
Human KCNE2 gene variants are also associated with atrial fibrillation; in this case the variants caused a gain of function of KCNQ1-KCNE2 channels when studied in vitro, consistent with a potential role in atrial fibrillation, which can be triggered by shortening of the atrial refractory period.91 It is not yet known whether KCNQ1-KCNE2 channels form in mammalian heart, but one possible role is for a background K+ conductance that could provide an essentially constant repolarizing force, possibly regulated by factors such as pH or cyclic AMP.
Hence, KCNE2 is thought to impact multiple aspects of cardiac excitability, including pacemaking and atrial and ventricular repolarization, by modifying the conductance, gating, trafficking and pharmacology of a host of Kv and HCN channels.
Hypothetical Neuronal Roles for KCNE2 Based Upon Evidence in Vitro
KCNE2 has also been found to functionally regulate the entire Kv3 subfamily of Kv α subunits, Kv3.1-Kv3.4 (KCNC1–4) in vitro.13,92,93 All these α subunits are expressed in the CNS, and Kv3.4 also in skeletal muscle.94 In the CNS, Kv3 subunits facilitate rapid firing of neurons owing to their extremely rapid activation kinetics, particularly of Kv3.1 and Kv3.2.95-97 These two delayed rectifier α subunits form heteromeric complexes with Kv3.4 and possibly Kv3.3, which in contrast are both N-type rapidly inactivating Kv α subunits.98-100 For example, Kv3.1-Kv3.4 heteromers are expressed in fast-spiking neurons, where they facilitate rapid firing. In general, Kv3.1-Kv3.4 heteromers generate a Kv current with inactivation kinetics intermediate between that of homomeric Kv3.1 and Kv3.4.101
KCNE2, as also observed for KCNE1, slows the activation and deactivation kinetics of Kv3.1 and Kv3.2, and accelerates their slow, C-type inactivation.92 In contrast, KCNE2 (and KCNE1, but not KCNE3) strongly suppresses Kv3.3 and Kv3.4 current, by sequestering these α subunits early in the secretory pathway and thus preventing them from passing K+ currents across the plasma membrane.13 When KCNE2 (or KCNE1) is co-expressed with both Kv3.1 and Kv3.4, something interesting occurs: Kv3.1-Kv3.4 channels are expressed at the plasma membrane and KCNE2 does not contribute to the surface-expressed complex.93 This appears to be a mechanism for regulating α subunit composition of surface expressed N-type channels, as the same phenomenon occurs with KCNE2 (or KCNE1) and Kv1.4 (KCNA4). Thus, Kv1.4 current is suppressed by KCNE2 but rescued by co-expression of Kv1.1 (KCNA1),93 a delayed rectifier α subunit which can form complexes with Kv1.4 because the two are in the same sequence similarity-defined subfamily, and therefore, have compatible N-terminal tetramerization domains.102,103
KCNE2 therefore prevents homomeric N-type α subunit channel formation, and instead ensures that only mixed N-type-delayed rectifier α subunit channels reach the cell surface.93 In this way, KCNE2 can potentially regulate cellular excitability by yet another mechanism, i.e., controlling inactivation kinetics via subunit composition, and therefore, controlling refractory period and after-hyperpolarizations resulting from post-inactivation recovery re-openings.104 One postulated physiological application for this would be in the rapid-firing neurons in which Kv3 channels are expressed. While neuronal KCNE2 protein expression has been resistant to detection, in situ hybridization studies indicated Kcne2 transcripts in the areas including the hippocampus, thalamus, hypothalamus (but predominantly in the CPe in the fourth and lateral ventricles, consistent with recent findings for KCNE2 protein).17,105
KCNE2 is postulated to regulate the KCNQ2-KCNQ3 channel, which generates the neuronal muscarinic (M) K+ current, based on gating effects and potential expression overlap.105 KCNQ1 is also reportedly neuronally expressed in the CNS,106 and could form complexes with KCNE2 there. Finally, it is possible that KCNE2 could associate with HCN channels in the brain. KCNE2 thus has the potential to shape neuronal excitability by influencing the trafficking, α subunit composition and gating of an assortment of Kv and HCN α subunits in the CNS.
