Abstract
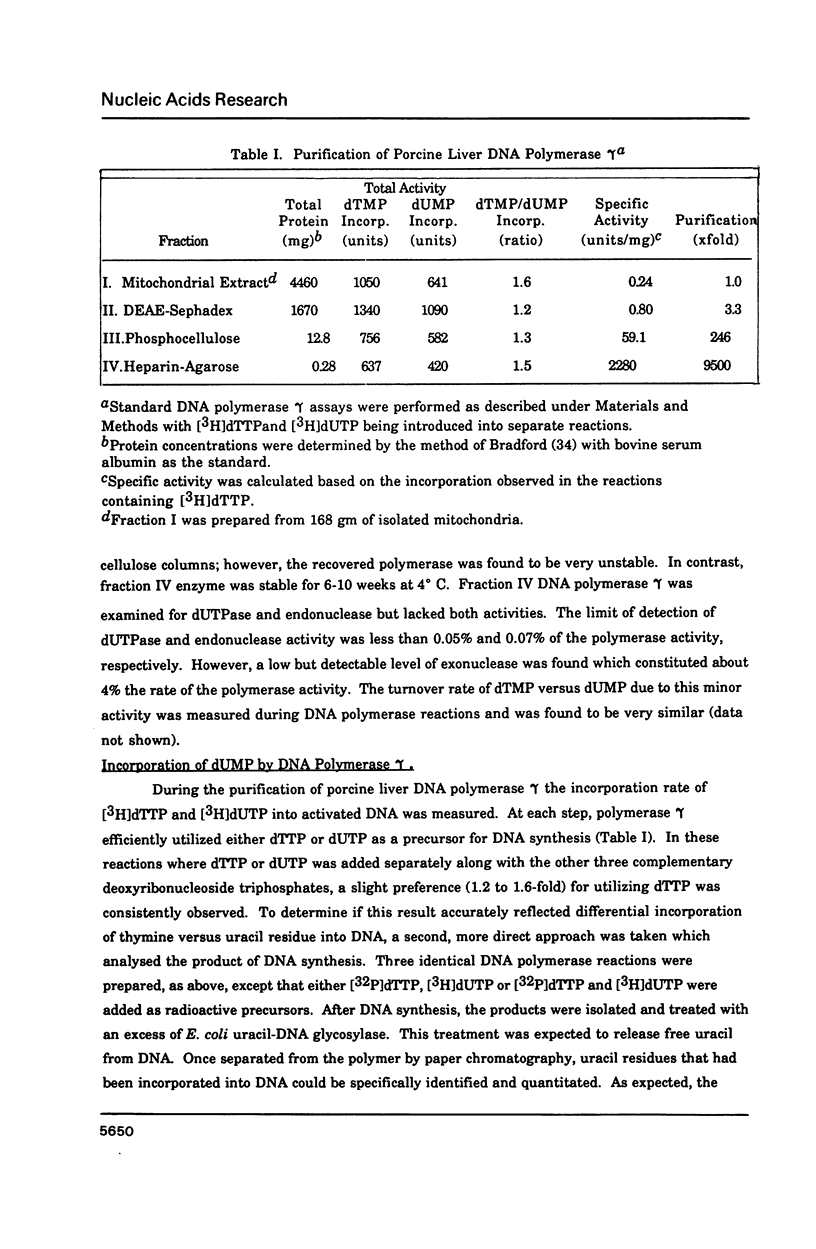
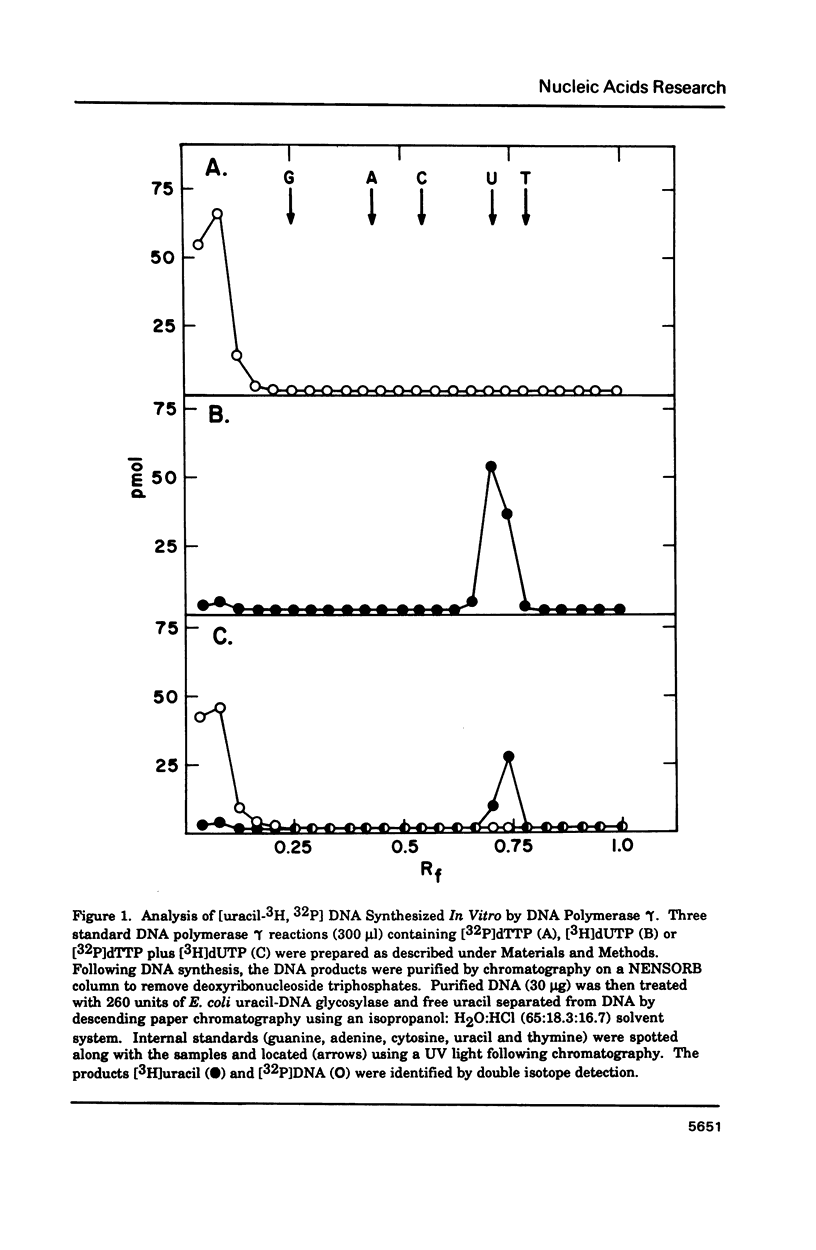
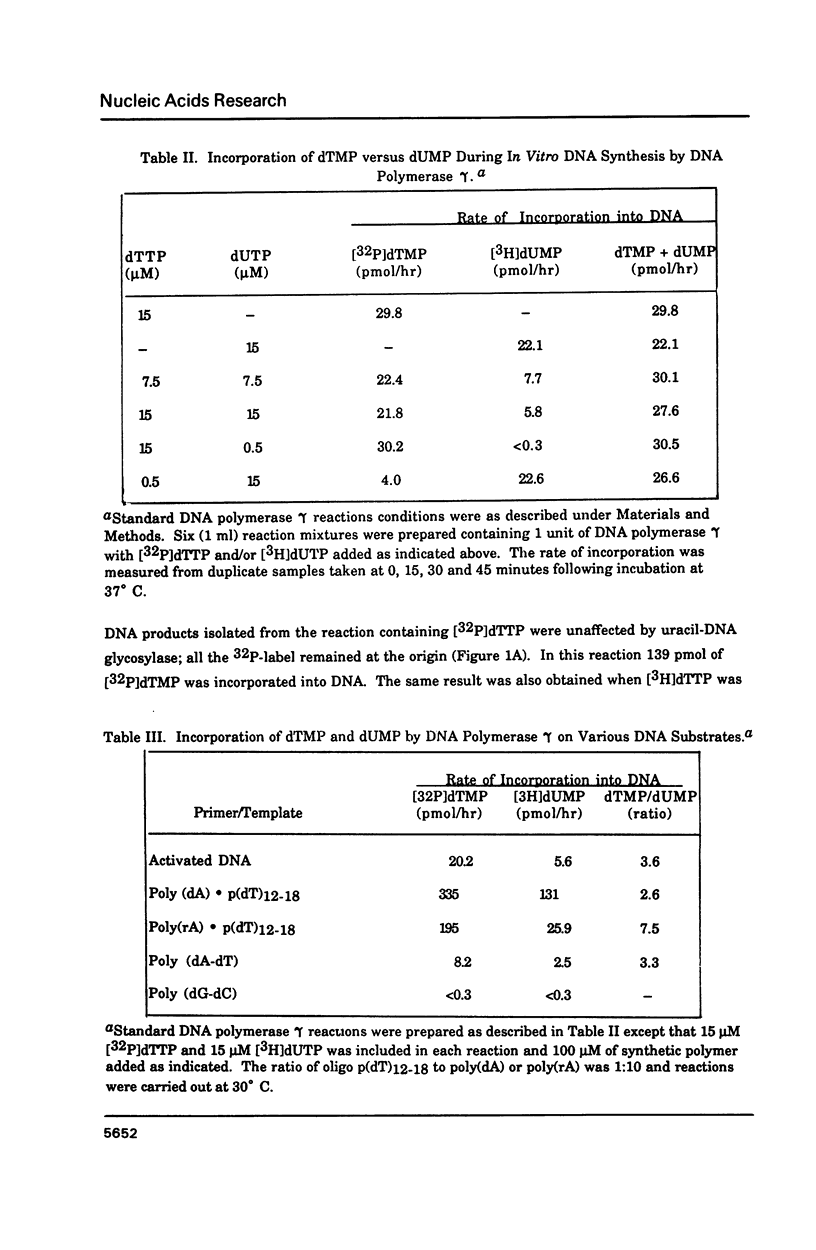
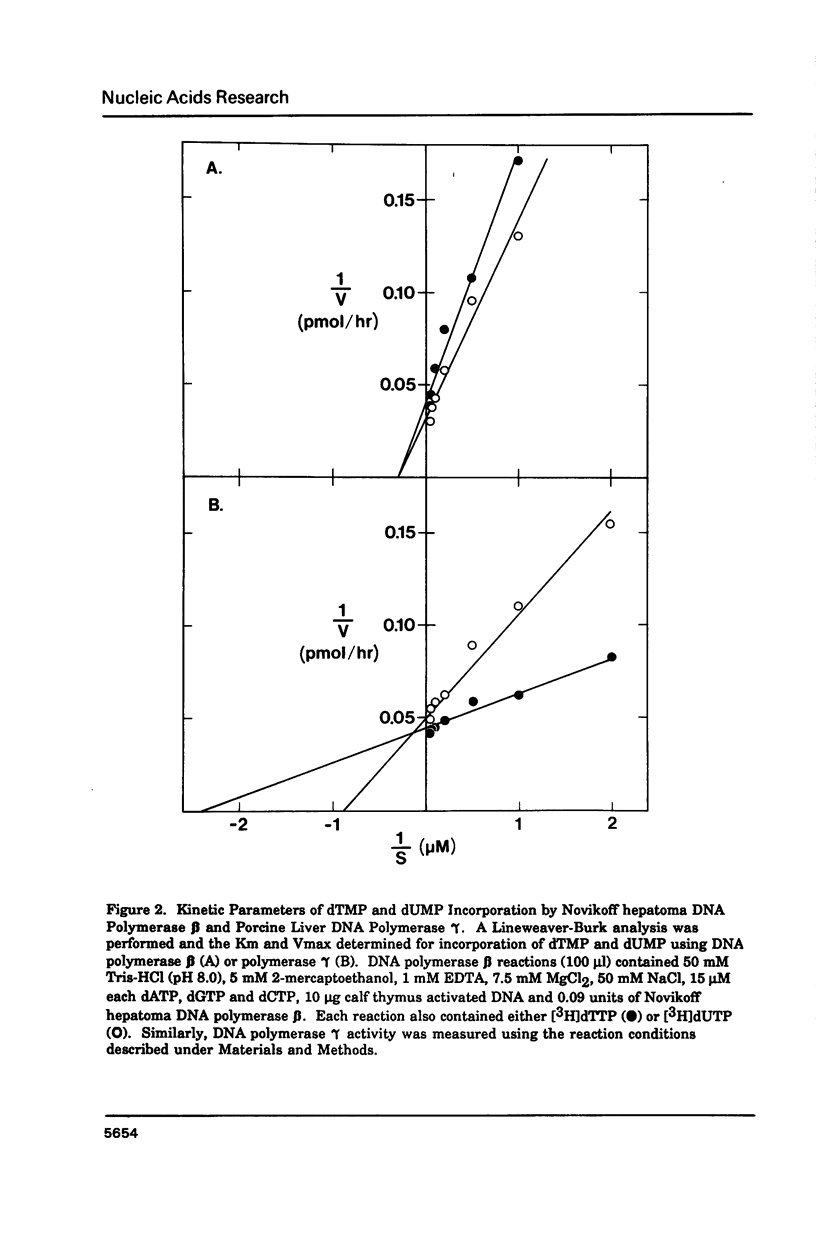
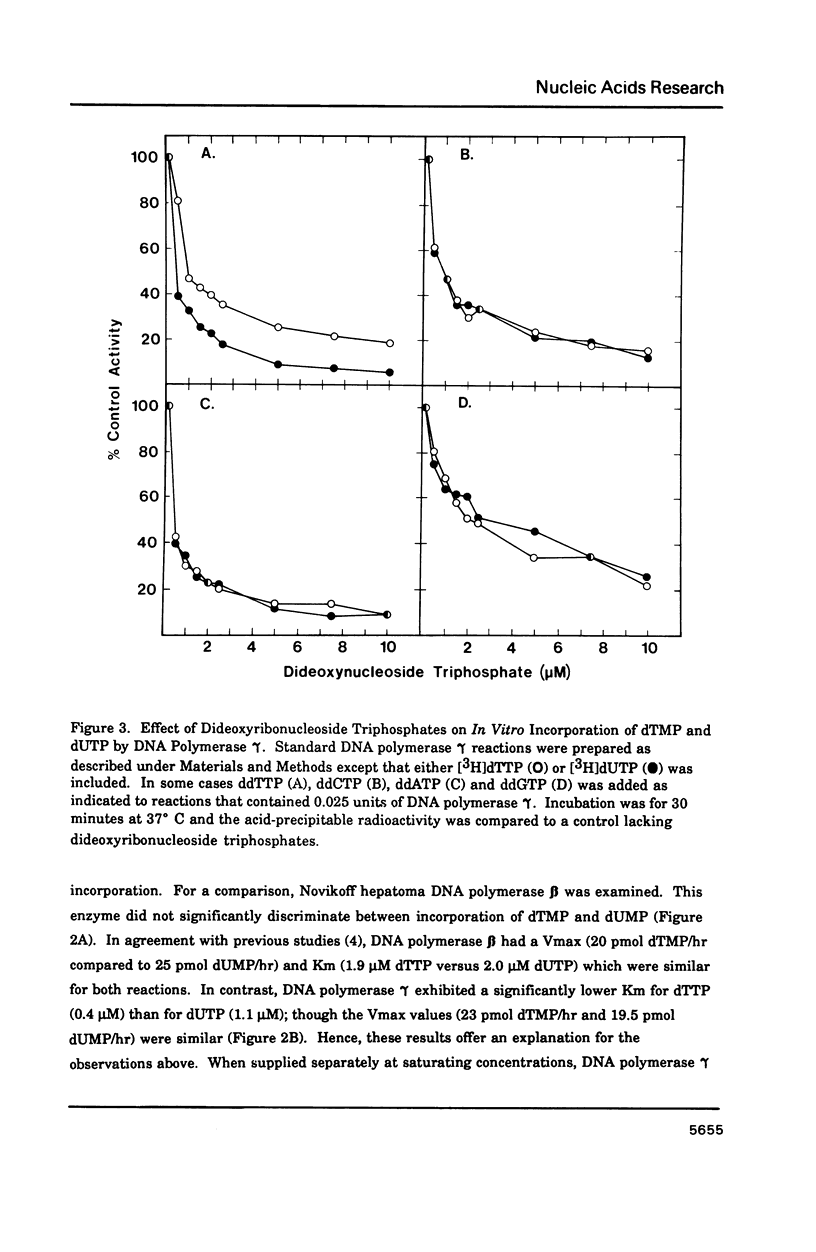
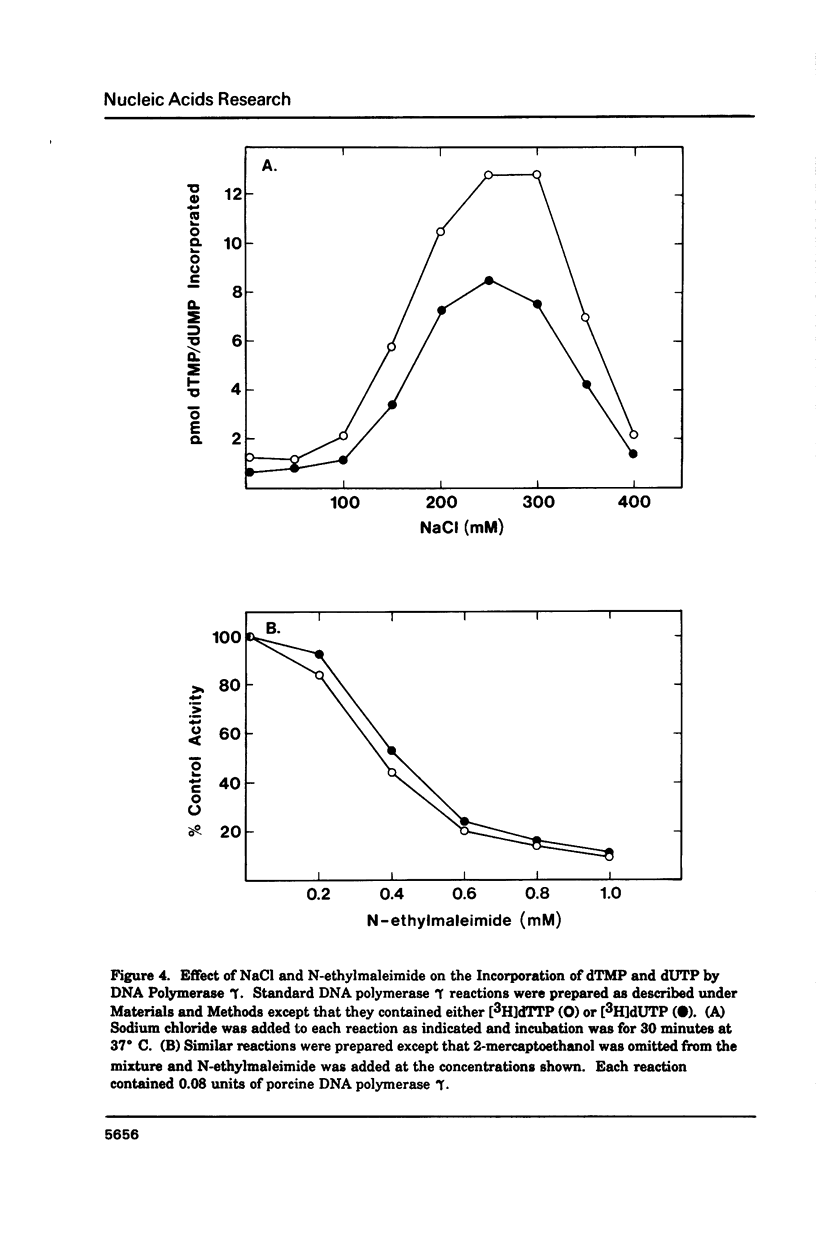
Porcine liver DNA polymerase gamma has been demonstrated to preferentially incorporate dTMP over dUMP during in vitro DNA synthesis. When polymerase activity was measured in standard reactions containing saturating levels of either dTTP or dUTP, the polymerization rate was slightly faster in the reaction containing dTTP. However, under conditions where both dTTP and dUTP competed, at an equal molar concentration, approximately 3-times more thymine residues were incorporated than uracil residues into DNA. Similarly, preferential incorporation of dTMP was observed on several substrates including poly (dA).oligo p(dT), poly (rA).oligo p(dT) and poly (dA-dT). The discrimination against dUMP incorporation was even more apparent with reduced levels of dUTP. These observations were consistent with the finding that the Km for DNA polymerase gamma was about 3-fold lower for dTTP (0.4 microM) than for dUTP (1.1 microM). On the other hand, the Vmax