Abstract
HSPA1A is a serum and intracellular heat shock protein with antiapoptotic and antithrombotic properties. The present study examines the hypothesis that a decrease in the synthesis of this protein in relation to certain polymorphisms of the regulatory region of the HSPA1A gene can define a vascular disease risk phenotype. A randomly selected population was studied and stratified into groups according to the degree of vascular risk. After applying the Task Force Chart to 452 people, the subjects were divided into three groups: group 0 (no vascular risk factor or risk < 5%), n = 239; group 1 (moderate (10–20%) risk, with no clinical cardiovascular disease), n = 161; and group 2 (overt atherosclerosis), n = 52. Serum and intragranulocytic HSPA1A was quantified, and direct Sanger sequencing was performed in all subjects. An analysis was made of the association of two single nucleotide polymorphisms (db rs1008438 −110A/C and db rs1043618 +190 G/C) with circulating and intragranulocytic HSPA1A and the risk of atherosclerosis. The atherosclerotic subjects showed significantly lower circulating HSPA1A levels than the other groups, regardless of the genotype. The patients with CC genotype for both polymorphisms showed significantly lower intragranulocytic HSPA1A levels than the other genotypes. Serum HSPA1A concentrations could be proposed as a biomarker of cardiovascular disease. CC homozygosis for polymorphisms db rs1008438 and db rs1043618 is associated with a decrease in the intragranulocytic production of HSPA1A. Given the antiatherogenic functions of intracellular HSPA1A, the −110A and +190 G alleles could constitute potential genetic biomarkers of a less severe clinical phenotype for the risk of developing atherosclerosis.
Keywords: Heat shock protein HSPA1A, Single nucleotide polymorphisms, Leukocytes, Vascular risk, Atherosclerosis
Introduction
Atherosclerosis is the main cause of cardiovascular diseases and the number one killer of the population in the developed countries (Domanski et al. 2011). Atherosclerosis is widely recognized as a chronic inflammatory disease that involves innate and adaptive immune responses (Jara et al. 2006). The antigens regarded as candidates for triggering the immune response in atherosclerosis include the heat shock proteins (HSPs) (Mehta et al. 2005).
Within the superfamiliy of HSPs, HSPA1A (Kampinga et al. 2009) is the main molecular chaperone. It has antiinflammatory and antiapoptotic functions (Yenari et al. 2005) and has been one of the proteins most widely studied in relation to the pathogenesis and development of atherosclerosis. Although for years HSPs were regarded as exclusively intracellular molecules, they are now also known to be circulating molecules, and the presence of HSPA1A has been demonstrated in the serum of normal individuals (Pockley et al. 1998; Jin et al. 2004). HSPA1A may be released into the extracellular compartment by several mechanisms: transport with proteins that possess transmembrane domains to the cell surface and its secretion into the extracellular environment, and their entry into secretary lysosomal endosomes and their cell surface and release of the contents into the extracellular space or by inhibiting phospholipase C activity (Asea 2008). Once in the extracellular space, stress proteins have immunobiological properties, both proinflammatory and antiinflammatory roles in physiological and pathophysiological situations (Pockley and Multhoff 2008). Taking into consideration that HSPA1A has antiinflammatory properties due to inhibition of the expression of proinflammatory cytokines (Luo et al. 2008) and of proinflammatory transcription factors such as the nuclear factor kappa-B (NF-κB) by blocking target activation and binding (Shimizu et al. 2002; Stice and Knowlton 2008), low levels of intracellular and circulating HSPA1A would promote a proinflammatory state and increase the vulnerability of the arterial wall to the damaging action of vascular risk factors involved in endothelial dysfunction—the first stage in the development of the atherosclerotic plaque.
Two single intronless genes (HSPA1A and HSPA1B) encode copies of the 641-amino acid protein Hsp70 which only differ by two amino acids (Kampinga et al. 2009). HSPA1A is expressed constitutively at low level and after thermal shock, and HSPA1B is expressed only after heat induction (Cascino et al. 1993). They are located in tandem along a ~15-kb region of chromosome 6p23.1 in the class III region of the major histocompatibility complex: The coding regions of HSPA1A and HSPA1B are identical except for six single base substitutions. The promoter and 3′ untranslated region (UTR) of the genes have considerable sequence differences, probably due to their distinct regulation of translation and transcription (Smith et al. 2007). Since single-nucleotide polymorphisms (SNPs) of HSPA1A have been described in relation to susceptibility towards certain diseases, such as essential hypertension (Li et al. 2009) or ischemic stroke (Liu et al. 2007), we hypothesized that possible polymorphisms of the regulatory region of HSPA1A could affect HSPA1A protein synthesis, determining diminished, normal, or increased HSPA1A-producing phenotypes. The hypo-producing phenotype would entail a greater risk of developing atherosclerotic disease. The present study was made to identify SNPs in the regulatory region of HSPA1A gene and evaluate whether any of them could affect HSPA1A synthesis in a randomly selected population which was later stratified into different groups according to the degree of vascular risk.
Methods
Study population and design
The description of the study population and the research design were done in our previous study (Dulin et al. 2010). Briefly, this was an observational, cross-sectional epidemiological study on the incidence of classical and candidate vascular risk factors, carried out from January 2004 to June 2009. Inclusion criteria include randomly selected voluntary subjects of both sexes aged 40–60 years, employees of Gregorio Marañón University General Hospital (HGUGM) in Madrid, Spain, who signed the informed consent. The study was approved by the Clinical Research Ethics Committee of Gregorio Marañón University General Hospital. All participants provided a clinical history and answered an epidemiological survey including age, personal and family medical history, smoking (number of cigarettes per year and smoking duration; if former smokers, number of years elapsed since smoking cessation), alcohol intake (if yes, grams of alcohol daily), treatments, and occurrence or presence of disease of atherosclerotic etiology. Blood pressure (BP) of all participants was measured with an automated BP recording device after an individual had been sitting quietly for 5 min. Blood was taken for the appropriate laboratory measurements. Exclusion criteria included pregnancy or breastfeeding, any systemic infection in the past 3 months, current oncological disease or radiotherapy/chemotherapy, autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, sarcoidosis), endocrine disorders (except for diabetes), liver disease, renal failure, glomerulonephritis, congenital heart disorder, oncohematological disease, or allergic disorders.
