Abstract
Adult reproductive success can account for a large fraction of male fitness, however, we know relatively little about the susceptibility of reproductive traits to mutation-accumulation (MA). Estimates of the mutational rate of decline for adult fitness and its components are controversial in Drosophila melanogaster, and post-copulatory performance has not been examined. We therefore separately measured the consequences of MA for total male reproductive success and its major pre-copulatory and post-copulatory components: mating success and sperm competitive success. We also measured juvenile viability, an important fitness component that has been well studied in MA experiments. MA had strongly deleterious effects on both male viability and adult fitness, but the latter declined at a much greater rate. Mutational pressure on total fitness is thus much greater than would be predicted by viability alone. We also noted a significant and positive correlation between all adult traits and viability in the MA lines, suggesting pleiotropy of mutational effect as required by ‘good genes’ models of sexual selection.
Keywords: mutation-accumulation, sexual selection, sperm competition, viability, fitness
1. Introduction
The effects of individual mutations may be too small to detect individually, however, their impact on total fitness is a fundamental quantity in populations genetics. Mutation-accumulation (MA) experiments, where selection is relaxed to allow new mutations to fix, can reveal their cumulative effects [1]. In Drosophila melanogaster, the most commonly used organism in MA studies, experiments have typically examined a single fitness trait: juvenile survival. In comparison, a handful of studies have measured adult fitness, with conflicting results [2–6]. In the Ives (IV) population of D. melanogaster, adult fitness is disproportionately important for males, accounting for 84 per cent of inbreeding depression for net fitness on the autosomes [7]. In the same population, new X-linked mutations are deleterious for both sexes but have a stronger impact on males than females [5]. Unfortunately, little is known about the susceptibility of specific reproductive traits like sperm competitive success and mating success to MA in any population, despite their importance for male fitness [8,9].
The magnitude and pattern of mutational change in these traits informs us about their relative importance to fitness and their genetic architecture. For example, a decrease in multiple traits accompanied by a stronger genetic correlation between traits indicates pleiotropy. The correlation between male reproductive traits and juvenile viability has been of particular interest in sexual selection research: a positive correlation is the most common test of additive benefits of sexual selection to offspring [10]. We therefore performed 50 generations of MA on D. melanogaster haploid genomes from an outbred laboratory-adapted population and measured its impact on juvenile viability, lifetime male reproductive success and mating success, as well as providing the first estimates of mutational effects for post-copulatory traits. The effects of MA were assessed genome-wide, in the normal condition of expression for new mutations in males (hemizygous on the X, heterozygous on the autosomes).
2. Methods
Haploid genomes originated from IV, a long-term laboratory-adapted population, and were isolated with the Drosophila hemiclone system (reviewed in [11]; figure 1a). The same set of 21 hemiclone lines was used to found both control (C) and MA groups. For the MA lines, we reduced the effective population size to a single haploid genome per generation, propagated without recombination (figure 1b). The same crosses with larger population sizes were used to maintain the C lines. We kept the controls as moderate-sized populations without recombination to limit adaptation, a persistent concern in MA experiments [12,13]. This may allow some MA in the controls, and will make our estimates of mutational impact conservative.
Figure 1.
