Abstract
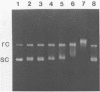
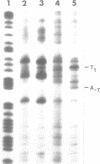
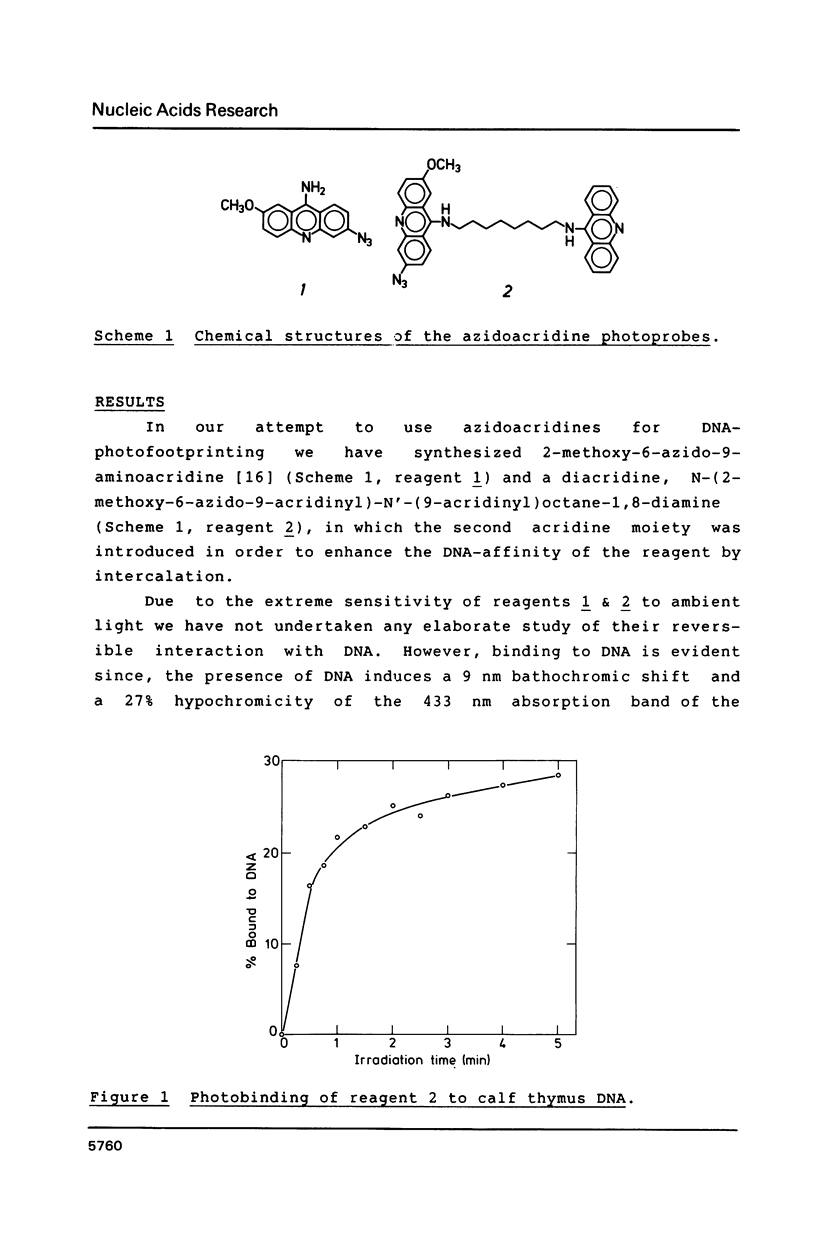
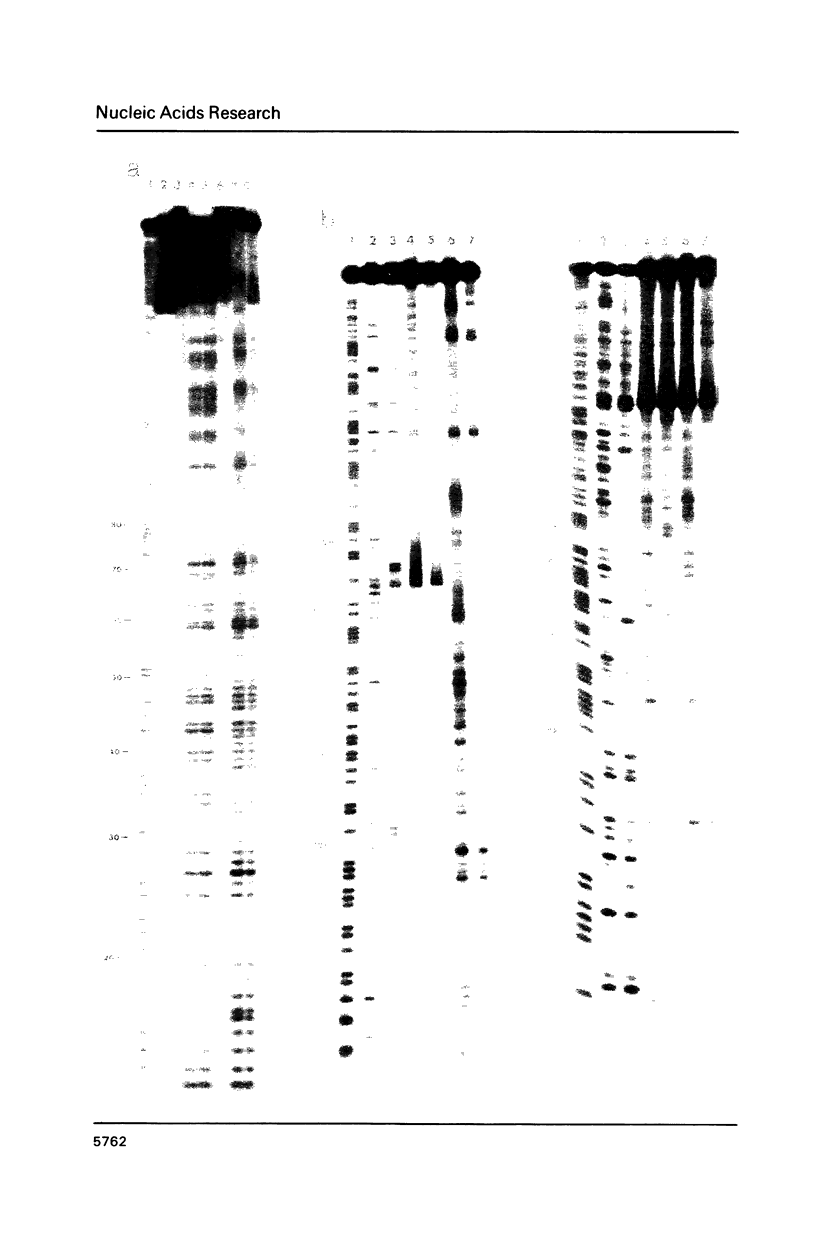
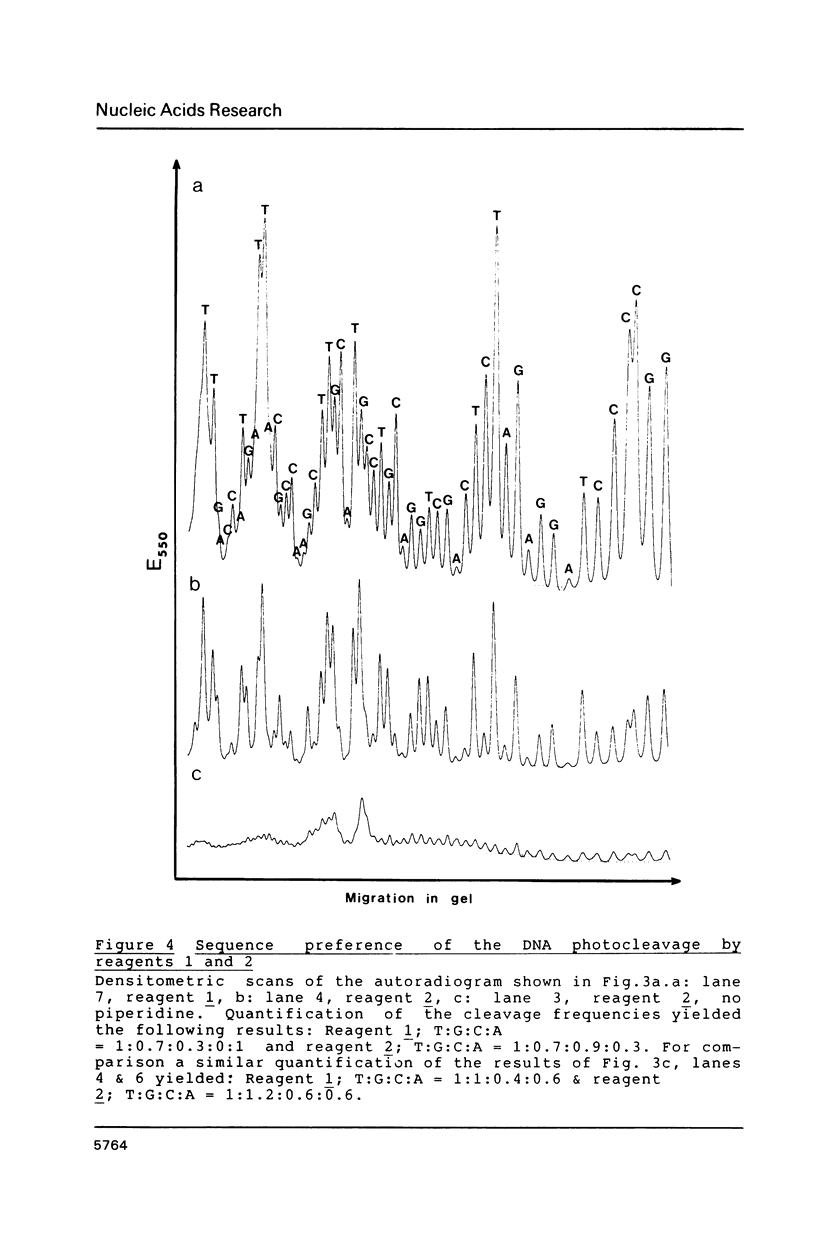
The long-wavelength ultraviolet (lambda approximately 420 nm) radiation induced reaction between 6-azido-2-methoxy-9-acridinylamines and supercoiled plasmid DNA results in single strand scissions and formation of covalent adducts (ratio approximately 1:10). By treating azidoacridine-photomodified DNA with piperidine at 90 degrees C, additional strand scissions are observed in a complex sequence dependent manner with an overall preference for T greater than or equal to G greater than C much greater than A. The resulting DNA fragments migrate as 5'-phosphates in polyacrylamide gels. Photofootprinting of the binding site of RNA-polymerase on promoter DNA is demonstrated with an azido-9-acridinylamino-octamethylene-9-aminoacridine. Similar experiments using 9-amino-6-azido-2-methoxyacridine indicate that this reagent recognizes changes in the DNA conformation induced by RNA polymerase binding, in relation to open complex formation.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. M., Wang J. C. Use of light for footprinting DNA in vivo. Nature. 1984 Jun 21;309(5970):682–687. doi: 10.1038/309682a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Elsner H., Buchardt O., Møller J., Nielsen P. E. Photochemical crosslinking of protein and DNA in chromatin. II. Synthesis and application of psoralen-cystamine-arylazido photocrosslinking reagents. Anal Biochem. 1985 Sep;149(2):575–581. doi: 10.1016/0003-2697(85)90616-5. [DOI] [PubMed] [Google Scholar]