The Department of Epidemiology and Preventive Medicine of the Hospital calculated the sample size. In order to determine whether there is a correlation between serum and intragranulocytic levels of HSPA1A and the development or presence of atherosclerotic disease and to estimate the size of the study cohort, the following premises were assumed: (1) an estimated incidence of the disease of 12.5% in the group with a high serum and intracellular concentration of HSPA1A and (2) this population has half the risk of the population with a low concentration of developing the disease (RR 0.5); error protection: alpha 0.05 (bilateral), beta: 0.20.
Assessment of vascular risk and classification of subjects
The calculation of the absolute risk of developing cardiovascular disease (CVD) in a given period of time, usually 10 years, is estimated based on the presence of prior coronary heart disease and the joint assessment of risk factors present (Grundy et al. 1999, Grundy 2007). For this purpose, there are several tables based on follow-up of the Framingham study population (D’Agostino et al. 2001). One of the most widely used is the Task Force Coronary Risk Chart (Wood et al. 1998). According to the coronary risk chart criteria, the studied population was classified into three groups: group 0 (G0): subjects with no vascular risk factor, or risk < 5%; group 1 (G1): subjects with moderate vascular risk (10–20%) who do not have clinical CVD; and group 2 (G2): subjects with clinically established atherosclerotic disease; all of them suffered one of the specific atherosclerotic cardiovascular disease events: coronary heart disease, cerebrovascular disease, peripheral vascular disease, or heart failure (D’Agostino et al. 2008; Graham et al. 2007).
Laboratory tests
Venous blood was drawn after a 12-h fasting period and centrifuged, and serum samples were frozen at −70°C for subsequent testing. Total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, and glucose were quantified with a Hitachi Modular Analytic SVA autoanalyzer (Roche Diagnostics S.L., Barcelona, Spain).
Isolation of polymorphonuclear neutrophil leukocytes (PMNs)
Twenty milliliters of blood was drawn using Vacuette™ tubes (Greiner bio-one A-4550 Kremsmünster, Austria). Five milliliters of whole blood was layered onto 3.5 mL of Polymorphprep™ (Nycomed, Oslo, Norway) in 10-mL polystyrene centrifuge tubes (Nunc, Roskilde, Denmark) and centrifuged (Heraeus, Labofuge 400 e, Kendro Laboratory Products, Newton, USA) at 450–500 g for 30 min at room temperature. In the gradient that formed, erythrocytes were located at the bottom. Above, two bands were seen; the lower one, corresponding to the PMNs, was gently removed in 10 mL of phosphate-buffered and centrifuged at 700 g for 5 min, and the supernatant was discarded. An aliquot of 10 × 106 cells was stored as dry pellet for DNA extraction, and the precipitate containing the PMNs was resuspended in 100 μL of RIPA buffer supplemented with protease and phosphatase inhibitors. Cell lysates where homogenized by 40 passages in a Kontes homogenizer and centrifuged at 12,000 g for 15 min at 4°C. The supernatant was collected and stored at −70°C until further processing. Protein concentration was measured by the Lowry microassay method, using the DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA).
Serum and intracellular HSPA1A
HSPA1A was quantified in diluted serum 1:5 using the Hsp70 ELISA kit (EKS-715, Assay-Designs-Stressgen, Ann Arbor, Michigan, USA) in accordance with the instructions of the manufacturer. The EKS-715 test recognizes recombinant and native plasma and serum HSPA1A, and there is no cross-reactivity with constitutive Hsp70 (HSPA8) or human Hsp60 (HSPD1) (Multhoff and Hightower, 2011). Results were expressed in nanograms per milliliter. The working range (linearity) for HSPA1A was 0.34–6.25 ng/ml, with a sensitivity of 0.30 ng/mL. The inter-assay and intraassay coefficients of variation of the assays were < 10%.
The concentration of intracellular HSPA1A was quantified using the Hsp70 ELISA kit for cell lysates (EKS-700B, Assay-Designs-Stressgen, Ann Arbor, Michigan, USA) in identical 20-μg aliquots of protein from the cell lysate. The results were expressed as ng HSPA1A/μg total protein. The assay is certified for use in detection of HSPA1A in human cell lysates and tissue (Multhoff and Hightower, 2011).
Sequencing of the regulatory region of the HSPA1A gene
A 1,053-bp sequence comprising the promoter, the 5′UTR region, and part of the HSPA1A gene coding region was amplified by PCR in three fragments from the genomic DNA isolated from the PMNs of each of the study subjects. Given the length of the fragment, the latter was sequenced in the form of three sub-fragments, followed by coupling of the sub-sequences obtained. To this effect, use was made of primers specifically designed from the published sequence (GenBank, NT_007592, gi: 224514668): For the first sub-fragment, the sequences of the forward and reverse primers were 5′-ACTGCACAACCGGGGTCCCC-3′ and 5′-AGTCGTCACGGAGACCCGCC-3′, respectively. For the second sub-fragment, they were 5′- GGCGGCACTCTGGCCTCTGA-3′ and 5′-GGCCATGCCGGTTCCCTGCT-3′, and for the third, the used primers were 5′ GGCGGCACTCTGGCCTCTGA-3′ and 5′-GCCAGTGCTTCATGTCCGAC-3′.