for these two reactions was very similar. Discrimination against dUMP incorporation was also observed during inhibition of polymerase gamma by dideoxyribonucleoside triphosphates. Dideoxythymidine triphosphate preferentially inhibited dUMP incorporation compared to that of dTMP, whereas ddATP, ddCTP and ddGTP inhibited both reactions equally.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. T., Friedberg E. C. The presence of nuclear and mitochondrial uracil-DNA glycosylase in extracts of human KB cells. Nucleic Acids Res. 1980 Feb 25;8(4):875–888. [PMC free article] [PubMed] [Google Scholar]
- Bessman M. J., Lehman I. R., Adler J., Zimmerman S. B., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. III. THE INCORPORATION OF PYRIMIDINE AND PURINE ANALOGUES INTO DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):633–640. doi: 10.1073/pnas.44.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Mathews C. K. Unusual compartmentation of precursors for nuclear and mitochondrial DNA in mouse L cells. J Biol Chem. 1982 Aug 25;257(16):9305–9308. [PubMed] [Google Scholar]
- Bestwick R. K., Moffett G. L., Mathews C. K. Selective expansion of mitochondrial nucleoside triphosphate pools in antimetabolite-treated HeLa cells. J Biol Chem. 1982 Aug 25;257(16):9300–9304. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynolf K., Eliasson R., Reichard P. Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell. 1978 Mar;13(3):573–580. doi: 10.1016/0092-8674(78)90330-6. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J. Differential inhibitors of DNA polymerases alpha and delta. Biochem Biophys Res Commun. 1985 Oct 30;132(2):628–634. doi: 10.1016/0006-291x(85)91179-9. [DOI] [PubMed] [Google Scholar]
- Caradonna S. J., Adamkiewicz D. M. Purification and properties of the deoxyuridine triphosphate nucleotidohydrolase enzyme derived from HeLa S3 cells. Comparison to a distinct dUTP nucleotidohydrolase induced in herpes simplex virus-infected HeLa S3 cells. J Biol Chem. 1984 May 10;259(9):5459–5464. [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison B. L., Leighton T., Rabinowitz J. C. Purification of Bacillus subtilis RNA polymerase with heparin-agarose. In vitro transcription of phi 29 DNA. J Biol Chem. 1979 Sep 25;254(18):9220–9226. [PubMed] [Google Scholar]
