Abstract
Chronic central neuropathic pain following CNS injuries remains refractory to therapeutic interventions. A novel approach would be to target key intracellular signaling proteins that are known to contribute to continued activation by phosphorylation of kinases, transcription factors, and/or receptors that contribute to changes in membrane excitability. We demonstrate that one signaling kinase, calcium/calmodulin-dependent kinase II (CaMKII), is critical in maintaining aberrant dorsal horn neuron hyperexcitability in the neuropathic pain condition following spinal cord injury (SCI). Following T10 contusion SCI, activated CaMKII (phosphorylated, pCAMKII) expression is significantly upregulated in the T7/8 spinal dorsal horn in neurons, but not glial cells, and in oligodendrocytes in the dorsal column in the same rats that displayed at-level mechanical allodynia. Furthermore, identified spinothalamic neurons demonstrated significant increases of pCaMKII after SCI compared to sham controls. However, neither astrocytes nor microglia showed pCaMKII expression in either sham or SCI rats. To demonstrate causality, treatment of SCI rats with KN-93, which prevents CaMKII activation, significantly attenuated at-level mechanical allodynia and aberrant WDR neuronal activity evoked by brush, pressure, pinch stimuli and a graded series of von Frey stimuli, respectively. This is the first evidence that persistent CaMKII activation contributes to chronic central neuropathic pain by mechanisms that involve maintained hyperexcitability of WDR dorsal horn neurons. Furthermore, targeting key signaling proteins is a novel, useful therapeutic strategy for treating chronic central neuropathic pain.
Keywords: CaMKII, central neuropathic pain, electrophysiology, immunocytochemistry, mechanical allodynia, Western Immunoblotting
INTRODUCTION
Over 70 % of people with spinal cord injury (SCI) suffer intractable and chronic central neuropathic pain (CNP) [2,7,45,46]. Neurosurgical treatment is generally disappointing [31] and management with ion channel blockers [18], morphine [1,44], anticonvulsants [19], antidepressants [5] and other strategies [4,25,48,51] including acupuncture [15] have limited efficacy due to the lack of understanding the detailed pathophysiological mechanisms.
A novel mechanism of CNP following SCI is activation of intracellular signaling cascades in the spinal dorsal horn [11,12], similar to those that result in hippocampal long-term potentiation (LTP), that may contribute to neuropathic pain [27,47,55]. In LTP, glutamate receptor mediated activation of a number of intracellular signaling kinases lead to increased mitogen activated protein kinases (MAPKs; e.g., ERK1/2, p38 MAPK) and phosphorylation of transcription factors such as cAMP responsive element binding protein (CREB) and Elk-1 [28, 30,32]. Activation of MAPK signaling pathways and CREB following peripheral injection of chemical irritants (e.g., formalin, capsaicin) or peripheral nerve injury [33,36,37] are linked indirectly to central sensitization, a persistent increase in electrophysiological activity (background and to evoked stimuli) of spinal dorsal horn neurons. We previously reported increases in spinal levels of the phosphorylated (and thus activated) forms of the MAPKs, extracellular signal related kinase (ERK) 1/2 and p38 MAPK, and the transcription factor, CREB, in SCI rats experiencing at-level mechanical allodynia [11,12]. However, upstream signaling pathways that lead to the spinal LTP-like pathways after SCI are not known.
A critical key signaling molecule in LTP is calcium/calmodulin-dependent protein kinase II (CaMKII), a molecule phosphorylated through IP3 pathways initiated by glutamate receptor activation, which regulates calcium signaling, influences synaptic function by phosphorylation of membrane receptors and is an important upstream enzyme to MAP kinase signaling pathways, including ERK1/2, p38 MAPK, and transcription factors such as CREB [33,42,43]. Phosphorylation of CaMKII allows continued activation of downstream pathways even after the calcium influx, that is known to accompany SCI, subsides [34]. Given the known roles of SCI produced glutamate receptor activation [38,39], the roles of MAP kinases [11,12] and the pivotal role of CaMKII in LTP signaling pathways, the current studies test and provide the first converging evidence (combining behavioral, protein, cytochemical and electrophysiological analyses) that persistent activation of CaMKII plays a critical role in “at level” mechanical allodynia, or chronic CNP following SCI.
EXPERIMENTAL PROCEDURES
Subjects
Male Sprague Dawley rats (n=100) weighing between 225-250 g (Harlan Inc, Houston TX) received a contusion injury at spinal level T10 using an Infinite Horizon impactor (150 kdynes, 1 s dwell) or were given a laminectomy only to serve as sham controls, or received no manipulation to serve as age-matched naïve rats. Only data from SCI rats that developed “pain” were examined as a SCI group (this level of injury routinely produces 90% of SCI rats that develop pain). SCI rats that did not develop “pain” were excluded from these analyses. Following surgery, injured rats were given supplemental injections of Baytril (enrofloxacin, 0.03%) twice daily for 3 days to prevent infection, and bladders were also expressed twice daily until the rats began to void on their own. All procedures were reviewed by the UTMB Animal Care and Use Committee and are consistent with the guidelines of the International Association for the Study of Pain and the NIH Guide for the Care and Use of Laboratory Animals.
Behavioral Procedures
For the study involving measurement of at-level mechanical allodynia, age matched rats were divided into 3 groups: 1) 10 naïve rats (naïve), 2) 10 rats given a laminectomy at T10 (sham) and 3) 10 injured rats (SCI). During the course of recovery, naïve, sham and injured rats were examined for the development of mechanical allodynia rostral to the injury using the procedure outlined in Crown and colleagues [10,11] with a few modifications. Briefly, a grid map of the girdle zone for allodynic responses was made on the rats using an indelible marker. A von Frey hair with bending force of 204.14 mN (26 gm-force) was applied to each point on the grid, and supraspinally mediated nociceptive responses (e.g., escape, biting, or vocalization) were recorded and mapped onto a grid map of that animal. The scoring of these complex behaviors excludes simple hyperreflexia, which is a segmental response [51,56,57]. Since animals do not normally display supraspinal responses to this stimulus, a positive response was interpreted to demonstrate that a noxious stimulus was experienced. In mapping the area of response, the number of responses was recorded (Nr) and normalized by the following formula: (Nr x 100)/total number of applications, indicating the percent responding out of the total number of applications. For both behavioral studies, data were analyzed only for the dermatome (T5-T10, between mid thoracic area and upper abdominal area) corresponding to the site of injury.
To test whether increases in activated CaMKII expression corresponded to the maintenance of at-level mechanical allodynia, animals from each condition (naïve, sham and SCI) were baseline tested prior to injury and then were tested weekly until 35 days post injury. The percent of supraspinal responses to a 4 mm blunt probe, a more natural stimulus, were also recorded and analyzed using the same method. In all cases, test order (26 g force von Frey filament or probe) was counterbalanced across conditions, with half the subjects in each condition being exposed to the probe first and half the subjects being exposed to the probe last. At none of the testing sessions did animals respond in the absence of mechanical stimulation. In addition, behavior was assessed by an experimenter blinded to experimental condition. For behavioral studies of the effects of CaMKII inhibition on at-level mechanical allodynia, three doses of KN-93 (a CaMKII inhibitor, 1, 10, or 100 μM in 50 μl saline, EMD Biosciences, San Diego) or the inactive enatiomer KN-92 (100 μM in 50 μl saline, EMD Biosciences, San Diego) were administered by intraspinal injection into the lumbar L4 space to SCI rats (n=5) over the course of ten days starting at 35 days post injury (drug dose was counterbalanced across groups and one day was allowed in between exposure for drug washout). Sham rats (n=5) were tested at the same time intervals but received the highest dose of KN-93 (100 μM in 50 μl). As an additional control, sham and SCI rats also received KN93’s inactive enantiomer, KN-92; at a dose that was equivalent to the highest dose of KN-93 (100 μM in 50 μl saline). Each SCI and sham rat received every dose of the drug over the course of the experiment starting at postinjury day 35 (dose order was randomized across the subjects to ensure there were no carryover effects from test session to test session on nociceptive reactivity in a cross over study design).