The optimized PCR reaction mixture in a final volume of 100 μl consisted of 50 μl of PCR Master MIX (Promega, WI, USA), 50 pM of each forward and reverse primer, and 400 ng of the DNA template. All reactions were performed using an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA). The cycle conditions were 95°C for 2 min, followed by 40 cycles of 94°C for 30 s (denaturation), 62°C for 30 s (annealing), and 72°C for 45 s (extension). The PCR products were purified using the High Pure PCR Product Purification Kit (Roche, WI, USA), separated by 0.6% agarose gel electrophoresis and visualized under UV light using ethidium bromide staining. Direct Sanger sequencing was carried out on an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Inc., Foster City, CA, USA). Sequence alignment and polymorphism identification were performed using Chromas 2.3 software (Technelysium Pty Ltd., Tewantin QLD, USA). A search of the region sequenced was performed in dbSNP (www.ncbi.nlm.nih.gov) to record all polymorphisms reported in the region.
Statistical analysis
Normal distribution of data was checked by the Kolmogorov–Smirnov normality test. Data with a normal distribution were compared by the Student’s t-test or one-way ANOVA with Bonferroni correction. Those with unequal variance or without a normal distribution were analyzed using a nonparametric Mann–Whitney rank sum test or Kruskal–Wallis test, depending on the amount of variables. The χ2 test or Fisher’s exact test was used to assess the goodness-of-fit between the observed allele frequencies and the expected counterparts by Hardy–Weinberg equilibrium and to evaluate the differences in allele distributions between groups. The associations between variants and atherosclerosis risk were estimated by odds ratios (ORs) and 95% confidence intervals (CIs) using binary logistic regression analysis. A p-value of less than 0.05 was considered statistically significant, while p-values of 0.05 and 0.1 were considered indicative of a trend. All data analyses were carried out using the SPSS version 12.0 statistical package.
Results
General characteristics of the subjects
The general characteristics of subjects were described in our previous study (Dulin et al. 2010). Briefly, a total of 452 subjects who met the inclusion criteria and gave their informed consent were included, of which 234 were females (51.8%) with a mean age of 49.95 ± 6.89 (x ± SD) years and 218 were males (48.2%) with a mean age of 48.86 ± 7.27 years. After applying the Task Force Coronary Risk Chart (Wood et al.1998), the subjects participating in the study were distributed in the next way: group 0 (G0): subjects with no vascular risk factor or a risk lower than 5%, n = 239; group 1 (G1): subjects with moderate vascular risk (10–20%) without clinical atherosclerosis, n = 161; and group 2 (G2): subjects with overt atherosclerotic disease (coronary heart disease, cerebrovascular disease, peripheral vascular disease or heart failure) n = 52 (D’Agostino et al. 2008; Graham et al.2007).
SNPs identification in the regulatory region of HSPA1A
Sequencing of the regulatory region of the HSPA1A gene revealed 7 SNPs. Referring to the gene transcription start site as +1, SNP −110A/C (dbSNP accession number rs1008438) in the core promoter region and +190 G/C (dbSNP accession number rs1043618) in the 5′UTR region were the most frequent SNPs, and both of them were found in 445 of the 451 cases studied (98.66%) (Table 1). That was the reason why the analyses were made only for polymorhism −110 A/C.
Table 1.
HSPA1A polymorphism location and frequencies
| Positiona | dbSNP accesionb | Nucleotide position in gene | Genotype | Frequency | % |
|---|---|---|---|---|---|
| −399 G/A | NA | 31722967 | GG | 450 | 99.13 |
| GA | 1 | 0.86 | |||
| AA | 0 | 0 | |||
| −365 G/A | rs4713489 | 31722943 | GG | 422 | 93.4 |
| GA | 27 | 6.0 | |||
| AA | 2 | 0.4 | |||
| −110 A/C | rs1008438 | 31723208 | AA | 147 | 32.5 |
| AC | 211 | 46.7 | |||
| CC | 93 | 20.6 | |||
| +27 G/A | rs34397183 | 31712343 | GG | 449 | 99.6 |
| GA | 2 | 0.4 | |||
| AA | 0 | 0 | |||
| +58 C/A | rs33934112 | 31723376 | CC | 439 | 97.3 |
| CA | 12 | 2.7 | |||
| AA | 0 | 0 | |||
| +120 T/C | rs11557922 | 31723437 | TT | 412 | 91.4 |
| TC | 39 | 8.6 | |||
| CC | 0 | 0 | |||
| +190 G/C | rs1043618 | 31723507 | GG | 153 | 33.8 |
| GC | 211 | 46.7 | |||
| CC | 87 | 19.2 |
In bold, the most frequent SNPs
NA not available
aPosition relative to the ATG start codon, where the first base in the start codon is +1
bIdentified in dbSNP (www.ncbi.nlm.nih.gov)
Allelic frequencies
There were no significant differences in allele frequencies of −110A/C and +190 G/C (data not shown).
Table 2 shows the allele and genotype frequencies both in global population and in each group without significant differences among them. The genotype frequencies for the studied polymorphisms respected the Hardy–Weinberg equilibrium (p > 0.05) in all groups. Similar genotype/allele distributions were noted by gender stratification (data not shown).
Table 2.