- Domena J. D., Mosbaugh D. W. Purification of nuclear and mitochondrial uracil-DNA glycosylase from rat liver. Identification of two distinct subcellular forms. Biochemistry. 1985 Dec 3;24(25):7320–7328. doi: 10.1021/bi00346a045. [DOI] [PubMed] [Google Scholar]
- Dresler S. L., Frattini M. G. DNA replication and UV-induced DNA repair synthesis in human fibroblasts are much less sensitive than DNA polymerase alpha to inhibition by butylphenyl-deoxyguanosine triphosphate. Nucleic Acids Res. 1986 Sep 11;14(17):7093–7102. doi: 10.1093/nar/14.17.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S. L., Kimbro K. S. 2',3'-Dideoxythymidine 5'-triphosphate inhibition of DNA replication and ultraviolet-induced DNA repair synthesis in human cells: evidence for involvement of DNA polymerase delta. Biochemistry. 1987 May 19;26(10):2664–2668. doi: 10.1021/bi00384a002. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Kunkel T. A., Seal G., Loeb L. A. Distinctive properties of mammalian DNA polymerases. Biochim Biophys Acta. 1979 Feb 27;561(2):369–382. doi: 10.1016/0005-2787(79)90145-x. [DOI] [PubMed] [Google Scholar]
- Geider K. DNA synthesis in nucleotide-permeable Escherichia coli cells. The effects of nucleotide analogues on DNA synthesis. Eur J Biochem. 1972 Jun 9;27(3):554–563. doi: 10.1111/j.1432-1033.1972.