Conclusions
The variety of native K+ currents is staggering, arising from a multiplicity of factors and combinatorial complexity. In this review the contribution to this complexity of just one ancillary subunit, KCNE2, has been discussed. KCNE2 has a broad influence on native cellular excitability owing to its promiscuity, ubiquitous expression, and cornucopia of effects on K+ channel biology. What has not been covered comprehensively here is that the relative contribution to native cell physiology of subunits such as KCNE2 is dynamically regulated and altered during, e.g., development, gestation and disease states, and by electrical and chemical stimuli during normal cellular functioning. The challenges ahead include how to condense the extraordinary and ever-increasing wealth of information on the biology of KCNE2 and other ion channel subunits into cellular and tissue-level models (including computational models) that can provide a useful framework for further enhancing our understanding of physiology and disease and for development of ion channel-targeted therapeutics.
Acknowledgments
G.W.A. is grateful for financial support from the US National Heart, Lung and Blood Institute, National Institutes of Health (R01 HL079275; R01HL101190).
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/19126
References
- 1.Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–84. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 3.Barry DM, Nerbonne JM. Myocardial potassium channels: electrophysiological and molecular diversity. Annu Rev Physiol. 1996;58:363–94. doi: 10.1146/annurev.ph.58.030196.002051. [DOI] [PubMed] [Google Scholar]
- 4.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Archiv. 2001;442:896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 5.Song P, Groos S, Riederer B, Feng Z, Krabbenhoft A, Smolka A, et al. KCNQ1 is the luminal K+ recycling channel during stimulation of gastric acid secretion. J Physiol. 2009;587:3955–65. doi: 10.1113/jphysiol.2009.173302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Um SY, McDonald TV. Voltage-gated potassium channels: regulation by accessory subunits. Neuroscientist. 2006;12:199–210. doi: 10.1177/1073858406287717. [DOI] [PubMed] [Google Scholar]
- 8.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–5. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 10.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey GK. (V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 11.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 12.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–87. doi: 10.1016/S0092-8674(00)80728-X. [DOI] [PubMed] [Google Scholar]
- 13.Kanda VA, Lewis A, Xu X, Abbott GW. KCNE1 and KCNE2 inhibit forward trafficking of homomeric N-type voltage-gated potassium channels. Biophys J. 2011;101:1354–63. doi: 10.1016/j.bpj.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Um SY, McDonald TV. Differential association between HERG and KCNE1 or KCNE2. PLoS ONE. 2007;2:e933. doi: 10.1371/journal.pone.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrasekhar KD, Bas T, Kobertz WR. KCNE1 subunits require co-assembly with K+ channels for efficient trafficking and cell surface expression. J Biol Chem. 2006;281:40015–23. doi: 10.1074/jbc.M604398200. [DOI] [PubMed] [Google Scholar]
- 16.McCrossan ZA, Lewis A, Panaghie G, Jordan PN, Christini DJ, Lerner DJ, et al. MinK-related peptide 2 modulates Kv2.1 and Kv3.1 potassium channels in mammalian brain. J Neurosci. 2003;23:8077–91. doi: 10.1523/JNEUROSCI.23-22-08077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roepke TK, Kanda VA, Purtell K, King EC, Lerner DJ, Abbott GW. KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. FASEB J. 2011;25:4264–73. doi: 10.1096/fj.11-187609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roepke TK, King EC, Purtell K, Kanda VA, Lerner DJ, Abbott GW. Genetic dissection reveals unexpected influence of beta subunits on KCNQ1 K+ channel polarized trafficking in vivo. FASEB J. 2011;25:727–36. doi: 10.1096/fj.10-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roepke TK, Kontogeorgis A, Ovanez C, Xu X, Young JB, Purtell K, et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f) FASEB J . 2008;22:3648–60. doi: 10.1096/fj.08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Kim LA, Rajan S, Xu S, Goldstein SA. Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40:15–23. doi: 10.1016/S0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 21.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112:651–63. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang KW, Goldstein SA. Subunit composition of minK potassium channels. Neuron. 1995;14:1303–9. doi: 10.1016/0896-6273(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 23.Morin TJ, Kobertz WR. Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc Natl Acad Sci USA. 2008;105:1478–82. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajo K, Ulbrich MH, Kubo Y, Isacoff EY. Stoichiometry of the KCNQ1 - KCNE1 ion channel complex. Proc Natl Acad Sci USA. 2010;107:18862–7. doi: 10.1073/pnas.1010354107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Xia J, Kass RS. MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J Biol Chem. 1998;273:34069–74. doi: 10.1074/jbc.273.51.34069. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Kanda VA, Choi E, Panaghie G, Roepke TK, Gaeta SA, et al. MinK-dependent internalization of the IKs potassium channel. Cardiovasc Res. 2009;82:430–8. doi: 10.1093/cvr/cvp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda VA, Purtell K, Abbott GW. Protein kinase C downregulates I(Ks) by stimulating KCNQ1-KCNE1 potassium channel endocytosis. Heart Rhythm. 2011;8:1641–7. doi: 10.1016/j.hrthm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Wang Y, Jiang M, Zankov DP, Chowdhury S, Kasirajan V, et al. KCNE2 protein is more abundant in ventricles than in atria and can accelerate hERG protein degradation in a phosphorylation-dependent manner. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00691.2011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopic S, Geibel JP. Update on the mechanisms of gastric acid secretion. Curr Gastroenterol Rep. 2010;12:458–64. doi: 10.1007/s11894-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 30.Malinowska DH, Sherry AM, Tewari KP, Cuppoletti J. Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K+ channels. Am J Physiol Cell Physiol. 2004;286:C495–506. doi: 10.1152/ajpcell.00386.2003. [DOI] [PubMed] [Google Scholar]
- 31.Kaufhold MA, Krabbenhoft A, Song P, Engelhardt R, Riederer B, Fahrmann M, et al. Localization, trafficking, and significance for acid secretion of parietal cell Kir4.1 and KCNQ1 K+ channels. Gastroenterology. 2008;134:1058–69. doi: 10.1053/j.gastro.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 2002;111:957–65. doi: 10.1016/S0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–55. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elso CM, Lu X, Culiat CT, Rutledge JC, Cacheiro NL, Generoso WM, et al. Heightened susceptibility to chronic gastritis, hyperplasia and metaplasia in Kcnq1 mutant mice. Hum Mol Genet. 2004;13:2813–21. doi: 10.1093/hmg/ddh307. [DOI] [PubMed] [Google Scholar]
- 35.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281:23740–7. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 36.Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS ONE. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston P, Wartosch L, Gunzel D, Fromm M, Kongsuphol P, Ousingsawat J, et al. Disruption of the K+ channel beta-subunit KCNE3 reveals an important role in intestinal and tracheal Cl- transport. J Biol Chem. 2010;285:7165–75. doi: 10.1074/jbc.M109.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 39.Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J. 2000;19:6326–30. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melman YF, Domenech A, de la Luna S, McDonald TV. Structural determinants of KvLQT1 control by the KCNE family of proteins. J Biol Chem. 2001;276:6439–44. doi: 10.1074/jbc.M010713200. [DOI] [PubMed] [Google Scholar]
- 41.Melman YF, Krumerman A, McDonald TV. A single transmembrane site in the KCNE-encoded proteins controls the specificity of KvLQT1 channel gating. J Biol Chem. 2002;277:25187–94. doi: 10.1074/jbc.M200564200. [DOI] [PubMed] [Google Scholar]