Allele and genotype frequencies of rs1008438 in general population and in groups according to vascular risk degree
| rs1008438 -110 A/C | Allele frequency | Allele distribution | Genotype | ||||
|---|---|---|---|---|---|---|---|
| p(A) | q(C) | A (AA + AC) | C (AC + CC) | AA | AC | CC | |
| General population (N = 451) | 0.56 | 0.44 | 505 | 397 | 147(32.6%) | 211(46.8%) | 93(20.6%) |
| Group 0 (N = 238) | 0.54 | 0.46 | 255 | 221 | 74 (31.1%) | 107 (45%) | 57(23.9%) |
| Group 1 (N = 161) | 0.61 | 0.39 | 194 | 126 | 59 (36.6%) | 76 (47.2%) | 26(16.2%) |
| Group 2 (N = 52) | 0.54 | 0.46 | 56 | 48 | 14 (26.9%) | 8(53.8%) | 10(19.2%) |
The presence of genotype CC in polymorphism −110 A/C was associated to a relative risk of 1.75 (95%CI 0.98–3.11, p = 0.058) of undergo vascular risk factors and a relative risk of 1.56 (95% CI 0.92–2.65, p = 0.066) of presenting vascular risk factors and clinically established atherosclerotic disease (Table 3).
Table 3.
Association between variants and atherosclerosis risk
| Genotype | G0 (n = 238) | G1 (n = 161) | OR | 95% CI | P |
|---|---|---|---|---|---|
| AA | 1.75 | 0.98–3.11 | 0.058 | ||
| AC | 1.58 | 0.90–2.70 | 0.114 | ||
| CC | 1 | ||||
| G0 (n = 238) | G2 (n = 52) | OR | 95% CI | ||
| AA | 1.08 | 0.45–2.60 | 0.867 | ||
| AC | 1.49 | 0.68-3.29 | 0.321 | ||
| CC | 1 | ||||
| G1 (n = 161) | G2 (n = 52) | OR | 95% CI | ||
| AA | 0.62 | 0.24–1.57 | 0.311 | ||
| AC | 0.92 | 0.41–2.24 | 0.921 | ||
| CC | 1 | ||||
| G0 (n = 232) | G1 + G2 (n = 213) | OR [95% CI] | |||
| AA | 1.56 | 0.92–2.65 | 0.066 | ||
| AC | 1.54 | 0.94–2.53 | 0.088 | ||
| CC | 1 |
OR (odds ratio), 95% CI (95% confidence interval), and P value between groups were calculated using binary logistic regression analysis
Serum HSPA1A
Serum levels of HSPA1A were detectable in 444 subjects, representing 98.23% of the cases, and the data were not normally distributed. The median concentration of circulating HSPA1A was 1.21 ng/mL (range 0.00–29.55 ng/mL). No statistically significant differences were found in the concentration of circulating HSPA1A according to the genotype in either the general population or on stratifying by risk groups. The concentration of circulating HSPA1A was significantly lower in the group with established atherosclerotic disease than in the other two groups—both combined (p = 0.028, Kruskal–Wallis test) and on considering group 0 (Mann–Whitney test p = 0.010) and group 1 (Mann–Whitney test p = 0.010) separately, regardless of the genotype involved (Table 4).
Table 4.
Circulating HSPA1A levels (ng/mL) according to genotype in the general population and stratified by risk groups
| AA | Pa | AC | Pa | CC | Pb | Pa | Pc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General population (N = 146) | General population (N = 207) | General population (N = 90) | |||||||||||
| 1.18 (0.00–7.54) | 1.21 (0.05–29.55) | 1.27 (0.28–5.23) | NS | ||||||||||
| G0 (N = 74) | G1 (N = 58) | G2 (N = 14) | G0 (N = 104) | G1 (N = 75) | G2 (N = 28) | G0 (N = 56) | G1 (N = 25) | G2 (N = 9) | |||||
| 1.24 (0.19–4.22) | 1.25 (0.0–7.54) | 1.02 (0.31–3.48) | NS (P = 0.21) | 1.21 (0.23–29.55) | 1.46 (0.10–6.76) | 1.14 (0.05–3.90) | NS (P = 0.4) | 1.42 (0.33–3.15) | 1.15 (0.69–5.23) | 1.09 (0.28–1.76) | NS (P = 0.13) | P = 0.028 | |
Data are expressed as median (range)
aP: differences according to genotypes, the grouping variable being vascular risk (Kruskal–Wallis test, P = NS)
bP: differences according to genotype in the general population (Kruskal–Wallis test, P = NS)
cP: differences according to vascular risk group. Serum HSPA1A concentration was significantly lower in the group of patients with established atherosclerosis disease than in the other two groups regardless of the genotype involved (Kruskal–Wallis test)
Intragranulocytic HSPA1A
In all the studied subjects we were able to quantify the intracellular HSPA1A concentration—no significant differences being observed in relation to the degree of vascular risk (Table 5). In contrast, the subjects with CC minor allele frequency (MAF) showed significantly lower intragranulocytic HSPA1A levels than the other genotypes (p < 0.001, Kruskal–Wallis test). On analyzing according to the vascular risk group, the decrease in intracellular HSPA1A concentration persisted in the CC genotype carriers regardless of the vascular risk group they belong to (p < 0.05, Kruskal–Wallis test) (Table 6).
Table 5.
Dose–response relationship of variations on serum and intracellular HSPA1A levels in different groups of vascular risk
| Group 0 | Group 1 | Group 2 | P | ||||
|---|---|---|---|---|---|---|---|
| Serum HSPA1A (ng/mL) | N = 235 | 1.26 (0.19–29.55) | N = 158 | 1.22 (0.00–7.54) | N = 51 | 1.09 (0.05–3.90) | 0.028* |
| Intracellular HSPA1A (ng/μg protein) | N = 239 | 38.96 (1.56–107.01) | N = 161 | 40.45 (5.61–160.75) | N = 52 | 38.62 (7.92–88.41) | NS** |
*P < 0.05 group 2 vs group 0 and group 1 combined (Kruskal–Wallis test) and separately: group 2 vs group 0 (Mann–Whitney test p = 0.010) and group 2 vs group 1 (Mann–Whitney test, p = 0.010); **P = NS (Kruskal–Wallis test, p = 0,587)
Table 6.