All rats were tested for supraspinal nociceptive responses (escape behaviors, biting, or vocalization, as above) to a 26 g force von Frey hair and a 4 mm blunt probe prior to drug administration (baseline). Briefly, rats were given inhalation anesthesia with isofluorane (1.5%) and the L4/L5 vertebral segment was identified by determining the location of the iliac crest. The injection needle was inserted into the L4/L5 space for drug delivery and a tail flick response to needle insertion was taken as evidence for successful lumbar puncture. To ensure that drug delivery into the lumbar space affected responses in the midthoracic region, a group of naïve rats were injected with 50 μl of a 2% lidocaine solution and tested for the loss of reflexive responses to a 100 gm-force von Frey stimulus applied to the rat’s dorsum. This volume of lidocaine was confirmed to produce a loss of reflexive and supraspinal responses throughout the region to be tested (data not shown). Following drug administration, rats were tested at 15, 45 and 75 minutes post injection.
Western Immunoblotting
At a time point consistent with the beginning of behavioral testing (35 days post SCI; when the maximal level of allodynia was observed), a separate age matched sham, naïve and SCI group of rats was sacrificed for Western immunoblotting. To test for persistent changes in pCaMKII expression following spinal cord contusion injury by Western Blot, all subjects were overdosed with pentobarbital (100 mg/kg) and perfused intracardially with 250 ml cold heparinized (1ml/1L) saline (0.9%) and 0.5 cm segments of spinal cord tissue immediately rostral to the injury site (the site corresponding to T8) were removed and dissected while on dry ice. For the tissue used in Western immunoblotting, the dorsal aspects of the spinal cord were microdissected and frozen on dry ice for subsequent analysis (Note: for immunocytochemical analyses the entire spinal cord at this level was analyzed to look at differences in gray and white matter expression of activated CaMKII, pCaMKII). The collected tissue was mechanically homogenized in ice-cold tris-buffered saline containing 40 mM Tris-HCl (pH 7.5), 2% SDS, 2 mg/ml aprotinin, 2 mg/ml antipain, 2 mg/ml chymostatin, 2 mg/ml bestatin, 2 mg/ml pepstatin-A, 2 mg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and 1 mM EDTA. Homogenates were centrifuged at 10,000 x g for 10 min. The supernatant was collected and centrifuged again at 10,000 x g for 10 min and then stored at −80°C. Protein concentrations of the homogenate were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). We have shown previously that this extraction method is efficient at collecting both the cytoplasmic and nuclear protein fractions [11,12].
Five samples from each group (naïve, sham, SCI) were used for Western immunoblotting. The samples were heated for 4 min at 95°C in an equal volume of sample buffer (100 mM Tris, pH 6.8, and 2% SDS, 2% 2-mercaptoethanol, 0.001% bromophenol blue, 20% glycerol) and then loaded onto a polyacrylamide gel in equal protein amounts (10 μg per lane). The stacking gel was 4% acrylamide, prepared in 0.13 M Tris, pH 6.8, and 0.1% SDS, and the separating gel was 10% acrylamide, prepared in 0.38 M Tris, pH 8.8, and 0.1% SDS. Samples were separated by electrophoresis in Tris-glycine buffer (25 mM Tris, 250 mM glycine, 0.1% SDS) at 300 V for approximately 30 min. Proteins were transferred overnight (12-14 hrs) to a PVDF membrane at 30 V in transfer buffer containing 20% MeOH, 20 mM Tris, 150 mM glycine, pH 8.0. Membranes were incubated for one hour at room temperature in blocking buffer containing 5% non-fat powdered milk in tris buffered saline (TBS)-Tween (20 mM Tris, 137 mM NaCl, 0.1% Tween-20), then washed for 10 min in TBS-Tween. Membranes were incubated overnight with primary antibodies to both the phosphorylated and nonphosphorylated forms of the alpha subunit of CaMKII (1:1000; Upstate Biotechnologies). Serial dilutions of the primary antibody were first tested on the protein samples using dotblotting to ensure that the results were not influenced by a ceiling effect. To control for equal protein loading, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:5000; Upstate Biotechnologies) immunoreactivity was used to verify equal loading of proteins on the PVDF membrane and this method found no significant differences between the naïve, sham, and SCI rats (all Fs < 1.0, p > 0.05). For statistical analyses, the data from each subject was normalized to the value for GAPDH expression. After washing off the primary antibody, membranes were incubated in horseradish peroxidase-conjugated anti-rabbit IgG diluted 1:20000 for one hour and washed 3 times in TBS for 5 min. Peroxidase activity was detected using an Amersham ECL Plus detection system, images were collected by exposing the membranes (exposure time varied from 30 s to 5 min) on chemiluminescence film (Hyperfilm ECL, Amersham Pharmacia Biotech, England), and integrated density values were calculated using LabWorks software (UVP, Upland, CA). Each membrane contained samples from sham, naïve, and SCI rats to allow for valid intergel comparisons. In addition, all membranes were exposed at the same time following CaMKII, p-CaMKII and GAPDH immunoblotting to allow for valid intragel comparisons.
Immunocytochemistry
For immunocytochemical studies, 5 age matched sham rats were compared to 10 injured rats. Thirty-five days after injury, rats were overdosed with pentobarbital (100 mg/kg) and perfused intracardially first with 300 ml of heparinized warm 0.9% saline followed by 500 ml of cold 4% paraformaldehyde. The T8 spinal cord was removed and post fixed for 4 hours in 4% paraformaldehyde prior to protection for 2 days in 30% sucrose at 4°C. The tissue was then embedded in OCT compound, frozen, mounted, and sectioned with a sliding microtome (model HM 400, Microm International, Waldorf Germany). Thirty micron sections from the T8 tissue were blocked in 5% normal goat serum for 30 minutes and incubated overnight in rabbit polyclonal pCaMKII antibody (alpha subunit, 1:200, Upstate Biotechnologies). The sections were then rinsed in phosphate buffered saline (PBS) and incubated in goat anti-rabbit antiserum conjugated to Alexa Fluor 568-Red (1:400, Molecular Probes). After rinsing, the floating sections were mounted on gelatin-coated slides and coverslipped with non-fade media. Expression of pCaMKII was quantified in the spinal cord gray and white matter rostral to the site of injury (T7/8) and in the individual lamina of the dorsal horn [42] in sham and injured rats by measuring the density of the reaction product for activated CaMKII and comparing between sham and SCI rats using MetaMorph Software (Universal Imaging Corporation, Downingtown, PA). To examine the cellular populations within the spinal cord that expresses pCaMKII, double labeling with either the neuronal marker NeuN (Chemicon, MAB377, 1:500), the astrocytic marker GFAP (Chemicon, MAB360, 1:1000), the microglial marker OX-42 (Serotec Ltd, MCA275G, 1:100) or the oligodendrocytes marker APC (CC-1, Calbiochem, Ab-7, 1:100) was performed. Omission of the primary antibodies or use of non-specific secondary IgG’s in the immunostaining process resulted in negative staining of the tissue sections.
For immunofluorescent staining, data from three channels were collected by Sequential scan (BioRad Radiance 2100 Confocal Laser System coupled to a Nikon E800) to avoid bleeding through between channels. Localization of pCaMKII and one of the cell marker images were collected with Krypton lasers of 568 nm excitation and 488 nm excitation. Red emission (a result of excitation of the conjugated AlexaFluor 568 to the pCaMKII antibody) demonstrated localization of pCaMKII; whereas, green emission (a result of excitation of the conjugated AlexaFluor 488 to the appropriate antibody) demonstrated localization of immunoreaction product in neurons, microglia, astrocytes or oligodendrocytes. Blue emission (DAPI) demonstrated nuclear localization. The yellow structures in merged images indicated the co-localization of the two antigens (Red+Green). Digital images were collected, saved and processed with MetaMorph Software.
