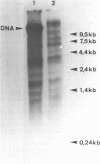
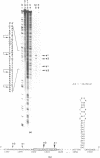
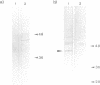
Abstract
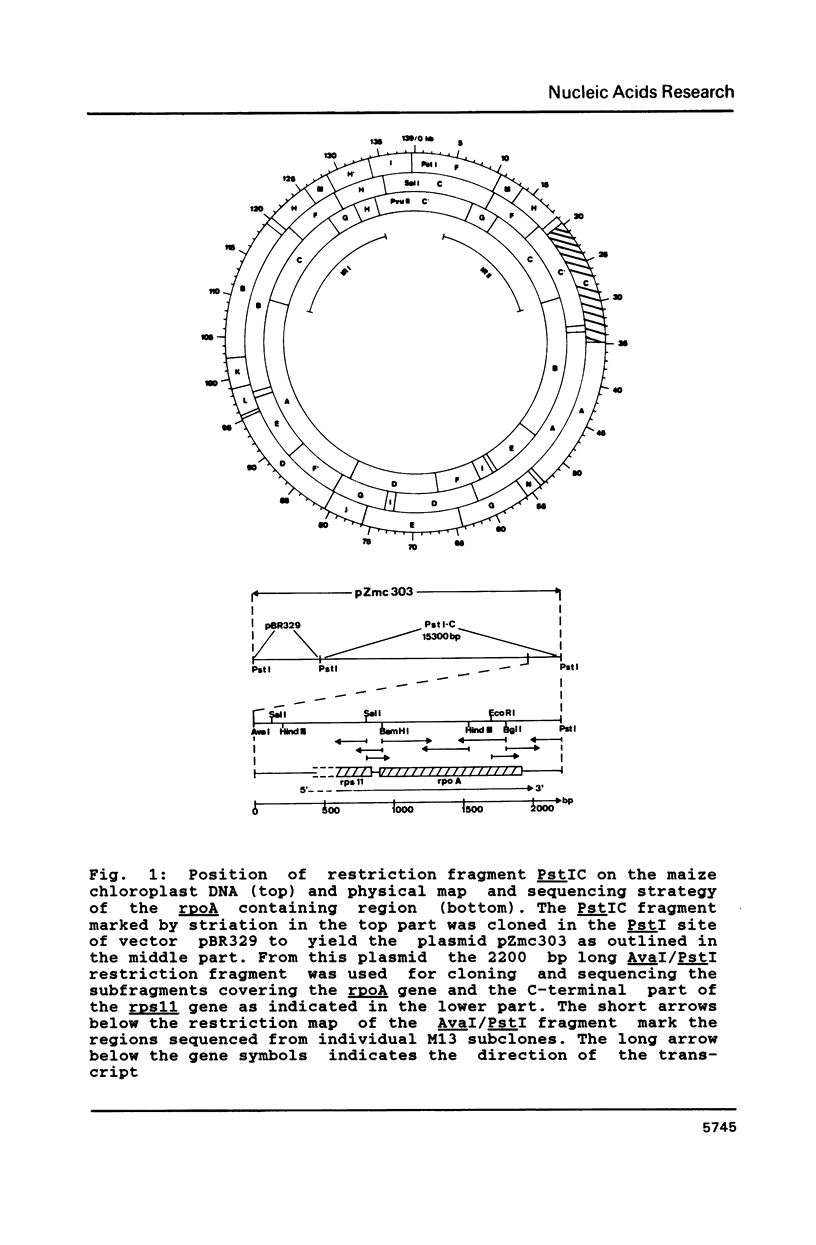
The rpoA gene coding for the alpha-subunit of DNA-dependent RNA polymerase located on the DNA of Zea mays chloroplasts has been characterized with respect to its position on the chloroplast genome and its nucleotide sequence. The amino acid sequence derived for a 39 Kd polypeptide shows strong homology with sequences derived from the rpoA genes of other chloroplast species and with the amino acid sequence of the alpha-subunit from E. coli RNA polymerase. Transcripts of the rpoA gene were identified by Northern hybridization and characterized by S1 mapping using total RNA isolated from maize chloroplasts. Antibodies raised against a synthetic C-terminal heptapeptide show cross reactivity with a 39 Kd polypeptide contained in the stroma fraction of maize chloroplasts. It is concluded that the rpoA gene is a functional gene and that therefore, at least the alpha-subunit of plastidic RNA polymerase, is expressed in chloroplasts.