Intragranulocytic HSPA1A levels (ng/μg protein) according to genotype in the general population and stratified by risk groups
| AA | Pa | AC | Pa | CC | Pb | Pa | Pc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General population (N = 147) | General population (N = 211) | General population (N = 93) | |||||||||||
| 42.63 (4.64–124.75) | 42.10 (2.62–160.75) | 29.74 (1.56–92.27) | P < 0.001 | ||||||||||
| G0 (N = 74) | G1 (N = 59) | G2 (N = 14) | G0 (N = 107) | G1 (N = 76) | G2 (N = 28) | G0 (N = 58) | G1 (N = 26) | G2 (N = 10) | |||||
| 44.85 (4.64–97.08) | 45.77 (5.61–124.75) | 39.91 (16.16–87.53) | NS (P = 0.457) | 41.56 (2.62–107.01) | 43.15 (5.67–160.75) | 43.85 (12.48–81.45) | NS (P = 0.866) | 30.25 (1.56–90.83) | 26.48 (7.07–92.27) | 28.03 (7.92–88.41) | NS (P = 0.873) | P = 0.024 | |
Data are expressed as median (range)
aP: differences according to vascular risk group, the grouping variable being genotype (Kruskal–Wallis test, P = NS)
bP: differences according to genotype in the general population (Kruskal–Wallis test). Subjects with CC genotype showed significantly lower intragranulocytic HSPA1A concentrations than the other genotypes
cP: differences according to genotypes between groups (Kruskal–Wallis test). The decrease in intracellular HSPA1A concentration persisted in the CC genotype carriers, regardless of the vascular risk group they belong to
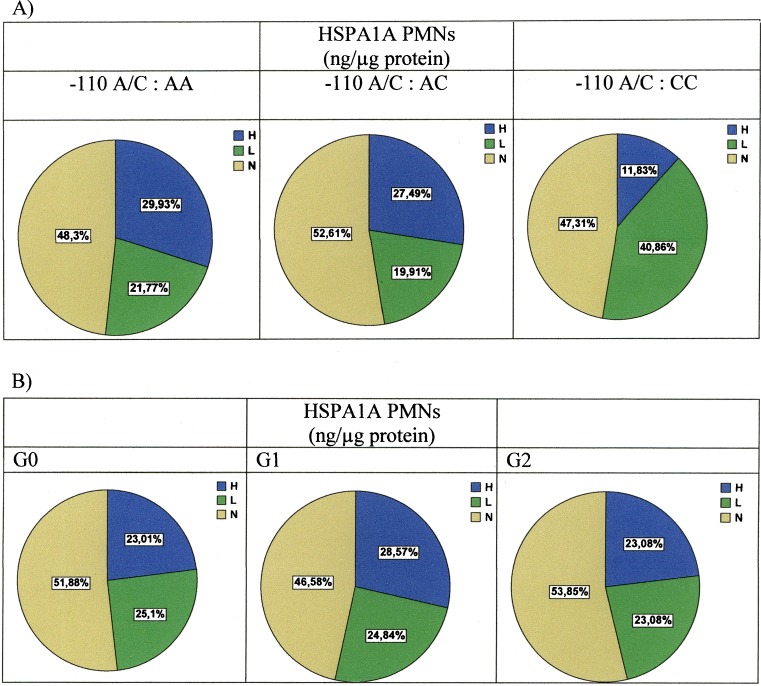
On the basis of the hypothesis that subjects who are hyper-producers of intracellular HSPA1A present a lesser vascular risk phenotype, we classified the individuals according to percentiles P25, P50, and P75. The individuals with intragranulocytic HSPA1A concentrations below P25 (<25.54 ng/μg protein) would be hypo-producers; those between P25 and P75 (P50 = 39.73 ng/μg protein) would be normal producers, and those with concentrations above P75 (> 52.94 ng/μg protein) would be classified as hyper-producers, with the purpose of analyzing them in relation to the −110A/C SNP of the promoter.
As can be seen in Fig. 1a, the greatest percentage of intragranulocytic HSPA1A hypo-producers corresponded to the subjects with the CC genotype. The assignment to a given vascular risk group revealed no significant differences in the percentages of hyper-, normal, or hypo-producing individuals (Fig. 1b).
Fig. 1.
a Percentage of intragranulocytic HSPA1A producers according to −110A/C genotype. H hyper-producers (subjects with intragranulocytic HSPA1A concentrations above P75), N normal producers (subjects with intragranulocytic HSPA1A concentrations between P25 and P75), and L hypo-producers (subjects with intragranulocytic HSPA1A concentrations below P25). CC genotype is a risk factor for low production of intracellular HSPA1A versus genotypes AA and CC (p < 0.05, χ2 test). b Percentage of intragranulocytic HSPA1A according to vascular risk group. No differences are observed between the groups in terms of percentage HSPA1A hypo-, normal, or hyper-producers
The CC genotype entails a risk of being a low intracytoplasmic HSPA1A producer with respect to normal production of 1.673 (95% CI 1.191–2.351, p = 0.004) versus the AC genotype and of 1.419 (95% CI 1.034–1.947, p = 0.033) versus the AA genotype. The CC genotype entails a risk of being a low producer with respect to high production of 2.980 (95% CI 1.654–5.638, p < 0.001) versus genotype AC and of 2.714 (95% CI 1.534–4.802, p < 0.001) versus the AA genotype
Discussion
Different studies have related low concentrations of HSPA1A at both intracellular level and in serum to the development of atherosclerosis (Pockley et al. 2009). Such low levels could be either a cause or a consequence of the disease (Hooper and Hooper, 2004; Pockley et al. 2004). The present study was designed to evaluate whether low levels of HSPA1A synthesis could be related to SNPs of the noncoding region of the HSPA1A gene, affecting its regulation at transcriptional level. To this effect, we sequenced the regulatory region comprising the promoter and the 5′UTR region, using DNA samples from peripheral blood PMNs. These cells were chosen because they are the most common type of leukocyte found in the circulation and due to their important role in the initiation and development of atherosclerosis. A close relationship has been demonstrated between a high PMN count and increased cardiovascular risk (Horne et al. 2005), and patients with unstable angina or acute myocardial infarction moreover present activated PMNs that release free oxygen radicals and inflammatory mediators which in turn interact with the endothelial cells—triggering the development of atherosclerosis (Baetta and Corsini 2010). Furthermore, circulating PMNs have been shown to be activated in a number of conditions associated with a high risk of developing atherosclerosis, such as hypertension (Ramasamy et al. 2010), type 2 diabetes (Hand et al. 2007), or hyperlipidemia (Mazor et al. 2008). The causal role of PMNs in atherogenesis and their implication in plaque vulnerability (Leclercq et al. 2007) are presently widely accepted.