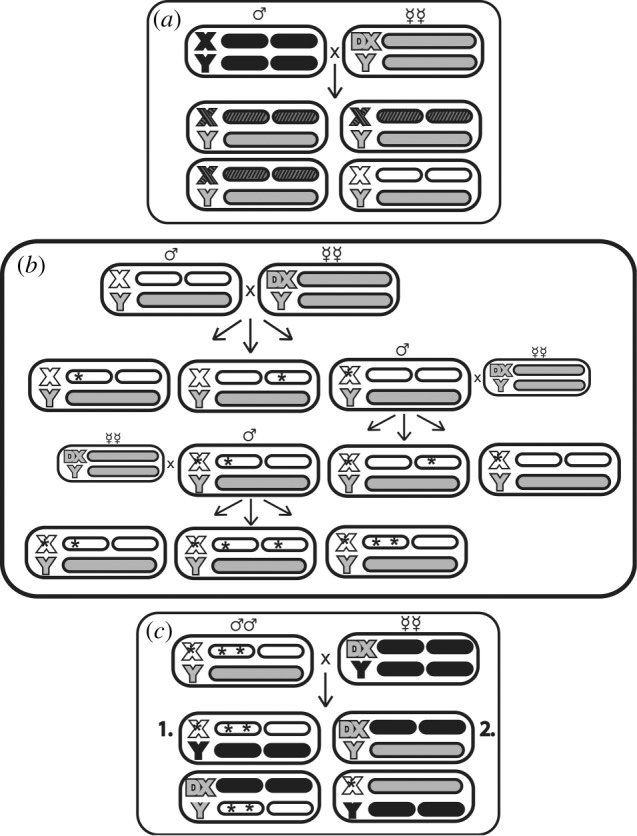
Crosses employed. (a) Random IV hemiclones were isolated by crossing wild-type males individually to groups of clone-generator (CG) females bearing attached-X chromosomes (DX) and homozygous for a marked autosomal translocation (T(2: 3) rdgc st in ri pp bw, grey bars). A single son from these crosses (white genotype) was then selected to fix a different haploid genome within each hemiclonal line. The MA and C populations were founded from the same initial group of hemiclones. (b) Mutation-accumulation (three generations shown). A single male from each line was mated to a group of CG females, creating a single-genome bottleneck and fixing the mutations present in the parent (black asterisks). Three sons from this cross were each mated to CG females in separate vials, one of which was randomly chosen to found the next generation (the other vials are kept as backups). Controls were maintained using identical crosses, but at larger population sizes (16–25 males) to allow selection. (c) Generation of experimental flies. MA or C hemiclones were crossed to DX females with wild-type autosomes. Hemiclonal males with MA or C hemiclones heterozygous for a set of random IV genotypes (1.) were used to assay performance. Females without any C or MA chromosomes (2.) were used to standardize viability.
For viability assays, we generated C/MA males by crossing males from 19 lines with DX-IV females, allowing them to lay approximately 100 viable eggs. The expected yield is 25 per cent hemiclonal males, and 25 per cent brown-eyed females that do not carry C/MA-derived chromosomes (figure 1c). The females were thus used to standardize viability. We measured 10 vials per line/treatment combination, 380 in total.
To measure adult fitness, we transferred single hemiclonal C/MA males from 19 lines to age-synchronized vials of a competitor (IVbw) reared under standard conditions (approx. 100 individuals/vial, 25°C, 50% relative humidity), during peak eclosion (day 9). The vials were left undisturbed for 5 days. On day 14, the entire population was placed under 2.5 min CO2 to simulate normal culture, then transferred to oviposition vials until approximately 100 eggs were laid (25–30 min). Red-eyed progeny emerging from these vials represent the lifetime reproductive success of the hemiclonal males under normal IV culture conditions. We measured 20 males for each line/treatment combination, 760 in total.
Mating success was measured by competing hemiclonal C/MA males from 20 lines with IV males for virgin IV females. We collected virgin hemiclonal males, as well as virgin IV females and competitor IV males on day 9 post-oviposition in same-sex groups. On day 11, the males were transferred to medium containing red or blue food-dyed yeast paste, which colours their underbelly. We then transferred pairs of opposite-coloured competitors (C/MA males with IV males) to female vials without anaesthesia, observing until mating took place. We performed 10 trials for each line/treatment/colour combination, 800 in total, including reciprocal dye treatments of all male genotypes.