- Fahr A., Hucho F. A stopped-flow apparatus for photoaffinity labeling studies in the milliseconds time range. Application in investigations of the nicotinic acetylcholine receptor. J Neurosci Methods. 1986 Mar;16(1):29–38. doi: 10.1016/0165-0270(86)90005-1. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., von Sprecken R. S., Yielding K. L., Yielding L. W. Ethidium binding sites on plasmid DNA determined by photoaffinity labeling. J Biol Chem. 1984 Sep 10;259(17):11090–11097. [PubMed] [Google Scholar]
- Kopacz S. J., Mueller D. M., Lee C. P. Photoaffinity labelling of submitochondrial membranes with the 3-azido analogue of 9-amino-3-chloro-7-methoxyacridine. Biochim Biophys Acta. 1985 May 3;807(2):177–188. doi: 10.1016/0005-2728(85)90121-5. [DOI] [PubMed] [Google Scholar]
- Kuhlmann K. F., Charbeneau N. J., Mosher C. W. Synthesis, DNA-binding and biological activity of a double intercalating analog of ethidium bromide. Nucleic Acids Res. 1978 Jul;5(7):2629–2641. doi: 10.1093/nar/5.7.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Hansen J. B., Buchardt O. Photochemical cross-linking of protein and DNA in chromatin. Synthesis and application of a photosensitive cleavable derivative of 9-aminoacridine with two photoprobes connected through a disulphide-containing linker. Biochem J. 1984 Oct 15;223(2):519–526. doi: 10.1042/bj2230519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Jeppesen C., Egholm M., Buchardt O. Adenosine-guanosine preferential photocleavage of DNA by azido-benzoyl- and diazocyclopenta-dienylcarbonyloxy derivatives of 9-aminoacridine. Nucleic Acids Res. 1988 May 11;16(9):3877–3888. doi: 10.1093/nar/16.9.