- 42.Melman YF, Um SY, Krumerman A, Kagan A, McDonald TV. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42:927–37. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Panaghie G, Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol. 2007;129:121–33. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panaghie G, Tai KK, Abbott GW. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J Physiol. 2006;570:455–67. doi: 10.1113/jphysiol.2005.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heitzmann D, Grahammer F, von Hahn T, Schmitt-Graff A, Romeo E, Nitschke R, et al. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J Physiol. 2004;561:547–57. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotte A, Pasham V, Mack AF, Bhandaru M, Qadri SM, Eichenmuller M, et al. Ca2+ activated K+ channel Kca3.1 as a determinant of gastric acid secretion. Cell Physiol Biochem. 2011;27:597–604. doi: 10.1159/000329981. [DOI] [PubMed] [Google Scholar]
- 47.Song P, Groos S, Riederer B, Feng Z, Krabbenhoft A, Manns MP, et al. Kir4.1 channel expression is essential for parietal cell control of acid secretion. J Biol Chem. 2011;286:14120–8. doi: 10.1074/jbc.M110.151191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright EM. Transport processes in the formation of the cerebrospinal fluid. Rev Physiol Biochem Pharmacol. 1978;83:3–34. [PubMed] [Google Scholar]
- 49.Wright EM, Saito Y. The choroid plexus as a route from blood to brain. Ann N Y Acad Sci. 1986;481:214–20. doi: 10.1111/j.1749-6632.1986.tb27152.x. [DOI] [PubMed] [Google Scholar]
- 50.Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 2010;25:239–49. doi: 10.1152/physiol.00011.2010. [DOI] [PubMed] [Google Scholar]
- 51.Kotera T, Brown PD. Evidence for two types of potassium current in rat choroid plexus epithelial cells. Pflugers Archiv. 1994;427:317–24. doi: 10.1007/BF00374540. [DOI] [PubMed] [Google Scholar]
- 52.Speake T, Kibble JD, Brown PD. Kv1.1 and Kv1.3 channels contribute to the delayed-rectifying K+ conductance in rat choroid plexus epithelial cells. Am J Physiol Cell Physiol. 2004;286:C611–20. doi: 10.1152/ajpcell.00292.2003. [DOI] [PubMed] [Google Scholar]
- 53.Speake T, Whitwell C, Kajita H, Majid A, Brown PD. Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech. 2001;52:49–59. doi: 10.1002/1097-0029(20010101)52:1<49::AID-JEMT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 54.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, et al. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001;98:13942–7. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cahalan MD, Chandy KG. Ion channels in the immune system as targets for immunosuppression. Curr Opin Biotechnol. 1997;8:749–56. doi: 10.1016/S0958-1669(97)80130-9. [DOI] [PubMed] [Google Scholar]
- 56.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MDK. + channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–9. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De La Vieja A, Dohan O, Levy O, Carrasco N. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 2000;80:1083–105. doi: 10.1152/physrev.2000.80.3.1083. [DOI] [PubMed] [Google Scholar]
- 58.Moreno JC, Visser TJ. New phenotypes in thyroid dyshormonogenesis: hypothyroidism due to DUOX2 mutations. Endocr Dev. 2007;10:99–117. doi: 10.1159/000106822. [DOI] [PubMed] [Google Scholar]
- 59.Roepke TK, King EC, Reyna-Neyra A, Paroder M, Purtell K, Koba W, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat Med. 2009;15:1186–94. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fröhlich H, Boini KM, Seebohm G, Strutz-Seebohm N, Ureche ON, Foller M, et al. Hypothyroidism of gene-targeted mice lacking Kcnq1. Pflugers Archiv. 2011;461:45–52. doi: 10.1007/s00424-010-0890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–55. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 62.Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, et al. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol. 1996;7:2533–42. doi: 10.1681/ASN.V7122533. [DOI] [PubMed] [Google Scholar]
- 63.Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol. 2006;291:C59–67. doi: 10.1152/ajpcell.00433.2005. [DOI] [PubMed] [Google Scholar]
- 64.Pourrier M, Zicha S, Ehrlich J, Han W, Nattel S. Canine ventricular KCNE2 expression resides predominantly in Purkinje fibers. Circ Res. 2003;93:189–91. doi: 10.1161/01.RES.0000084851.60947.B5. [DOI] [PubMed] [Google Scholar]
- 65.Jiang M, Zhang M, Tang DG, Clemo HF, Liu J, Holwitt D, et al. KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation. 2004;109:1783–8. doi: 10.1161/01.CIR.0000124225.43852.50. [DOI] [PubMed] [Google Scholar]