For FluoroGold labeling, 5 age matched sham rats were compared to 5 injured rats. Seven days prior to sacrifice, rats used for the spinothalamic tract cell (STT) immunocytochemical colocalization study were given injections of FluoroGold (2%) into the ventral posterior lateral (VPL) nucleus of the thalamus to co-localize retrogradely labeled STT cells with immunocytochemical markers for the alpha subunit of pCaMKII. Briefly, while rats were deeply anesthetized and 0.1 μl injections of Fluorogold were delivered into one hemisphere of the VPL using the following the stereotaxic coordinates (distance from bregma, distance from the midline, distance from the surface of the skull) based on Paxinos and Watson [42]: -3.0 mm, 2.8 mm, 5.7/5.4 mm; -3.5 mm, 2.2 mm, 6.0/5.6 mm; -3.5 mm, 2.6 mm, 6.0/5.6 mm; -3.5 mm, 3.3 mm, 5.5/5.2 mm; -4.0 mm, 2.2 mm, 6.0/5.6 mm; -4.0 mm, 2.6 mm, 6.0/5.6 mm; -4.0 mm, 3.3 mm, 5.5/5.2 mm. FluoroGold labeled STT cells in sham and injured rats were confirmed under a fluorescence microscope (E1000, Nikon) and tested for the intensity of pCaMKII expression. Briefly, dorsal horn sections were viewed at 400X magnification and the FluoroGold positive STT cells were identified using a 330-380 nm wave band filter cube. Pictures of spinothalamic tract cells were captured using MetaMorph software on the CoolSnap digital camera, following which a 532-587 nm wave band (red) filter cube was used to visualize the expression of pCaMKII positive cells. Pictures were then taken of STT cells through the red filter cube to localize expression of pCaMKII within the same STT cells. All pictures for imaging pCaMKII expression were taken with the same exposure conditions. The average intensity of pCaMKII expression was measured with MetaMorph for sham and injured rats. Increases in the staining intensity of pCaMKII in STT cells are interpreted as an increase in the expression of pCaMKII within that cell type. Note that for both Western immunoblotting and immunocytochemistry, the same antibody was used to detect activated CaMKII expression.
Electrophysiology
In vivo extracellular single-unit recordings were made from age matched sham rats (n=10) and spinally contused rats 35 days after injury (n=10). Rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and supplemented with sodium pentobarbital (5 mg/kg/h) infused intravenously through a jugular vein catheter. Adequacy of anesthesia was monitored by the lack of withdrawal reflexes to noxious stimuli and the absence of corneal blink reflexes. Core temperature was maintained at 37°C by a thermostatically controlled heating blanket. Laminectomy was performed to expose the thoracic spinal segments T8-T10. Cells were isolated from thoracic segments (T7-T8) rostral to the site of contusion injury (T10). In vivo extracellular single-unit recordings were made with a low impedance (0.4-0.8 M at 1KHz; Kation Scientific) glass carbon fiber microelectrode. After isolation of single wide dynamic range (WDR) neuron, which is characterized by graded response patterns to increased stimuli intensity [8,50], background activity was measured followed by cutaneous receptive field mapping with von Frey filaments and brief pinches. Peripheral stimuli during electrophysiology included the following: brush (with a makeup brush; force range [±SEM]= 2-4 [±0.5] g), pressure (with a large arterial clip at 144 g/mm2), pinch (with a small arterial clip at 583 g/mm2), and von Frey filament application (with 5 filaments: 0.6 gm, 2 gm, 6 gm, 26 gm and 60 gm). Each stimulus was applied for 10 seconds with a 20 second break between stimuli.
Electrical signals were amplified and input to a window discriminator, displayed on analog and digital storage oscilloscopes, processed by a data collection system (CED 1401+; Cambridge Instruments, Cambridge, UK), and stored on a computer (Pentium Computer, Dell, Austin, TX, USA) to construct peristimulus time histograms. The stored digital record of unit activity was analyzed offline with Spike 2 software (v4.07, Cambridge Electronic Design, Cambridge, UK). Responsiveness to peripheral stimulus was calculated by subtracting the baseline activity from evoked activity to calculate a net increase in discharge rate. The effects of spinally applied KN-93 (100 μM in 50 μl) or KN-92 (the inactive enantiomer) were tested on cells rostral to the site of contusion at 35 days post injury. The 35 day time point was chosen for two reasons: 1) all SCI rats developed maximal at-level mechanical allodynia by this time and 2) we find activated CaMKII expression is significantly upregulated. Recordings in response to the set of peripheral stimuli were made and the same cells then received KN-93 or KN-92 application and were recorded at 15, 45 and 75 minutes after application. To ensure a single WDR unit was held for the duration of the recording experiment, we used the Spike2 program to compare the action potential shape and amplitude. All recorded single WDR neurons used in this study had the same reproducible shape and amplitude, or signature amplitude, throughout the recording period [10,21].
Statistics
Supraspinal responses during the girdling test for at-level mechanical allodynia were analyzed by a one way repeated measures analysis of variance (ANOVA) using SPSS 11.5 for Windows (SPSS; Chicago, IL). The effects of KN-93 administration on at level mechanical allodynia were analyzed using a one way repeated measures analysis of covariance (ANCOVA; with baseline reactivity as the covariate). To compare the group means for the electrophysiological data for reactivity to brush, press, and pinch, 1 between-1 within repeated measures ANOVAs were performed. The group means for the Western immunoblot data were analyzed using one way ANOVAs. To compare average intensity of pCaMKII alpha subunit expression in both the spinal cord and STT cells, Student’s t tests were performed using SigmaPlot 9 (Systat Software, Point Richmond CA). Laminar distributions of pCaMKII positive cells in the dorsal horn were analyzed using 1 between-1 within ANOVAs. Neuronal responses to von Frey stimuli applied just rostral to the site of injury were analyzed using 1 between-1within ANCOVAs with baseline reactivity as the covariate. Post hoc tests were performed using Duncan’s New Multiple Range Test. In all cases, the alpha level for statistical significance was set at p < 0.05. All data are represented as mean ± SEM.
RESULTS
SCI Rats Display At-Level Mechanical Allodynia by 35 Days After Injury
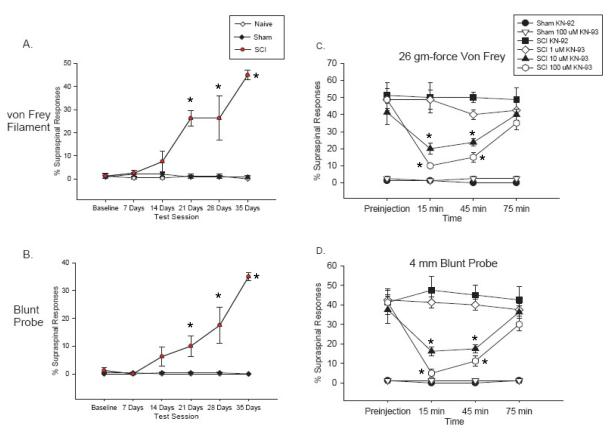
Spinal T10 contusion injured rats developed mechanical allodynia over the course of 35 days, as evidenced by a significant increase in the percentage of supraspinal responses (e.g., escape, biting, vocalization) to both the 26 gm-force von Frey filament and the 4 mm blunt probe applied to the dermatomes of the body trunk between mid thoracic areas and upper abdominal areas of the back (T5-T10) corresponding to the area rostral to the site of injury compared to presurgical and sham control responses (p < 0.0001, repeated measures ANOVAs, Figure 1A&B). Analyses of the baseline scores for supraspinal responses to the von Frey and blunt probe stimuli found no significant differences between the groups prior to injury (p > 0.05).