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E. Photoaffinity labeling of chromatin. Synthesis and properties of arylazido derivatives of 9-aminoacridine: potential photolabels for chromatin studies. Eur J Biochem. 1982 Feb;122(2):283–289. doi: 10.1111/j.1432-1033.1982.tb05878.x. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Zhen W. P., Henriksen U., Buchardt O. Sequence-influenced interactions of oligoacridines with DNA detected by retarded gel electrophoretic migrations. Biochemistry. 1988 Jan 12;27(1):67–73. doi: 10.1021/bi00401a012. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Gilbert W. DNA-binding site of lac repressor probed by dimethylsulfate methylation of lac operator. J Mol Biol. 1979 Aug 25;132(4):709–728. doi: 10.1016/0022-2836(79)90384-x. [DOI] [PubMed] [Google Scholar]
- Sage E., Moustacchi E. Sequence context effects on 8-methoxypsoralen photobinding to defined DNA fragments. Biochemistry. 1987 Jun 16;26(12):3307–3314. doi: 10.1021/bi00386a010. [DOI] [PubMed] [Google Scholar]
- Selleck S. B., Majors J. Photofootprinting in vivo detects transcription-dependent changes in yeast TATA boxes. Nature. 1987 Jan 8;325(7000):173–177. doi: 10.1038/325173a0. [DOI] [PubMed] [Google Scholar]
- Singer P., Wu C. W. Promoter search by Escherichia coli RNA polymerase on a circular DNA template. J Biol Chem. 1987 Oct 15;262(29):14178–14189. [PubMed] [Google Scholar]
- Spassky A., Kirkegaard K., Buc H. Changes in the DNA structure of the lac UV5 promoter during formation of an open complex with Escherichia coli RNA polymerase. Biochemistry. 1985 May 21;24(11):2723–2731. doi: 10.1021/bi00332a019. [DOI] [PubMed] [Google Scholar]