- 66.Bendahhou S, Marionneau C, Haurogne K, Larroque MM, Derand R, Szuts V, et al. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc Res. 2005;67:529–38. doi: 10.1016/j.cardiores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 67.McCrossan ZA, Roepke TK, Lewis A, Panaghie G, Abbott GW. Regulation of the Kv2.1 potassium channel by MinK and MiRP1. J Membr Biol. 2009;228:1–14. doi: 10.1007/s00232-009-9154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brandt MC, Endres-Becker J, Zagidullin N, Motloch LJ, Er F, Rottlaender D, et al. Effects of KCNE2 on HCN isoforms: distinct modulation of membrane expression and single channel properties. Am J Physiol Heart Circ Physiol. 2009;297:H355–63. doi: 10.1152/ajpheart.00154.2009. [DOI] [PubMed] [Google Scholar]
- 69.Qu J, Kryukova Y, Potapova IA, Doronin SV, Larsen M, Krishnamurthy G, et al. MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J Biol Chem. 2004;279:43497–502. doi: 10.1074/jbc.M405018200. [DOI] [PubMed] [Google Scholar]
- 70.Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, et al. MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res. 2001;88:E84–7. doi: 10.1161/hh1201.093511. [DOI] [PubMed] [Google Scholar]
- 71.Abbott GW, Goldstein SA. Disease-associated mutations in KCNE potassium channel subunits (MiRPs) reveal promiscuous disruption of multiple currents and conservation of mechanism. FASEB J. 2002;16:390–400. doi: 10.1096/fj.01-0520hyp. [DOI] [PubMed] [Google Scholar]
- 72.Scherer CR, Lerche C, Decher N, Dennis AT, Maier P, Ficker E, et al. The antihistamine fexofenadine does not affect I(Kr) currents in a case report of drug-induced cardiac arrhythmia. Br J Pharmacol. 2002;137:892–900. doi: 10.1038/sj.bjp.0704873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui J, Melman Y, Palma E, Fishman GI, McDonald TV. Cyclic AMP regulates the HERG K(+) channel by dual pathways. Curr Biol. 2000;10:671–4. doi: 10.1016/S0960-9822(00)00516-9. [DOI] [PubMed] [Google Scholar]
- 74.Cui J, Kagan A, Qin D, Mathew J, Melman YF, McDonald TV. Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J Biol Chem. 2001;276:17244–51. doi: 10.1074/jbc.M010904200. [DOI] [PubMed] [Google Scholar]
- 75.Balijepalli RC, Delisle BP, Balijepalli SY, Foell JD, Slind JK, Kamp TJ, et al. Kv11.1 (ERG1) K+ channels localize in cholesterol and sphingolipid enriched membranes and are modulated by membrane cholesterol. Channels (Austin) 2007;1:263–72. doi: 10.4161/chan.4946. [DOI] [PubMed] [Google Scholar]
- 76.Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–6. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 77.Sanguinetti MC, Jurkiewicz NK. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am J Physiol. 1991;260:H393–9. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- 78.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 79.Rudy Y. Molecular basis of cardiac action potential repolarization. Ann N Y Acad Sci. 2008;1123:113–8. doi: 10.1196/annals.1420.013. [DOI] [PubMed] [Google Scholar]
- 80.Weerapura M, Nattel S, Chartier D, Caballero R, Hebert TE. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current. Is MiRP1 the missing link? J Physiol. 2002;540:15–27. doi: 10.1113/jphysiol.2001.013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dupuis DS, Klaerke DA, Olesen SP. Effect of beta-adrenoceptor blockers on human ether-a-go-go-related gene (HERG) potassium channels. Basic Clin Pharmacol Toxicol. 2005;96:123–30. doi: 10.1111/j.1742-7843.2005.pto960206.x. [DOI] [PubMed] [Google Scholar]
- 82.Park KH, Kwok SM, Sharon C, Baerga R, Sesti F. N-Glycosylation-dependent block is a novel mechanism for drug-induced cardiac arrhythmia. FASEB J. 2003;17:2308–9. doi: 10.1096/fj.03-0577fje. [DOI] [PubMed] [Google Scholar]
- 83.Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, et al. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci USA. 2000;97:10613–8. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Witchel HJ, Hancox JC. Familial and acquired long qt syndrome and the cardiac rapid delayed rectifier potassium current. Clin Exp Pharmacol Physiol. 2000;27:753–66. doi: 10.1046/j.1440-1681.2000.03337.x. [DOI] [PubMed] [Google Scholar]