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. The effect of methotrexate on levels of dUTP in animal cells. J Biol Chem. 1980 Nov 25;255(22):10630–10637. [PubMed] [Google Scholar]
- Grafstrom R. H., Tseng B. Y., Goulian M. The incorporation of uracil into animal cell DNA in vitro. Cell. 1978 Sep;15(1):131–140. doi: 10.1016/0092-8674(78)90089-2. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Dickey L., Goulian M. DNA fragmentation and cytotoxicity from increased cellular deoxyuridylate. Biochemistry. 1986 Jun 3;25(11):3225–3230. doi: 10.1021/bi00359a022. [DOI] [PubMed] [Google Scholar]
- Lansman R. A., Clayton D. A. Selective nicking of mammalian mitochondrial DNA in vivo: photosensitization by incorporation of 5-bromodeoxyuridine. J Mol Biol. 1975 Dec 25;99(4):761–776. doi: 10.1016/s0022-2836(75)80183-5. [DOI] [PubMed] [Google Scholar]
- Leblanc J. P., Laval J. Comparison at the molecular level of uracil-DNA glycosylases from different origins. Biochimie. 1982 Aug-Sep;64(8-9):735–738. doi: 10.1016/s0300-9084(82)80120-x. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Mahagaokar S., Orengo A., Rao P. N. The turnover of deoxyuridine triphosphate during the HeLa cell cycle. Exp Cell Res. 1980 Jan;125(1):86–94. doi: 10.1016/0014-4827(80)90192-5. [DOI] [PubMed] [Google Scholar]