Sequencing of the regulatory region of HSPA1A detected 7 SNPs, of which six are referenced in the dbSNP database. Despite the practically identical sequence of the coding region of HSPA1A and HSPA1B, the putative 3′ and 5′ promoters are dissimilar, with sequence variations (Cascino et al. 1993)—this allows us to affirm that the sequenced regulatory region is exclusively that corresponding to HSPA1A. The two most frequent SNPs, −110A/C db rs1008438 and +190 G/C db rs1043618, were found together in 98.66% of the cases; as a result, the analyses were made on a unitary basis for −110A/C. The genotype frequencies for both polymorphisms were in Hardy–Weinberg equilibrium in each group. Our population was homogeneous with respect to gender, age, ethnicity, and geographic regions, thus minimizing the possible biases related to population. Moreover, the sample is representative of the Spanish population because volunteers come from different social classes and have different living standards and educational levels. In our study, patients suffering from some type of atherosclerotic disease showed significantly lower levels of circulating HSPA1A, regardless of the −110A/C and +190 G/C genotype. This agrees with prior studies (Pockley et al. 2003, Zhu et al. 2003, Martin-Ventura et al. 2007, Armutcu et al. 2008), and it has been discussed in our previous publication (Dulin et al. 2010). In contrast to these works, Zhang et al (2010) reported that high extracellular Hsp70 levels were associated with the increase and the severity of acute coronary syndrome (ACS), and recently, it has been reported that serum Hsp70 levels correlate with the severity of atherosclerosis in patients with peripheral artery disease of the lower extremities and carotid artery disease (Krepuska et al. 2011). The discrepancy between the results of the different studies could have been due, as the authors propose, to differences in population characteristics, the difference of samples (serum versus plasma), and different kinds of ELISA used. Anyway, further investigations to elucidate the potential mechanisms which could explain this controversy must be conducted. Although we could not show significant differences in the concentrations of circulating HSPA1A according to the genotype, median HSPA1A levels were higher in control subjects carrying the −110A/C CC genotype and +190 G/C CC genotype, which agrees with the studies of Zhang et al. (2011), in their case with statistically significant differences that could be explained because of the greater sample size in their study. They reported that this significant association was found only for −190 C/C genotype and not for −110A/C. In our study, both polymorphisms were associated in the same way to changes in serum and intragranulocytic HSPA1A concentrations; these differences could be due to ethnic differences between the studied populations, which could explain the differences in the frequencies of the genotype −110A/C.
The intragranulocytic HSPA1A concentrations showed no significant differences between the disease-free population and the subjects with moderate vascular risk or established atherosclerotic disease. However, the individuals presenting the CC genotype for SNPs −110 and +190 had significantly lower intracellular HSPA1A levels. Intracellular HSPA1A is a cytoprotective molecule with chaperone function. An increase in intracellular HSPA1A concentration exerts antiinflammatory effects through negative regulation of the transcription of proinflammatory cytokines, decreased levels of TNF-α induced inter-cellular adhesion molecule (ICAM-1), and the in vivo inhibition of leukocyte adhesion to the endothelium (Pockley et al. 2009). Furthermore, HSPA1A possesses antithrombotic properties since it prevents the endothelial cell apoptosis (Schmitt et al. 2007) that contributes to atherosclerosis and atherothrombotic events (Bielecka-Dabrowa et al. 2009). Based on our results, homozygosity for −110A/C and +190 G/C SNPs entails a risk of presenting moderate vascular risk or declared atherosclerosis—probably as a result of diminished intracellular HSPA1A synthesis and a consequent partial loss of its antiinflammatory and antithrombotic properties. Previous studies evaluating the activity of the promoter of HSPA1A gene through luciferase reporter assays have demonstrated that polymorphism rs1008438 modifies the activity of the promoter, the −110A allele showing significantly higher promoter activity than the −110 C allele (Qi et al. 2009). He et al. (2009) demonstrated that the +190 C allele is also associated to a reduction in relative luciferase activity (RLA), from which it can be concluded that the +190 C allele in the 5′UTR region of the HSPA1A gene reduces the activity of the promoter and probably diminishes HSPA1A protein synthesis through translation efficiency or post-transcriptional regulation. This reduction in RLA is reinforced when the −110 C and +190 C alleles coexist. As has been demonstrated in our study, this would cause genotypes AA-110 and GG + 190 to be associated with an increased production of HSPA1A versus the CC genotypes. The AA genotype would represent the healthiest genotype from the perspective of atherosclerotic disease, as a result of its association to high intracellular HSPA1A levels—affording a strong protection against the initiation and development of the disease. In coincidence with other authors (Li et al. 2009; Qi et al, 2009; He et al. 2009, Buraczynska et al. 2009, Zhang et al. 2011), this leads us to postulate the −110 A and +190 G alleles as possible genetic markers of less severe clinical phenotypes for the risk of developing atherosclerosis.