- 85.Gordon E, Panaghie G, Deng L, Bee KJ, Roepke TK, Krogh-Madsen T, et al. A KCNE2 mutation in a patient with cardiac arrhythmia induced by auditory stimuli and serum electrolyte imbalance. Cardiovasc Res. 2008;77:98–106. doi: 10.1093/cvr/cvm030. [DOI] [PubMed] [Google Scholar]
- 86.Christe´ G, Theriault O, Chahine M, Millat G, Rodriguez-Lafrasse C, Rousson R, et al. A new C-terminal hERG mutation A915fs+47X associated with symptomatic LQT2 and auditory-trigger syncope. Heart Rhythm. 2008;5:1577–86. doi: 10.1016/j.hrthm.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res. 1996;79:79–85. doi: 10.1161/01.res.79.1.79. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M, Jiang M, Tseng GN. minK-related peptide 1 associates with Kv4.2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circ Res. 2001;88:1012–9. doi: 10.1161/hh1001.090839. [DOI] [PubMed] [Google Scholar]
- 89.Proenza C, Angoli D, Agranovich E, Macri V, Accili EA. Pacemaker channels produce an instantaneous current. J Biol Chem. 2002;277:5101–9. doi: 10.1074/jbc.M106974200. [DOI] [PubMed] [Google Scholar]
- 90.Liang Q, Yang L, Wang Z, Huang S, Li S, Yang G. Tanshinone IIA selectively enhances hyperpolarization-activated cyclic nucleotide-modulated (HCN) channel instantaneous current. J Pharmacol Sci. 2009;110:381–8. doi: 10.1254/jphs.08334FP. [DOI] [PubMed] [Google Scholar]
- 91.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–4. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 92.Lewis A, McCrossan ZA, Abbott GW, Min K. MiRP1, and MiRP2 diversify Kv3.1 and Kv3.2 potassium channel gating. J Biol Chem. 2004;279:7884–92. doi: 10.1074/jbc.M310501200. [DOI] [PubMed] [Google Scholar]
- 93.Kanda VA, Lewis A, Xu X, Abbott GW. KCNE1 and KCNE2 provide a checkpoint governing voltage-gated potassium channel alpha-subunit composition. Biophys J. 2011;101:1364–75. doi: 10.1016/j.bpj.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949–72. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, et al. Contributions of Kv3 channels to neuronal excitability. Ann N Y Acad Sci. 1999;868:304–43. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- 96.Perney TM, Kaczmarek LK. Localization of a high threshold potassium channel in the rat cochlear nucleus. J Comp Neurol. 1997;386:178–202. doi: 10.1002/(SICI)1096-9861(19970922)386:2<178::AID-CNE2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 97.Ding S, Matta SG, Zhou FM. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol. 2011;105:554–70. doi: 10.1152/jn.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudy B, Sen K, Vega-Saenz de Miera E, Lau D, Ried T, Ward DC. Cloning of a human cDNA expressing a high voltage-activating, TEA-sensitive, type-A K+ channel which maps to chromosome 1 band p21. J Neurosci Res. 1991;29:401–12. doi: 10.1002/jnr.490290316. [DOI] [PubMed] [Google Scholar]
- 99.Desai R, Kronengold J, Mei J, Forman SA, Kaczmarek LK. Protein kinase C modulates inactivation of Kv3.3 channels. J Biol Chem. 2008;283:22283–94. doi: 10.1074/jbc.M801663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernandez FR, Morales E, Rashid AJ, Dunn RJ, Turner RW. Inactivation of Kv3.3 potassium channels in heterologous expression systems. J Biol Chem. 2003;278:40890–8. doi: 10.1074/jbc.M304235200. [DOI] [PubMed] [Google Scholar]
- 101.Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–66. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- 102.Xu J, Yu W, Jan YN, Jan LY, Li M. Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits. J Biol Chem. 1995;270:24761–8. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- 103.Zerangue N, Jan YN, Jan LY. An artificial tetramerization domain restores efficient assembly of functional Shaker channels lacking T1. Proc Natl Acad Sci USA. 2000;97:3591–5. doi: 10.1073/pnas.060016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruppersberg JP, Frank R, Pongs O, Stocker M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 1991;353:657–60. doi: 10.1038/353657a0. [DOI] [PubMed] [Google Scholar]
- 105.Tinel N, Diochot S, Lauritzen I, Barhanin J, Lazdunski M, Borsotto M. M-type KCNQ2-KCNQ3 potassium channels are modulated by the KCNE2 subunit. FEBS Lett. 2000;480:137–41. doi: 10.1016/S0014-5793(00)01918-9. [DOI] [PubMed] [Google Scholar]
- 106.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]