For post-copulatory success, we collected virgin IVbw females, IVbw males and C/MA males from 20 lines on day 9. On day 12, groups of 18 males (P1) were combined with 12 virgin females and allowed to interact for 1.5 h: nearly all females mate once under these conditions. The first mates were removed using light CO2 and 12 males (P2) were added after a 30 min female recovery period. The flies interacted overnight (18 h), and we then placed females in individual 13 × 100 mm test tubes containing fresh media to oviposit for 20 h. Progeny were scored for paternity 11–14 days later. Male performance was divided into two components: P1 and P2, depending on whether the focal males (C/MA) or the competitor (IVbw) males mated first. We assessed paternity in 50 females for each line/treatment/order combination, 3800 in total.
Statistical inferences were performed using normalized likelihoods [14], using R, v. 2.12.0 [15]. Normalized likelihoods are equivalent to Bayesian analyses using flat priors, and can also be used to generate standard p-values and confidence intervals [16] (electronic supplementary material). All statistics were based on line means.
3. Results
Results for all performance measures are summarized in table 1. Line means and between-line variance estimates along with their confidence intervals are presented in the electronic supplementary material for all traits. For mating success, we verified that the ratio of red to blue-dyed success was not significantly different from unity for the MA and C males before combining them (MA red/blue = 1.10 (0.88–1.39), C red/blue = 0.85 (0.71–1.02)). For P2, where we did not observe matings over the entire 18 h interaction window, we excluded females having produced no offspring from the P2 male before calculating line means to ensure that sperm competition occured. Females mated to MA males had slightly but significantly larger broods. This was true whether the MA males were the P1 (MA/C = 1.05, 95% confidence interval (CI) = (1.02, 1.08), p < 0.0001) or P2 (MA/C = 1.04, 95% CI = (1.01, 1.07), p = 0.01) male. Correlations between adult traits and viability are shown in table 2.
Table 1.
Fitness declines associated with MA, based on group means (95% confidence intervals in brackets). (Per-generation rates of declines were calculated assuming multiplicative fitness effects between mutations.)
| trait | mean | % decline | per-generation decline (%) | p-value (two-tailed) |
|---|---|---|---|---|
| viability | ||||
| C | 85.1% (80.5–89.9) | 30.8 (24.9–36.1) | 0.73 (0.57–0.89) | <0.0001 |
| MA | 58.9% (55.4–62.6) | |||
| adult fitness | ||||
| C | 3.06 (2.89–3.24) | 53.6 (48.5–58.2) | 1.52 (1.32–1.73) | <0.0001 |
| MA | 1.42 (1.30–1.55) | |||
| mating success | ||||
| C | 45.6% (41.0–50.2) | 20.8 (7.2–32.5) | 0.46 (0.15–0.79) | 0.0032 |
| MA | 36.1% (31.7–40.5) | |||
| P1 | ||||
| C | 10.1% (9.5–10.7) | 42.1 (36.5–47.3) | 1.09 (0.90–1.27) | <0.0001 |
| MA | 5.8% (5.4–6.3) | |||
| P2 | ||||
| C | 85.9% (85.2–86.5) | 21.6 (22.9–20.2) | 0.48 (0.45–0.52) | <0.0001 |
| MA | 67.3% (66.3–68.4) | |||
| total fitness | ||||
| C | 2.60 (2.40–2.82) | 67.8 (63.3–71.8) | 2.24 (1.98–2.50) | <0.0001 |
| MA | 0.84 (0.75–0.93) | |||
Table 2.
Correlations between male fitness traits and viability, based on line means (95% confidence intervals in brackets).
| traits | C | MA | p-value for difference between C and MA (two-tailed) |
|---|---|---|---|
| P1 | 0.14 (−0.13, 0.42) | 0.43 (0.23, 0.61) | 0.10 |
| P2 | 0.21 (−0.07, 0.47) | 0.57 (0.45, 0.67) | 0.012 |
| mating success | 0.13 (−0.22, 0.47) | 0.42 (0.17, 0.64) | 0.19 |
| adult fitness | 0.098 (−0.16, 0.38) | 0.49 (0.31, 0.65) | 0.021 |
4. Discussion
Adult male fitness declined significantly with MA. We previously estimated that MA on the X chromosome depressed adult fitness by 0.8 per cent per generation [5], assuming multiplicative effects of mutations on fitness, so the heterozygous autosomes contributed roughly 0.7 per cent per generation to the decline in adult male fitness in this experiment. To our knowledge, this is the first reported estimate for the effects of MA on heterozygous male reproductive success. Adult male fitness declined at more than double the rate of viability, accounting for roughly two-thirds of the rate of decline in total fitness. The cost of mutation for IV males is thus much greater than viability alone would predict, as others have hypothesized [3,17].