Full text
PDF






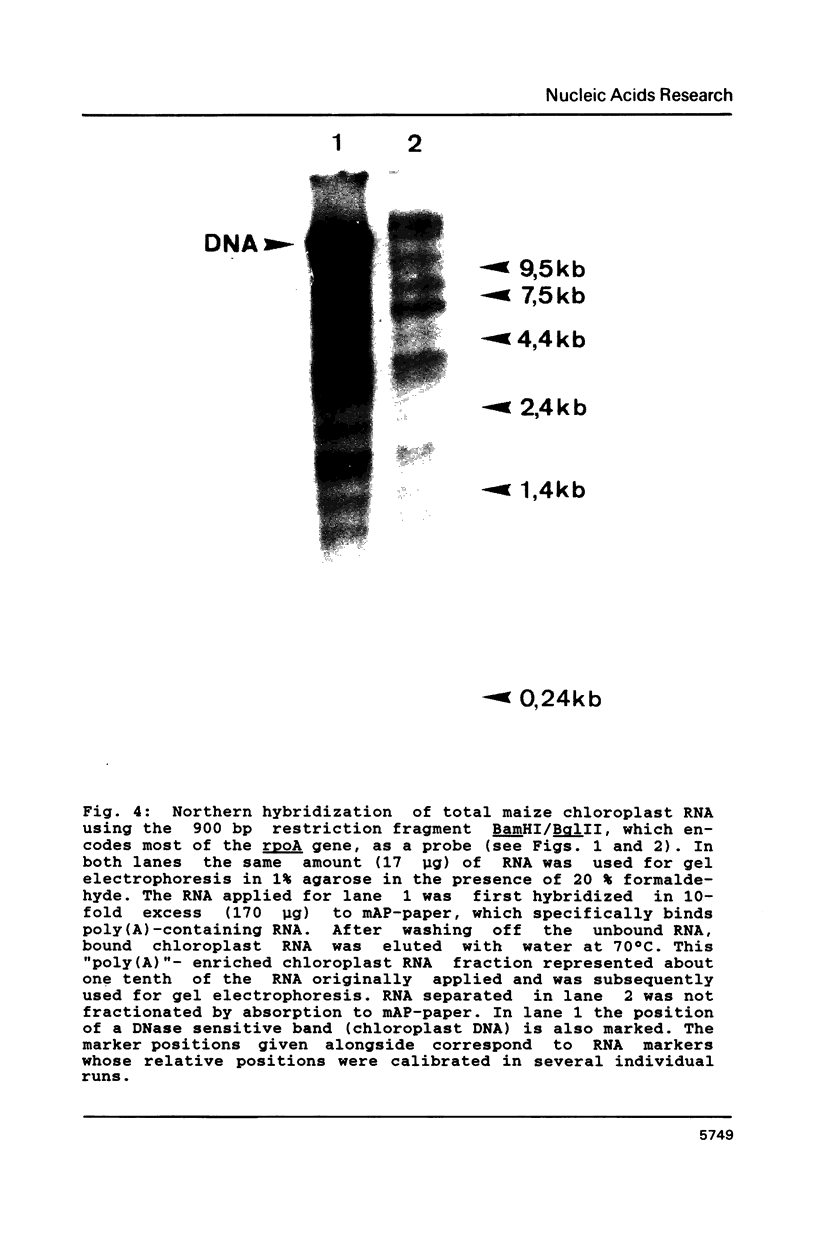

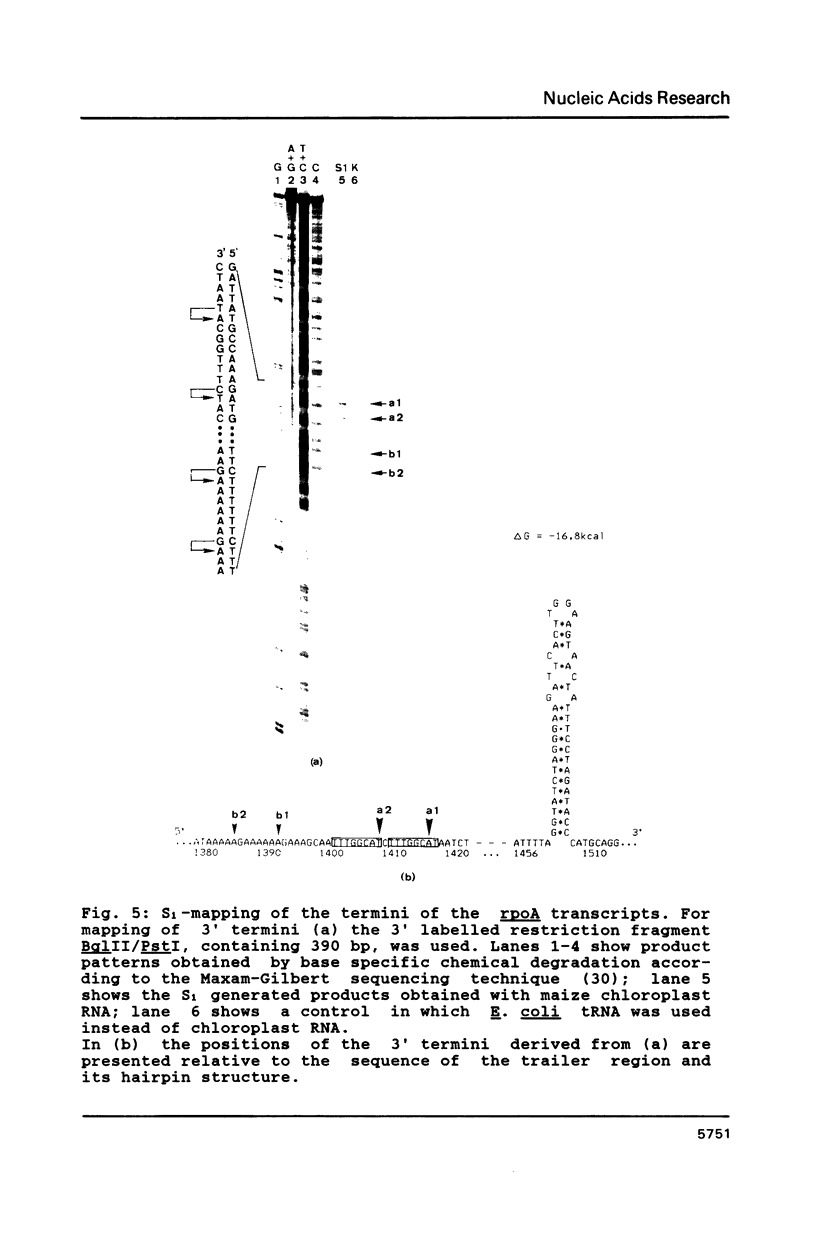



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard J., Bourque D. P., Hildebrand M., Zaitlin D. In vitro expression of chloroplast genes in lysates of higher plant chloroplasts. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3983–3987. doi: 10.1073/pnas.82.12.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Lescure A. M., Mache R. Transcription of the chloroplast DNA: a review. Biochimie. 1986 Jul-Aug;68(7-8):981–990. doi: 10.1016/s0300-9084(86)80041-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Johnson M. S., Doolittle R. F. Aligning amino acid sequences: comparison of commonly used methods. J Mol Evol. 1984;21(2):112–125. doi: 10.1007/BF02100085. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Narita J. O., Greenberg B. M., Prescott D. M., Hallick R. B. Selective in vitro transcription of chloroplast genes. J Cell Biochem. 1983;22(1):31–46. doi: 10.1002/jcb.240220104. [DOI] [PubMed] [Google Scholar]
- Lerbs S., Bräutigam E., Parthier B. Polypeptides of DNA-dependent RNA polymerase of spinach chloroplasts: characterization by antibody-linked polymerase assay and determination of sites of synthesis. EMBO J. 1985 Jul;4(7):1661–1666. doi: 10.1002/j.1460-2075.1985.tb03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Attardi G., Trovato D., Strong D. D., Doolittle R. F. Antibodies against synthetic peptides reveal that the unidentified reading frame A6L, overlapping the ATPase 6 gene, is expressed in human mitochondria. Cell. 1983 Apr;32(4):1269–1277. doi: 10.1016/0092-8674(83)90308-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Hayward R. S. Nucleotide sequence of the rpoA-rplQ DNA of Escherichia coli: a second regulatory binding site for protein S4? Nucleic Acids Res. 1984 Jul 25;12(14):5813–5821. doi: 10.1093/nar/12.14.