Figure 1.
Attenuation of at-level mechanical allodynia by inhibition of pCaMKII activation following contusive SCI. The percentage of supraspinal nociceptive responses (y axis) made by naïve (Naïve), sham (Sham) and spinal injured rats (SCI) tested weekly for 35 days post injury is graphed. The SCI rats showed a significant increase in supraspinal responses to both von Frey stimulation (A, p < 0.0001) and stimulation with a 4 mm blunt probe (B, p < 0.0001). Prior to KN-93 injection, SCI rats displayed at-level mechanical allodynia (as evidenced by an increased percentage of supraspinal nociceptive responses). Administration of KN-93 dose dependently reversed this at-level mechanical allodynia to both von Frey stimulation (C) and stimulation with a 4 mm blunt probe (D). Administration of KN-93 had no effect on sham rats. *: significant differences between before and after KN-93 treatment.
At-Level Mechanical Allodynia is Reversed by the CaMKII Inhibitor, KN-93
To examine the effects of inhibiting the activation of CaMKII on at-level mechanical allodynia, SCI and sham rats were tested prior to drug administration, exposed to one of three doses of the CaMKII inhibitor KN-93 (1, 10 or 100 μM in 50 μl) and then tested at 15 min, 45 min and 75 min after drug application. Naïve rats were not significantly different compared to sham groups in terms of supraspinal behavioral reactivity or pCaMKII expression levels. First, we performed an analysis on the baseline data for reactivity to the von Frey and blunt probe stimuli of the SCI and sham rats over the course of the drug treatment regimen to ensure that repeated exposure to KN-93 did not have lingering effects on supraspinal behavioral responses that would interfere with the ability to observe the acute effects of KN-93 administration. These analyses found no significant differences between the groups for either of the tests at any day during the dosing regimen (p > 0.05, repeated measures ANOVA), indicating that drug administration did not have any carryover effects on behavioral reactivity.
Prior to KN-93 administration on 35 days post SCI, SCI rats displayed significant at-level mechanical allodynia relative to sham rats (p < 0.0001, ANOVA, Figure 1 C&D). Injections of KN-93 led to a significant dose-dependent reversal of the at-level mechanical allodynia in SCI rats over the 45 minute testing interval (p < 0.0001 for all comparisons; repeated measures ANOVAs with baseline reactivity serving as the covariate, Figure 1C and D). Post hoc analyses of the group means for reactivity to the von Frey and blunt probe stimuli determined that sham groups had significantly fewer responses compared to any of the SCI groups (p < 0.05 for all comparisons). In addition, administration of 10 μM and 100 μM of KN-93 led to a significant reversal of at-level mechanical allodynia, as these 2 groups had significantly fewer supraspinal responses to both the von Frey and blunt probe stimuli than either the SCI KN-92 or SCI 1 μM KN-93 groups (p < 0.05). Finally, the SCI KN-92 and SCI 1 μM KN-93 groups had significantly greater response values compared to all other groups to both the von Frey and blunt probe stimuli, (p < 0.05).
Increases in Activated CaMKII Protein Levels in Dorsal Spinal Cord Correspond to At-Level Mechanical Allodynia Following SCI
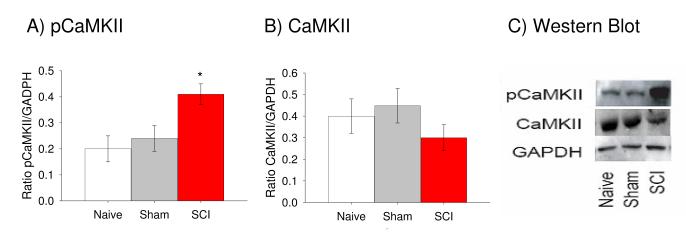
By 35 days post SCI, tissue was taken and used in Western immunoblot analyses to determine whether rats that displayed at-level mechanical allodynia also expressed an increase in the activated form of CaMKII. To control for differences in loading, each sample was normalized to GAPDH expression for the sample and statistics were performed on these ratios. SCI rats displayed significant upregulation in pCaMKII expression following injury relative to either sham or naïve rats (Figure 2A, p < 0.01, ANOVA). In addition, there were no significant differences between the groups in terms of nonphosphorylated CaMKII expression (Figure 2B, p > 0.05, ANOVA), suggesting that SCI does not cause an increase in the expression of CaMKII but instead leads to a change in the phosphorylation state of the kinase.
Figure 2.
Upregulation of pCaMKII is evident at 35 days after contusive SCI. (A) Western blot data from the same naïve, sham and SCI rats indicated that SCI rats had significantly greater levels of activated pCaMKII than the other groups (p < 0.0001). (B) No differences existed between the groups in terms of nonphosphorylated CaMKII expression (p > 0.05). (C) Representative Western blots for CaMKII (middle) and pCaMKII (top). GAPDH (bottom) is included as the loading control.
SCI Rats Demonstrate Increases in Activated CaMKII Protein Intensities in the Dorsal Spinal Cord Gray and White Matter, in Neurons, and in Spinothalamic Tract Cells
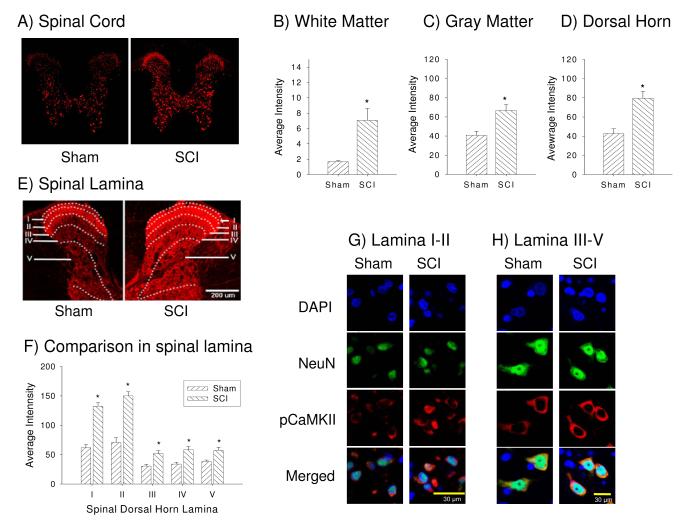
To determine whether CaMKII activation is related to at-level mechanical allodynia after SCI, the dorsal spinal cords of injured and sham rats were compared at 35 days post injury using immunocytochemistry. SCI rats had significantly greater expression of pCaMKII in the cytoplasmic compartment of neurons in the dorsal horn (sensitivity threshold set high to illustrate the cellular specificity of the most intense reaction product, Figure 3A and D) including white matter and gray matter (Figure 3C) compared to sham rats (p < 0.05; Student’s t-test). Note that sham specimens have considerably less staining in laminae I and II compared to SCI specimens (Figure A). With the image capture sensitivity threshold set to high laser intensity maximize all detectable labeling for regional comparisons (Figure 3E) pCaMKII expression was present in the white matter (dorsal column) and in neuronal cells but not microglial and astrocytes in the gray matter (see Figures 5A, B), whereas images taken with the laser intensity set low (Figure 3A) clearly showed neuronal cytoplasmic staining that was upregulated after SCI (Figure 3A, G, H). To further analyze changes within the dorsal horn of sham and SCI rats, pCaMKII expression was examined in each lamina of the dorsal horn, laminae I-V. Again, SCI rats displayed significant increases in pCaMKII expression in the all dorsal horn lamina relative to sham rats (p < 0.0001, repeated measures ANOVAs, Figure 3E-H).
Figure 3.