We separately measured components of male reproductive success, and found that all of them declined with MA. MA males were on average 20 per cent worse than C males at obtaining matings, indicating deleterious mutational effects on attractiveness and/or male–male competition. We are aware of only one other estimate of pre-copulatory mating success: Houle et al. [4], using a closely related IV population, did not find a significant reduction in male mating ability on homozygous second chromosomes after 44 generations of MA, but this was attributed to a lack of experimental power.
We show for the first time, to our knowledge, that MA is associated with a decline in post-copulatory success, for both P1 and P2. Our P2 measure excludes males that failed to produce any offspring: while ensuring that sperm competition did occur, this tends to underestimate the decline owing to MA. We attribute the decline in post-copulatory success to competitive exclusion rather than reduced survival of MA male offspring, because females mated to MA males did not produce smaller broods. In fact, females mated to MA males tended to have slightly more progeny, whether the males were in P1 or P2. Given that this increase was similar regardless of male position, and that the exposure time to P1 and P2 males was very different, we suggest that this result is unlikely to be caused by a reduction in male harassment/vigour with MA. Instead, the ejaculate of MA males might be less harmful. One possible mechanism is that MA males produce fewer harmful accessory peptides, indicating a trade-off between post-copulatory success and mate-harm.
Viability has been well characterized in MA studies using D. melanogaster [1]. Most studies have measured homozygous effects on a single autosome and extrapolated to haploid genomes: these estimates are usually 0.3–1% per generation. Considering that new mutations were heterozygous for 80 per cent of the genome in our experiment, our estimate of 0.73 per cent seems somewhat high. Using a different experimental design, Shabalina et al. [17] noted a 1 per cent per generation decline on larval survival in outbred populations. Their result is comparable to ours although their experimental conditions were much harsher, with mean larval survivals of only approximately 10 per cent. Overall, our result supports high mutation pressure on viability.
For all traits in the control lines, there was no significant association between male performance and viability. Mallet & Chippindale [7], and others [18,19], have interpreted this as suggesting a lack of viability benefits to offspring resulting from sexual selection. In the MA lines, however, we noted a significantly positive relationship between each of the male performance traits and viability, resulting in a significant difference in the correlations between C and MA for adult fitness and P2 success. New mutations thus appear to have pleiotropic effects on viability and male reproductive performance, representing one avenue for offspring to realize additive genetic benefits from sexual selection.
Acknowledgements
We thank members of the Chippindale Laboratory for help with data collection. Funding was provided by NSERC. We also thank the editor and anonymous reviewers for their helpful comments.