An observation of note in our study has been the distinct behaviors of serum HSPA1A and intragranulocytic HSPA1A in relation to the genotype of the polymorphisms of the regulatory region. The observed reduction in circulating HSPA1A levels in atherosclerotic subjects would not be due to a reduction in production of the protein secondary to the existence of these polymorphisms. Whether the level of HSPA1A is a biomarker of the progression of the disease or has an active role in the endothelial lesion must be elucidated in future prospective studies. In contrast, the polymorphisms of the HSPA1A regulatory region effectively have been related to a decrease in the production of intragranulocytic HSPA1A—representing a possible genetic marker of risk for the development of atherosclerosis. Thus, it may be postulated that intracellular HSPA1A and serum HSPA1A would play different roles in relation to the etiopathogenesis and evolution of atherosclerosis, as has already been suggested by other authors (Cai et al. 2010, Zhang et al. 2011) and has been shown in the response to other stressors (Magalhaes et al. 2010).
The present study should be interpreted within the context of its limitations. Firstly, it involves a cross-sectional design, which inevitability entails the limitations of studies of this kind, i.e., the inability to prove the existence of a causal relationship. Secondly, our study involves a relatively small sample size in each group, though each group yielded significant results. On the other hand, we sub-divided research subjects into control subjects without disease or vascular risk < 5% and subjects with moderate (10–20%) vascular risk at 10 years, but are unable to rule out the possibility that some of them may have presented sub-clinical atherosclerosis, since carotid artery ultrasound was not performed to measure intima-media thickness, and coronary angiography was not used to exclude possible silent myocardial infarction. Lastly, we only genotyped the regulatory region of HSPA1A and did not examine polymorphisms in other genes of the Hsp70 family which might be associated with atherosclerosis.
In summary, it has been demonstrated that the −110CC and +190CC genotypes of the HSPA1A gene entail a tendency towards increased vascular risk or risk of established atherosclerosis due to their significant correlation to diminished intragranulocytic HSPA1A synthesis and thus a reduction in its antiinflammatory and antithrombotic properties.
Acknowledgements
The authors are grateful to Dr. Jose Luis Bellón-Cano for valuable statistical consultations and to Mrs. Maria Jesús Sánchez for her invaluable help in sample processing. This study was supported by grant 03/1308 from Fondo de Investigaciones Sanitarias and by Fundación Mutua Madrileña.
Disclosures
None
References
- Armutcu F, Ataymen M, Atmaca H, Gurel A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med. 2008;46:785–790. doi: 10.1515/CCLM.2008.166. [DOI] [PubMed] [Google Scholar]
- Asea A (2008) Hsp70: a chaperokine. In: John Wiley & Sons Ltd (eds). The biology of extracellular molecular chaperones. Novartis Found Symp. West Sussex, UK. ;291:173-9; discussion 179-83, 221-224. [DOI] [PubMed]
- Baetta R, Corsini A. Role of polymorphonuclear neutrophils in atherosclerosis: current state and future perspectives. Atherosclerosis. 2010;210:1–13. doi: 10.1016/j.atherosclerosis.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Bielecka-Dabrowa A, Barylski M, Mikhailidis DP, Rysz J, Banach M. HSP 70 and atherosclerosis—protector or activator? Expert Opin Ther Targets. 2009;13:307–317. doi: 10.1517/14728220902725149. [DOI] [PubMed] [Google Scholar]
- Buraczynska M, Swatowski A, Buraczynska K, Dragan M, Ksiazek A. Heat-shock protein gene polymorphisms and the risk of nephropathy in patients with type 2 diabetes. Clin Sci (Lond) 2009;116:81–86. doi: 10.1042/CS20070411. [DOI] [PubMed] [Google Scholar]
- Cai WF, Zhang XW, Yan HM, Ma YG, Wang XX, Yan J, Xin BM, Lv XX, Wang QQ, Wang ZY, Yang HZ, Hu ZW. Intracellular or extracellular heat shock protein 70 differentially regulates cardiac remodelling in pressure overload mice. Cardiovasc Res. 2010;88:140–149. doi: 10.1093/cvr/cvq182. [DOI] [PubMed] [Google Scholar]
- Cascino I, D’Alfonso S, Cappello N, Giordano M, Pugliese A, Awdeh Z, Alper CA, Richiardi PM. Gametic association of HSP70-1 promoter region alleles and their inclusion in extended HLA haplotypes. Tissue Antigens. 1993;42:62–66. doi: 10.1111/j.1399-0039.1993.tb02238.x. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Sr, Gruñid SM, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Domanski M, Lloyd-Jones D, Fuster V, Grundy S (2011) Can we dramatically reduce the incidence of coronary heart disease? Rev Cardiol Nov 1. doi:10.1038/nrcardio.2011.158 [DOI] [PubMed]
- Dulin E, García-Barreno P, Guisasola MC. Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones. 2010;15:929–937. doi: 10.1007/s12192-010-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Suppl 2 et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14:E1–E40. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Cardiovascular and metabolic risk factors: how we can improve outcomes in the high-risk patients? Am J Med. 2007;120:S3–S9. doi: 10.1016/j.amjmed.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007;76:44–50. doi: 10.1016/j.diabres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- He M, Guo H, Yang X, Zhang X, Zhou L, Cheng L, Zeng H, Hu FB, Tanguay RM, Wu T. Functional SNPs in HSPA1A gene predict risk of coronary heart disease. PLoS One. 2009;4(3):e4851. doi: 10.1371/journal.pone.0004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Is low-heat shock protein 70 a primary or a second event in the development of atherosclerosis? Hypertension. 2004;43:e18. doi: 10.1161/01.HYP.0000118134.56524.b1. [DOI] [PubMed] [Google Scholar]
- Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein JB. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Jara LJ, Medina G, Vera Lastra O, Amigo MC. Accelerated atherosclerosis, immune response and autoimmune rheumatic diseases. Autoimmun Rev. 2006;5:195–201. doi: 10.1016/j.autrev.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jin X, Wang R, Xiao C, et al. Serum and lymphocyte levels of heat shock protein 70 in aging: a study in the normal Chinese population. Cell Stress Chaperones. 2004;9:69–75. doi: 10.1379/477.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guideline for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepuska M, Szeberin Z, Sótonyi P, Sarkadi H, Fehérvári M, Apor A, Rimely E, Prohászka Z, Acsády G. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones. 2011;16:257–265. doi: 10.1007/s12192-010-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq A, Houard X, Philippe M, Ollivier V, Sebbag U, Meilhac O, Michel JB. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc. 2007;82:1420–1429. doi: 10.1189/jlb.1106671. [DOI] [PubMed] [Google Scholar]
- Li JX, Tang BP, Sun HP, Feng M, Cheng ZH, Niu WQ. Interacting contribution of the five polymorphisms in three genes of Hsp70 family to essential hypertension in Uygur ethnicity. Cell Stress Chaperones. 2009;14:355–362. doi: 10.1007/s12192-008-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cheng J, Peng J, Han S, Yu L, Nie S. Effects of polymorphisms of heat shock protein 70 gene on ischemic stroke, and interaction with smoking in China. Clin Chim Acta. 2007;384:64–68. doi: 10.1016/j.cca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Luo X, Zuo X, Zhang B, Song L, Wei X, Zhou Y, Xiao X. Release of heat shock protein 70 and the effects of extracellular heat shock protein 70 on the production of IL-10 in fibroblast-like synoviocytes. Cell Stress Chaperones. 2008;13:365–373. doi: 10.1007/s12192-008-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães Fde C, Amorim FT, Passos RL, Fonseca MA, Oliveira KP, Lima MR, Guimarães JB, Ferreira-Júnior JB, Martini AR, Lima NR, Soares DD, Oliveira EM, Rodrigues LO. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB. Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atheroesclerosis. 2007;194:334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Mazor R, Shurtz-Swirski R, Farah R, Kristal B, Shapiro G, Dorlechter F, Cohen-Mazor M, Meilin E, Tamara S, Sela S. Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients. Atherosclerosis. 2008;197:937–943. doi: 10.1016/j.atherosclerosis.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Mehta TA, Greenman J, Ettelaie C, Venkatasubramaniam A, Chetter IC, McCollum PT. Heat shock proteins in vascular disease. A review. Eur J Vasc Endovasc Surg. 2005;29:395–402. doi: 10.1016/j.ejvs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Hightower LE. Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones. 2011;16:251–255. doi: 10.1007/s12192-010-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Multhoff G. Cell stress proteins in extracellular fluids: friend or foe? Novartis Found Symp. 2008;291:86–95. doi: 10.1002/9780470754030.ch7. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faide U, Frostegård J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegård J. Response: is low-heat shock protein 70 a primary or a secondary event in the development of atherosclerosis? Hypertension. 2004;43:e18–e19. doi: 10.1161/01.HYP.0000118134.56524.b1. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones. 2009;14:545–553. doi: 10.1007/s12192-009-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Niu WQ, Zhu TC, Liu JL, Dong WY, Xu Y, Ding SQ, Cui CB, Pan YJ, Yu GS, Zhou WY, Qiu CC. Genetic interaction of Hsp70 family genes polymorphisms with high-altitude pulmonary edema among Chinese railway constructors at altitudes exceeding 4000 meters. Clin Chim Acta. 2009;405:17–22. doi: 10.1016/j.cca.2009.03.056. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Maqbool M, Mohamed AL, Noah RM. Elevated neutrophil respiratory burst activity in essential hypertensive patients. Cell Immunol. 2010;263:230–234. doi: 10.1016/j.cellimm.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tamamori-Adachi M, Arai H, Tabuchi N, Tanaka H, Sunamori M. Lipopolysaccharide pretreatment attenuates myocardial infarct size: a possible mechanism involving heat shock protein 70-inhibitory kappaB alpha complex and attenuation of nuclear factor kappaB. J Thorac Cardiovasc Surg. 2002;124:933–941. doi: 10.1067/mtc.2002.122305. [DOI] [PubMed] [Google Scholar]
- Smith RS, Meyers DA, Peters SP, Moore WC, Wenzel SA, Bleecker ER, Hawkins GA. Sequence analysis of HSPA1A and HSPA1B in a multi-ethnic study population. DNA Seq. 2007;18:47–53. doi: 10.1080/10425170601060707. [DOI] [PubMed] [Google Scholar]
- Stice JP, Knowlton AA. Estrogen, NFkappaB, and the heat shock response. Mol Med. 2008;14:517–527. doi: 10.2119/2008-00026.Stice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D, Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of cornary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and others Societies on Coronary Prevention. Atherosclerosis. 1998;140:199–270. doi: 10.1016/S0021-9150(98)90209-X. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu Z, Zhou L, et al. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15:675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tanguay RM, He M, Deng Q, Miao X, Zhou L, Wu T. Variants of HSPA1A in combination with plasma Hsp70 and Anti-Hsp70 antibody levels associated with higher risk of acute coronary syndrome. Cardiology. 2011;119:57–64. doi: 10.1159/000329917. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055–1059. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]