Spinal cord injury increases pCaMKII expression within the whole spinal cord. (A) Example of phosphorylated CaMKII (pCaMKII) expression in sham (left panel) and SCI (right panel) rats with low laser intensity. Quantifications analysis show the significant increase in pCaMKII expression in white matter (B), gray matter (C) and spinal dorsal horn (D) following SCI (p < 0.05). (E) Example of the laminar distribution of pCaMKII expression in sham (left panel) and SCI (right panel) rats with high laser intensity. (F) Quantification of the significant increase in pCaMKII following SCI in lamina I-V of the dorsal horn (p < 0.05). Example of neuronal pCaMKII expression in both lamina I-II (G) and lamina III-V (H), respectively.
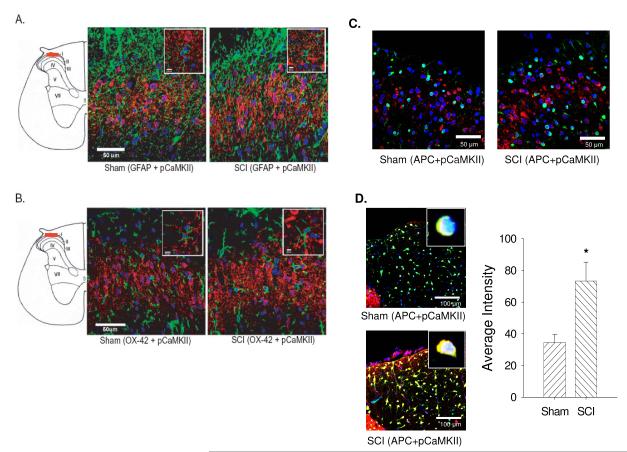
Figure 5.
Activated CaMKII expression in glial cells. pCaMKII (red) double immunofluorescent staining using astrocytes marker GFAP (green, A), microglia marker OX-42 (green, B) or oligodendrocytes marker CC-1 (green, C and D) indicated that activated CaMKII colocalized and showed significant difference in only oligodendrocytes in the dorsal column (p<0.05, D).
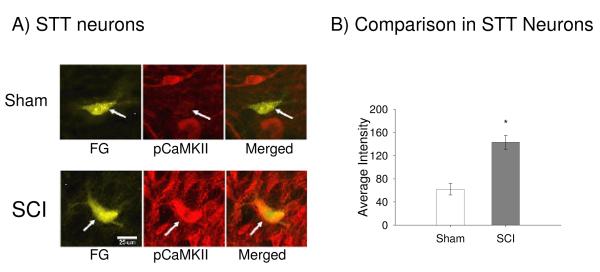
To further test whether neurons known to be involved in pain transmission upregulate pCaMKII following SCI, FluoroGold injections were made stereotaxically into the ventral posterior lateral (VPL) nucleus of the thalamus to localize retrogradely labelled spinothalamic (STT) cells rostral to the injury. Three to five STT cells were counted per single section from SCI and sham groups, respectively and the total number of STT cells (21 cells for SCI and 22 cells for Sham) did not show significant difference. Figure 4A depicts examples of FluoroGold labeled pCaMKII positive cells in the contralateral dorsal horn of both sham (top) and injured (bottom) rats. SCI produced a significant increase in the average intensity of pCaMKII expression within the labeled STT cells (p < 0.001; Students t-test, Figure 4B).
Figure 4.
Example of STT cells that express pCaMKII in sham and SCI rats. (A) Injections of Fluorogold into one hemisphere of the VPL showed STT cells in the spinal dorsal horn at T7/8 level. (B) Quantification of the significant increase in the average intensity of pCaMKII expression in STT cells in lamina V of injured rats compared to sham rats (p < 0.05).
In order to determine if glial cell populations expressed activated CaMKII in the spinal cord after SCI, double immunofluorescent staining was performed using antibodies that recognize GFAP (to localize astrocytes), OX-42 (to localize microglia) and APC (to localize oligodendrocytes). Following SCI, astrocytes (Figure 5A) and microglia (Figure 5B) did not show the localization of pCaMKII expression, despite lowering the threshold of laser capture to maximize the detection of immunoreactivity throughout the gray and white matter.. However, oligodendrocytes in the dorsal column (Figure 5D), but not in lamina I-V (Figure 5C), showed significantly increased localization of pCaMKII expression. These data suggest that the activation of CaMKII occurs exclusively in neurons in all dorsal lamina and in oligodendrocytes in the dorsal column.
SCI Produces Hyperexcitability in Dorsal Horn Neurons in the Thoracic Spinal Cord Rostral to the Site of Injury
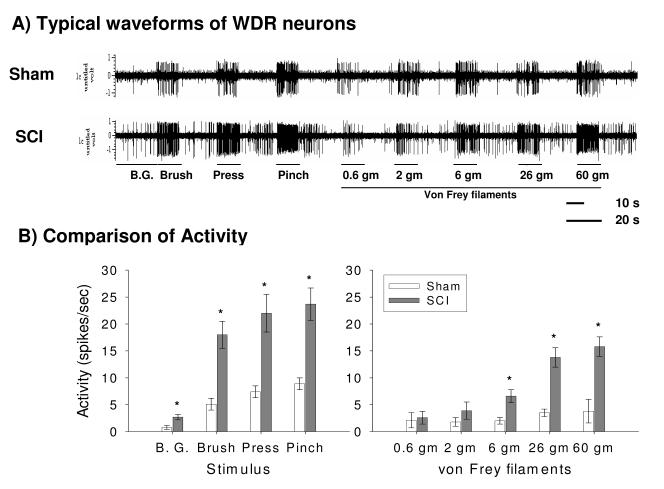
At 35 days post injury, background and neuronal activity in response to brush, pressure, pinch and graded von Frey filaments stimuli were compared between sham and SCI rats. Figure 6A shows typical waveforms of wide dynamic range (WDR) neuronal activity to gradual intensity of mechanical stimuli in sham (top) and SCI (bottom) rats. SCI rats prior to drug administration showed significantly greater neuronal activity in the absence of stimulation (background) and to brush, press, and pinch stimuli compared to sham rats (all ps < 0.05; ANOVA, Figure 6B). In addition, similar analyses of neuronal responding to a graded series of von Frey stimuli found that SCI rats prior to drug application displayed significantly increased neuronal activity to the 6 gm, 26 gm and 60 gm force stimuli compared to sham rats (p < 0.01, ANOVA, Figure 6B). There were no significant differences in responses between the groups for the 0.6 gm or 2 gm von Frey stimulus (p > 0.05).
Figure 6.
SCI produces dorsal horn neuronal hyperexcitability. (A) Typical waveforms of neuronal activity in sham (top) and SCI (bottom) rats. (B) SCI significantly increased background (B.G.) activity and evoked neuronal responses to brush, pressure, pinch, and 6 gm, 26 gm and 60 gm graded von Frey stimuli compared to sham rats (p < 0.001) as demonstrated by the histograms of dorsal horn neuronal responses in spikes/sec.
Inhibition of CaMKII with KN-93 Attenuated Neuronal Hyperreactivity to Brush, Pressure, and Pinch
The next goal of the current set of experiments was to determine the effects of KN-93 administration on neuronal hyperexcitability in SCI rats 35 days after injury. Our electrophysiological study of the effects of KN-93 focused on administration to the SCI rats, as the current study indicated that KN-93 had no effect on the behavior of sham rats at all doses tested, including the highest dose of KN-93 (100 μM), the dose that significantly diminished at-level mechanical allodynia after SCI in our behavioral study.