- Spassky A., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion. Conformational analysis and footprinting of the lac operon. Biochemistry. 1985 Dec 31;24(27):8050–8056. doi: 10.1021/bi00348a032. [DOI] [PubMed] [Google Scholar]
- Spassky A. Visualization of the movement of the Escherichia coli RNA polymerase along the lac UV5 promoter during the initiation of the transcription. J Mol Biol. 1986 Mar 5;188(1):99–103. doi: 10.1016/0022-2836(86)90484-5. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Aiba H., Schümperli D. The structure of tandem regulatory regions in the deo operon of Escherichia coli K12. EMBO J. 1982;1(3):317–322. doi: 10.1002/j.1460-2075.1982.tb01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Svenningsen B. A., Munch-Petersen A., Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitro system. Mol Gen Genet. 1978 Feb 16;159(2):191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Dervan P. B. Methidiumpropyl-EDTA.Fe(II) and DNase I footprinting report different small molecule binding site sizes on DNA. Nucleic Acids Res. 1983 Aug 25;11(16):5555–5567. doi: 10.1093/nar/11.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin L. P. Polyfunctional DNA intercalating agents. Med Res Rev. 1986 Jul-Sep;6(3):275–340. doi: 10.1002/med.2610060303. [DOI] [PubMed] [Google Scholar]
- Zhen W. P., Buchardt O., Nielsen H., Nielsen P. E. Site specificity of psoralen-DNA interstrand cross-linking determined by nuclease Bal31 digestion. Biochemistry. 1986 Oct 21;25(21):6598–6603. doi: 10.1021/bi00369a039. [DOI] [PubMed] [Google Scholar]