References
- 1.Halligan D. L., Keightley P. D. 2009. Spontaneous mutation accumulation studies in evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 40, 151–172 10.1146/annurev.ecolsys.39.110707.173437 (doi:10.1146/annurev.ecolsys.39.110707.173437) [DOI] [Google Scholar]
- 2.Mack P. D., Lester V. K., Promislow D. E. L. 2000. Age-specific effects of novel mutations in Drosophila melanogaster. II. Fecundity and male mating ability. Genetica 110, 31–41 10.1023/A:1017538505627 (doi:10.1023/A:1017538505627) [DOI] [PubMed] [Google Scholar]
- 3.Fry J. D., Heinsohn S. L., Mackay T. F. C. 1998. Heterosis for viability, fecundity, and male fertility in Drosophila melanogaster: comparison of mutational and standing variation. Genetics 148, 1171–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houle D., Hughes K. A., Hoffmaster D. K., Ihara J., Assimacopoulos S., Canada D., Charlesworth B. 1994. The effects of spontaneous mutation on quantitative traits. I. Variances and covariances of life history traits. Genetics. 138, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallet M., Bouchard J., Kimber C., Chippindale A. 2011. Experimental mutation-accumulation on the X chromosome of Drosophila melanogaster reveals stronger selection on males than females. BMC Evol. Biol. 11, 156. 10.1186/1471-2148-11-156 (doi:10.1186/1471-2148-11-156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez J., Lopez-Fanjul C. 1996. Spontaneous mutational variances and covariances for fitness-related traits in Drosophila melanogaster. Genetics 143, 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallet M. A., Chippindale A. K. 2011. Inbreeding reveals stronger net selection on Drosophila melanogaster males: implications for mutation load and the fitness of sexual females. Heredity 106, 994–1002 10.1038/hdy.2010.148 (doi:10.1038/hdy.2010.148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harshman L. G., Clark A. G. 1998. Inference of sperm competition from broods of field-caught Drosophila. Evolution 52, 1334–1341 10.2307/2411303 (doi:10.2307/2411303) [DOI] [PubMed] [Google Scholar]
- 9.Rybak F., Sureau G., Aubin T. 2002. Functional coupling of acoustic and chemical signals in the courtship behaviour of the male Drosophila melanogaster. Proc. R. Soc. Lond. B 269, 695–701 10.1098/rspb.2001.1919 (doi:10.1098/rspb.2001.1919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokko H., Jennions M. D., Brooks R. 2006. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37, 43–66 10.1146/annurev.ecolsys.37.091305.110259 (doi:10.1146/annurev.ecolsys.37.091305.110259) [DOI] [Google Scholar]
- 11.Abbott J. K., Morrow E. H. 2011. Obtaining snapshots of genetic variation using hemiclonal analysis. Trends Ecol. Evol. 26, 359–368 10.1016/j.tree.2011.03.011 (doi:10.1016/j.tree.2011.03.011) [DOI] [PubMed] [Google Scholar]
- 12.Keightley P. D., Halligan D. L. 2009. Analysis and implications of mutational variation. Genetica 136, 359–369 10.1007/s10709-008-9304-4 (doi:10.1007/s10709-008-9304-4) [DOI] [PubMed] [Google Scholar]
- 13.Keightley P. D. 1996. Nature of deleterious mutation load in Drosophila. Genetics 144, 1993–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shcherbinin A. F. 1987. The normalized likelihood method. Meas. Tech. 30, 1129–1134 10.1007/BF00864631 (doi:10.1007/BF00864631) [DOI] [Google Scholar]
- 15.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 16.Walley P. 2002. Reconciling frequentist properties with the likelihood principle. J. Stat. Plan. Infer. 105, 35–65 10.1016/S0378-3758(01)00203-8 (doi:10.1016/S0378-3758(01)00203-8) [DOI] [Google Scholar]
- 17.Shabalina S. A., Yampolsky L. Y., Kondrashov A. S. 1997. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Natl Acad. Sci. USA 94, 13 034–13 039 10.1073/pnas.94.24.13034 (doi:10.1073/pnas.94.24.13034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janhunen M., Kekäläinen J., Kortet R., Hyvärinen P., Piironen J. 2011. No evidence for an indirect benefit from female mate preference in Arctic charr Salvelinus alpinus, but female ornamentation decreases offspring viability. Biol. J. Linn. Soc. 103, 602–611 10.1111/j.1095-8312.2011.01659.x (doi:10.1111/j.1095-8312.2011.01659.x) [DOI] [Google Scholar]
- 19.Promislow D. E. L., Smith E. A., Pearse L. 1998. Adult fitness consequences of sexual selection in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 95, 10 687–10 692 10.1073/pnas.95.18.10687 (doi:10.1073/pnas.95.18.10687) [DOI] [PMC free article] [PubMed] [Google Scholar]