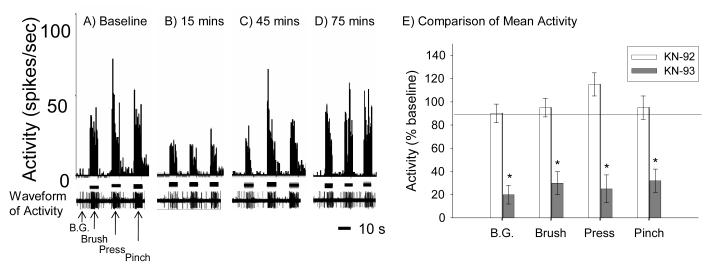
First, we sought to determine the effects of CaMKII inhibition on background activity as well as the evoked activity to brush, pressure and pinch stimuli (Figure 7A-D). The CaMKII inhibitor, KN-93, significantly decreased the background rate of neuronal firing in SCI rats (p < 0.05, repeated measures ANOVA). CaMKII inhibition also produced a significant decrease in neuronal responses to the brush, pressure and pinch stimuli in SCI rats treated with 100 μM KN-93 (all ps< 0.01, repeated measures ANOVA). Taken together, these data indicate that CaMKII inhibition with KN-93 diminishes neuronal hyperexcitability following SCI not only in the absence of stimulation (background) but also to both non-noxious (brush) and noxious (pressure, pinch) stimuli in dermatomes corresponding to the site rostral to SCI. Figure 7E depicts neuronal responses in spikes per second at the time point of maximal drug effectiveness, 15 minutes post injection.
Figure 7.
CaMKII inhibition significantly decreases background activity and evoked neuronal responses to brush, pressure, and pinch stimulation at the near rostral level of contusive SCI. (A-D) show spike frequency histograms of neuronal activity to brush, pressure and pinch stimuli before (A) and 15 mins (B), 45 mins (C) and 75 mins (D) after KN-93 administration. (E) Mean activity (±SEM) expressed as a percentage activity of baseline (background, B.G.) and to brush, pressure and pinch for KN-92 (open bars) and KN-93 treated (filled bars) rats at 15 min post administration. KN-93 not only significantly attenuated background activity but also produced significant decreases in neuronal responding to brush, pressure and pinch (p < 0.001). KN-92 had no effect on neuronal activity.
Inhibition of CaMKII with KN-93 Diminishes Neuronal Responses to von Frey Stimulation
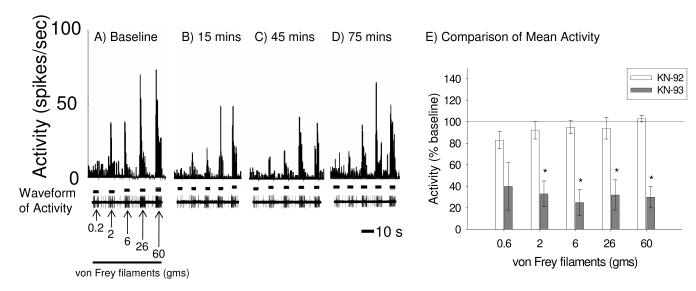
In addition to the pCaMKII inhibition on reactivity to brush, pressure, and pinch, we examined the effects of KN-93 on dorsal horn neuronal activity in response to graded von Frey stimuli applied to the dermatome rostral to the site of SCI. KN-93 treatments significantly reduced neuronal firing to the 2 gm, 6 gm, 26 gm and 60 gm von Frey stimuli (Figure 8 A-E, ps < 0.05 for all comparisons, repeated measures ANOVA with baseline reactivity as the covariate). However, KN-93 had no effect on reactivity to the 0.6 gm von Frey stimulus (Figure 8E, p > 0.05),
Figure 8.
CaMKII inhibition significantly decreases neuronal responses to graded von Frey stimulation at the near level of contusive SCI. (A-D) show spike frequency histograms of neuronal activity to graded von Frey stimuli before (A) and 15 mins (B), 45 mins (C) and 75 mins (D) after KN-93 administration. (E) Mean activity (±SEM) to 0.6 gm, 2 gm, 6 gm, 26 gm, and 60 gm von Frey stimuli applied to the skin rostral to the site of SCI 15 minutes after drug administration. KN-93 significantly attenuated neuronal activity to 0.6 gm, 2 gm, 6 gm, 26 gm and 60 gm von Frey stimuli (p < 0.001) whereas KN-92 had no effect.
DISCUSSION
These data provide the first convergent evidence that chronically activated calcium/calmodulin-dependent protein kinase II (CaMKII) contributes to central neuropathic pain following spinal cord injury (SCI).
We report that SCI rats show increased neuronal activation of CaMKII on POD 35 after SCI that play a causal role in at-level mechanical allodynia (a pathophysiological condition in which non-noxious stimuli becomes noxious) since we are able to demonstrate that an inhibitor of pCaMKII activation, KN-93, dose dependently reversed the mechanical allodynia. In colocalization studies, we report that SCI develops significant increases in phosphorylated CaMKII (pCaMKII) expression in neurons, but surprisingly not in astrocytes and microglia, rostral (in regions of the body trunk where at-level mechanical allodynia developed) to the spinal lesion site, that included spinothalamic tract cells in the dorsal horn gray matter. In addition, pCaMKII was upregulated in oligodendrocytes in the dorsal column, but not in dorsal horn spinal lamina at both at-level (T7/8, current study) and below-level regions (L4/5) [22] in rats with T10 contusive SCI compared to controls. These data suggest that SCI produces CamKII activation of both spinal gray matter neurons, including STT cells, and of dorsal column cells, including oligodendrocytes. These findings demonstrate altered intracellular mechanisms in sensory synaptic transmission results in central neuropathic pain after SCI. In electrophysiological studies, WDR dorsal horn neurons rostral to the site of injury displayed significant differences in both evoked and background neuronal activity to a broad range of stimuli (e.g., brush, pressure, pinch and graded von Frey stimuli) following spinal cord contusion injury compared to sham controls. Importantly, KN-93 significantly reduced the hyperexcitability of WDR dorsal horn neurons, indicating a pivotal role for CaMKII phosphorylation in the altered membrane excitability. Taken collectively, these novel results demonstrate that activation of CaMKII plays a pivotal role in intracellular pathways that underlie the neuronal hyperexcitability and provides the substrate for central neuropathic pain.
In the hippocampus and other CNS structures, synaptic efficacy can be modulated by NMDA receptor mediated activation of protein kinases by phosphorylation. One key signaling protein kinase, CaMKII, is activated by phosphorylation that is triggered by increased intracellular Ca2+ concentrations, which in part occurs through influx by NMDA receptor activation in response to increased extracellular amino acid concentrations by synaptic, and extrasynaptic mechanisms [9,47] that occur after SCI. Once CaMKII is phosphorylated, it remains active through autophosphorylation mechanisms [60] even after Ca2+ concentrations return to normal [34]. CaMKII increases the activation state of postsynaptic receptors, principally the NMDA and AMPA glutamate receptors (see below), by increased duration of activation state and/or an increase in activated receptor number leading to increased postsynaptic responses to excitatory transmission or long term potentiation (LTP).
LTP can last for hours and shares a number of molecular similarities with dorsal horn neuron hyperexcitability during central sensitization [16,27,54]. For example, both LTP and central sensitization involve activation of the NMDA receptor, followed by subsequent activation of downstream intracellular enzymatic cascades involving adenylyl cyclase, protein kinase A, protein kinase C, and/or CaMKII. Stimulation of these cascades leads to activation of a number of mitogen activated protein kinases (MAPKs), including ERK 1/2 and p38 MAPK which then, in turn, can lead to phosphorylation of transcription factors and changes in gene transcription [11,27,59]. The transcription factor CREB has received a great deal of attention for its role in the formation of long-term memory, largely due to the importance of CREB activation in the late phases of both long-term facilitation and long-term potentiation [3,14]. Recent research on models of central sensitization following peripheral insults (such as injections of formalin or capsaicin, or sciatic nerve injury) [17,29,40,41,58] and SCI [10,12] highlight the role of activated CaMKII, ERK 1/2, p38 MAPK and CREB in nociceptive hyperreactivity. However, the role of CaMKII and subsequent downstream pathways leading to transcriptional changes had not been described in a model of persistent pain (months to years) after SCI and the cellular localization of key signaling molecules was unknown. It is reasonable to expect the existence of persistent phosphorylated levels of CaMKII and other intracellular signaling molecules that would feedback to contribute to continued activation of glutamate receptors or other receptors, if continued neuronal hyperexcitability is expected. Additionally, phosphorylated CaMKII increases in specific pain projection neurons would be expected if activated CaMKII is involved in persistent pain, which is what we report in the present manuscript.