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Reiss T., Link G. Characterization of transcriptionally active DNA-protein complexes from chloroplasts and etioplasts of mustard (Sinapis alba L.). Eur J Biochem. 1985 Apr 15;148(2):207–212. doi: 10.1111/j.1432-1033.1985.tb08826.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben-Müller G., Hallick R. B., Alt J., Westhoff P., Herrmann R. G. Spinach plastid genes coding for initiation factor IF-1, ribosomal protein S11 and RNA polymerase alpha-subunit. Nucleic Acids Res. 1986 Jan 24;14(2):1029–1044. doi: 10.1093/nar/14.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Gruissem W. Control of plastid gene expression: 3' inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987 Dec 24;51(6):1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Strittmatter G., Gozdzicka-Jozefiak A., Kössel H. Identification of an rRNA operon promoter from Zea mays chloroplasts which excludes the proximal tRNAValGAC from the primary transcript. EMBO J. 1985 Mar;4(3):599–604. doi: 10.1002/j.1460-2075.1985.tb03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Obokata J, Chunwongse J, Shinozaki K, Sugiura M. Rapid splicing and stepwise processing of a transcript from the psbB operon in tobacco chloroplasts: determination of the intron sites in petB and petD. Mol Gen Genet. 1987 Oct;209(3):427–431. doi: 10.1007/BF00331145. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G. Production and use of antibodies against synthetic peptides. J Immunol Methods. 1986 Apr 17;88(2):149–161. doi: 10.1016/0022-1759(86)90001-3. [DOI] [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988 Feb 1;171(3):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]