- Meyer R. R., Simpson M. V. Deoxyribonucleic acid biosynthesis in mitochondria. Purification and general properties of rat liver mitochondrial deoxyribonucleic acid polymerase. J Biol Chem. 1970 Jul 10;245(13):3426–3435. [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Characterization of the action of Escherichia coli DNA polymerase I at incisions produced by repair endodeoxyribonucleases. J Biol Chem. 1982 Jan 10;257(1):575–583. [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Excision repair and DNA synthesis with a combination of HeLa DNA polymerase beta and DNase V. J Biol Chem. 1983 Jan 10;258(1):108–118. [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. Purification, properties, and use as a reagent to reduce uracil incorporation into DNA. J Biol Chem. 1978 May 10;253(9):3305–3312. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. HeLa cell R-deoxyribonucleic acid polymerases. Separation and characterization of two enzymatic activities. J Biol Chem. 1974 Sep 25;249(18):5809–5815. [PubMed] [Google Scholar]
- Stalker D. M., Mosbaugh D. W., Meyer R. R. Novikoff hepatoma deoxyribonucleic acid polymerase. Purification and properties of a homogeneous beta polymerase. Biochemistry. 1976 Jul 13;15(14):3114–3121. doi: 10.1021/bi00659a027. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Chien J., Lehman I. R., Duncan B. K., Warner H. R. Uracil incorporation: a source of pulse-labeled DNA fragments in the replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1978 Jan;75(1):233–237. doi: 10.1073/pnas.75.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Lehman I. R. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J Mol Biol. 1977 Dec 5;117(2):293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Demple B. F., Deutsch W. A., Kane C. M., Linn S. Apurinic/apyrimidinic endonucleases in repair of pyrimidine dimers and other lesions in DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4602–4606. doi: 10.1073/pnas.77.8.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wist E., Unhjem O., Krokan H. Accumulation of small fragments of DNA in isolated HeLa cell nuclei due to transient incorporation of dUMP. Biochim Biophys Acta. 1978 Sep 27;520(2):253–270. doi: 10.1016/0005-2787(78)90225-3. [DOI] [PubMed] [Google Scholar]