The role of phosphorylated CaMKII in pain involves several mechanisms that lead to increased receptor activation. The increased distribution of pCaMKII in laminae I and II of the spinal dorsal horn during activation of small diameter primary afferent neurons indicates its importance in nociceptive processing since this is the terminal projection laminae for nociceptive neurons [55]. Furthermore, phosphorylated CaMKII is upregulated through NMDA activation via ERK ½ pathways which in turn results in increased phosphorylation of CREB in the spinal dorsal horn [17,39]. In addition, there is evidence of a feedback mechanism since activated CaMKII induces phosphorylation of NMDA channels, increasing Ca2+ influx, leading to increased adenylate cyclase and subsequent increases in signaling pathways including ERK 1/2, which then leads to CaMKII activation [13,16]. Postsynaptic upregulation of pCaMKII activity in the spinal dorsal horn has been demonstrated in response to peptidergic primary afferent activity [6] and is thought to contribute to increased AMPA receptor trafficking, specifically the GluR1 subunit, by endosomal integration into the neuronal plasma of dorsal horn neurons, with the resultant increased post synaptic efficacy of those neurons [20]. Point mutation in mice of the alpha-CaMKII gene at position Thr-286 resulted in loss of the ongoing secondary pain produced by the formalin test, supporting a critical role of alpha-CaMKII autophosphorylation in spontaneous/ongoing pain syndromes [60]. Finally, more recent work has indicated a required role of pCaMKII in TRPV1 ligand binding [49]. TRPV1 is a ligand gated cationic channel expressed in small diameter sensory neurons, that is activated by heat, acid and capsaicin. Thus, a persistent increase in pCaMKII would be expected to result in increased nociceptive sensitivity via increased TRPV1 activation of the C fiber polymodal primary afferent population.
Our data indicates that a key kinase, CaMKII, upstream of ERK1/2 and p38 MAPK, remains persistently activated following SCI. Whereas our former studies determined a tight correlation between upregulation of activated ERK1/2 and p38 MAPK, and the expression of at-level mechanical allodynia [10,11], the current work demonstrates that spinal application of an inhibitor of CaMKII phosphorylation leads to significant decreases in both at-level mechanical allodynia and neuronal hyperexcitability rostral to the site of injury. In other studies, a causal role for one MAP kinase, p38 MAPK, in the maintenance of below-level pain was suggested [23] since application of minocycline led to decreased activated of p38 MAPK and diminished mechanical allodynia in the hindlimbs after moderate to severe spinal contusion injury. However, minocycline, which acts in part by inhibiting p38 MAPK signaling pathways, has a broad array of biological effects such as inhibition of inflammatory cytokines, free radicals and matrix metalloproteases, all which can contribute to improved recovery after SCI [51,52], including decreased pain symptoms [26]. Thus, specific inhibitors are desirable. Inhibiting the activation of ERK1/2 by PD98059 following excitotoxicity lesion of the spinal cord, diminishes the development of excessive grooming behavior, which is one indication of central neuropathic pain [59]. The findings in the current study indicate CaMKII and downstream cascades play a crucial role in persistent central neuropathic pain. Activated components of the intracellular signaling cascades associated with central sensitization and LTP, specifically activated ERK1/2, p38MAPK and the transcription factor CREB, are upregulated in animals that experience mechanical allodynia after SCI but not in SCI animals with no mechanical allodynia [11,12]. CaMKII is thought to activate CREB, a transcription factor at least in part by ERK pathways [40]. Furthermore, both activated forms of CaMKII and CREB are upregulated in spinothalamic tract neurons, which are part of the neural circuitry mediating pain [11, 55, present study]. These data provide further support for the hypothesis that intracellular signaling pathways provide critical substrates for central sensitization and persistent pain in the spinal dorsal horn after injury. Furthermore these pathways are similar to those that occur in LTP in the hippocampus and other CNS regions [16,47]. The paradox, however, is that LTP results in neuronal hyperexcitability that persists for minutes to hours; whereas, hyperexcitability of WDR dorsal horn neurons after SCI is persistent. For example, spinal dorsal horn neurons showed consistent neuronal hyperexcitability following SCI via loss of endogenous GABAergic tone [21], decreased serotonergic system [24] and glial activation [23].
However, the significance of the differential expression of pCaMKII in glial cells between gray matter and whiter matter in the present study is unclear; oligodendrocytes showed upregulated pCaMKII in the spinal dorsal column, but not in dorsal horn lamina whereas astrocytes and microglia did not show pCaMKII expression. In conclusion, our study suggests that pCaMKII is critical in determining the driving mechanisms underlying the persistent intracellular signaling pathways that contribute to neuronal membrane hyperexcitability after SCI.
Acknowledgements
This work was funded by CRPF grant CB1-0404-2 to EDC, Mission Connect of TIRR-Houston, the Dunn Foundation, the West Foundation, Mr. Frank Liddell and NIH grants NS11255 and NS39161 to CEH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No Conflict of Interest
Contributor Information
Eric D Crown, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555-1043..
Young S. Gwak, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, 77555-1043..
Zaiming Ye, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX77555-1043..
Huaiyu Tan, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, 77555-1043..
Kathia M Johnson, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, 77555-1043..
Guo-Ying Xu, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, 77555-1043..
David J McAdoo, Department of Neuroscience and Cell Biology, University of Texas Medical branch, Galveston, TX, 77555-1043.
Claire E Hulsebosch, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555-1043..
REFERENCES
- [1].Attal N, Guirimand F, Brasseur L, Gaude V, Chauvin M, Bouhassira D. Effects of IV morphine in central pain: a randomized placebo-controlled study. Neurology. 2002;58:554–563. doi: 10.1212/wnl.58.4.554. [DOI] [PubMed] [Google Scholar]
- [2].Belanger E, Levi AD. The acute and chronic management of spinal cord injury. J Am Coll Surg. 2000;190:603–618. doi: 10.1016/s1072-7515(00)00240-4. [DOI] [PubMed] [Google Scholar]
- [3].Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- [4].Capel ID, Dorrell HM, Spencer EP, Davis MW. The amelioration of the suffering associated with spinal cord injury with subperception transcranial electrical stimulation. Spinal Cord. 2003;41:109–117. doi: 10.1038/sj.sc.3101401. [DOI] [PubMed] [Google Scholar]
- [5].Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–373. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- [6].Choi SS, Seo YJ, Kwon MS, Shim EJ, Lee JY, Ham YO, Lee HK, Suh HW. Increase of phosphorylation of calcium/calmodulin-dependent protein kinase-II in several brain regions by substance P administered intrathecally in mice. Brain Res Bull. 2005;65:375–381. doi: 10.1016/j.brainresbull.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [7].Christensen MD. Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- [8].Chung JM, Surmeier DJ, Lee DJ, Sorkin LS, Honda CN, Tsong Y, Willis WD. Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J Neurophysiol. 1986;56:308–327. doi: 10.1152/jn.1986.56.2.308. [DOI] [PubMed] [Google Scholar]
- [9].Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP Kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [12].Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Westlund KN, Hulsebosch CE. Upregulation of the phosphorylated form of CREB in spinothalamic tract cells following spinal cord injury: relation to central neuropathic pain. Neurosci Lett. 2005;384:139–144. doi: 10.1016/j.neulet.2005.04.066. [DOI] [PubMed] [Google Scholar]
- [13].Dai Y, Wang H, Ogawa A, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Ca2+/calmodulin-dependent protein kinase II in the spinal cord contributes to neuropathic pain in a rat model of mononeuropathy. Eur J Neurosci. 2005;21:2467–2474. doi: 10.1111/j.1460-9568.2005.04091.x. [DOI] [PubMed] [Google Scholar]
- [14].Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- [15].Dyson-Hudson TA, Shiflett SC, Kirshblum SC, Bowen JE, Druin EL. Acupuncture and Trager psychophysical integration in the treatment of wheelchair user’s shoulder pain in individuals with spinal cord injury. Arch Phys Med Rehabil. 2001;82:1038–1046. doi: 10.1053/apmr.2001.24888. [DOI] [PubMed] [Google Scholar]
- [16].Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fang L, Wu J, Zhang X, Lin Q, Willis WD. Calcium/calmodulin dependent protein kinase II regulates the phosphorylation of cyclic AMP-responsive element-binding protein of spinal cord in rats following noxious stimulation. Neurosci Lett. 2005;374:1–4. doi: 10.1016/j.neulet.2004.10.014. [DOI] [PubMed] [Google Scholar]
- [18].Finnerup NB, Biering-Sørensen F, Johannesen IL, Terkelsen AJ, Juhl GI, Kristensen AD, Sindrup SH, Bach FW, Jensen TS. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–1030. doi: 10.1097/00000542-200505000-00023. [DOI] [PubMed] [Google Scholar]
- [19].Finnerup NB, Gottrup H, Jensen TS. Anticonvulsants in central pain. Expert Opin Pharmacother. 2002;3:1411–1420. doi: 10.1517/14656566.3.10.1411. [DOI] [PubMed] [Google Scholar]
- [20].Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–323. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- [21].Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Profentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gwak YS, Hassler SN, Hulsebosch CE. Reactive oxygen species modulate central neuropathic pain following Spinal cord injury. Annual Meeting for Society for Neuorscience; 2010; Abstract 80.9/TT15. [Google Scholar]
- [23].Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hains BC, Willis WD, Hulsebosch CE. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp Brain Res. 2003;149:174–186. doi: 10.1007/s00221-002-1352-x. [DOI] [PubMed] [Google Scholar]
- [25].Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, McCartney N. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- [26].Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hulsebosch CE. From discovery to clinical trials: treatment strategies for central neuropathic pain after spinal cord injury. Curr Pharm Des. 2005;11:1411–1420. doi: 10.2174/1381612053507864. [DOI] [PubMed] [Google Scholar]
- [28].Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- [29].Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Larsson M, Broman J. Pathway-specific bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II at spinal nociceptive synapses after acute noxious stimulation. J Neurosci. 2006;26:4198–4205. doi: 10.1523/JNEUROSCI.0352-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Levi R, Hultling C, Seiger A. The Stockholm Spinal Cord Injury Study: 2. Associations between clinical patient characteristics and post-acute medical problems. Paraplegia. 1995;33:585–594. doi: 10.1038/sc.1995.125. [DOI] [PubMed] [Google Scholar]
- [32].Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–348. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- [33].Ma W, Quirion R. Increased phosphorylation of cyclic AMP response element-binding protein (CREB) in the superficial dorsal horn neurons following partial sciatic nerve ligation. Pain. 2001;93:295–301. doi: 10.1016/S0304-3959(01)00335-9. [DOI] [PubMed] [Google Scholar]
- [34].Kitamura K, Kanasashi M, Suga C, Saito S, Yoshida S, Ikezawa Z. Cutaneous reactions induced by calcium channel blocker: high frequency of psoriasiform eruptions. J Dermatol. 1993;20:279–286. doi: 10.1111/j.1346-8138.1993.tb01392.x. [DOI] [PubMed] [Google Scholar]
- [35].Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- [36].Messersmith DJ, Kim DJ, Iadarola MJ. Transcription factor regulation of prodynorphin gene expression following rat hindpaw inflammation. Brain Res Mol Brain Res. 1998;53:260–269. doi: 10.1016/s0169-328x(97)00308-2. [DOI] [PubMed] [Google Scholar]
- [37].Miletic G, Pankratz MT, Miletic V. Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany thermal hyperalgesia following chronic constriction injury in rats. Pain. 2002;99:493–500. doi: 10.1016/S0304-3959(02)00242-7. [DOI] [PubMed] [Google Scholar]
- [38].Mills CD, Hulsebosch CE. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci Lett. 2002;319:59–62. doi: 10.1016/s0304-3940(01)02551-4. [DOI] [PubMed] [Google Scholar]
- [39].Mills CD, Johnson KM, Hulsebosch CE. Group I metabotropic glutamate receptors in spinal cord injury: Roles in neuroprotection and the development of chronic central pain. J Neurotrauma. 2002;19:23–42. doi: 10.1089/089771502753460213. [DOI] [PubMed] [Google Scholar]
- [40].Miyabe T, Miletic V. Multiple kinase pathways mediate the early sciatic ligation-associated activation of CREB in the rat spinal dorsal horn. Neurosci Lett. 2005;381:80–85. doi: 10.1016/j.neulet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [41].Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Paxinos G, Watson G. The rat brain in stereotaxic coordinates. 3rd edition Academic Press; New York: 1997. [Google Scholar]
- [43].Sandkuhler J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- [44].Siddall PJ, Gray M, Rutkowski S, Cousins MJ. Intrathecal morphine and clonidine in the management of spinal cord injury pain: a case report. Pain. 1994;59:147–148. doi: 10.1016/0304-3959(94)90059-0. [DOI] [PubMed] [Google Scholar]
- [45].Siddall P, Yezierski R, Loeser J. Pain following spinal cord injury: Clinical features, prevalence, and taxonomy. IASP Newsletter. 2000;3:3–7. [Google Scholar]
- [46].Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- [47].Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- [48].Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11:2687–2698. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- [50].Tan H, Johnson KM, Hulsebosch CE. Role of ionotropic glutamate receptors in a rodent model of central neuropathic pain. Neurosci. 2004 Abstr. 34. [Google Scholar]
- [51].Vincler M, Maixner W, Vierck CJ, Jr., Light AR. Effects of systemic morphine on escape latency and a hindlimb reflex response in the rat. J Pain. 2001;2:83–90. doi: 10.1054/jpai.2001.19560. [DOI] [PubMed] [Google Scholar]
- [52].Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–29. doi: 10.1191/0269215503cr581oa. [DOI] [PubMed] [Google Scholar]
- [53].Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Widerstrom-Noga EG, Duncan R, Felipe-Cuervo E, Turk C. Assessment of the impact of pain and impairments associated with spinal cord injuries. Arch Phys Med Rehabil. 2002;83:395–404. doi: 10.1053/apmr.2002.28028. [DOI] [PubMed] [Google Scholar]
- [55].Willis WD., Jr Long term potentiation in spinothalamic neurons. Brain Res Brain Res Rev. 2002;40:202–214. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- [56].Willis WD, Jr, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 3rd Ed Plenum Publishing; New York: 2004. [Google Scholar]
- [57].Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- [58].Wu J, Fang L, Lin Q, Willis WD., Jr The role of nitric oxide in the phosphorylation of cyclic adenosine monophosphate-responsive element-binding protein in the spinal cord after intradermal injection of capsaicin. J Pain. 2002;3:190–198. doi: 10.1054/jpai.2002.123653. [DOI] [PubMed] [Google Scholar]
- [59].Yu CG, Yezierski RP. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Brain Res Mol Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [60].Zeitz KP, Giese KP, Silva AJ, Basbaum AI. The contribution of autophosphorylated alpha-calcium-calmodulin kinase II to injury-induced persistent pain. Neuroscience. 2004;128:889–898. doi: 10.1016/j.neuroscience.2004.07.029. [DOI] [PubMed] [Google Scholar]