Abstract

Many peptides with the potential of therapeutic action for brain disorders are not in clinical use because they are unable to cross the blood−brain barrier (BBB) following peripheral administration. We have developed two potential strategies for the delivery of peptides to the brain and demonstrated their feasibility with enkephalins. In the first approach, designated induced reversible lipophilization, Leu/Met Enkephalins were converted to 9-fluorenylmethoxycarbonyl (Fmoc) derived lipophilic prodrug analogues, which undergo slow, spontaneous hydrolysis under physiological conditions, generating the native agonists. In contrast to Enkephalin, Fmoc-Met-Enkephalin was found to facilitate an analgesic effect following intraperitoneal administration in mice. Fmoc-Leu-Enkephalin was not analgesic. In the second approach, Enkephalin was linked to BBB transport vectors through an Fmoc based linker spacer, forming conjugates that slowly release Enkephalin under physiological conditions. A pronounced antinociceptive response was thus obtained following intraperitoneal administration of either cationized-human serum albumin-Fmoc-Enkephalin or polyethylene glycol5-Fmoc-Enkephalin. Derivatives of Enkephalin covalently linked to the same BBB-transport vectors through a stable (nonreversible) chemical bond were not analgesic. In summary, we have demonstrated that lipophilicity can be conferred to hydrophilic peptides to a degree permitting the permeation of the BBB by passive diffusion, without the drawback of agonist inactivation, which is often caused by irreversible derivatization. Similarly, in the second strategy, the conjugation to BBB-permeable vectors overcomes the obstacle of peptide inactivation by releasing the active form in the central nervous system.
Keywords: Neuropeptide, enkephalin, blood−brain barrier, prodrug, reversible-lypophilization, reversible-conjugation
A neuropeptide agonist should possess several characteristic features following peripheral administration to allow its arrival at the CNS and facilitate there a viable therapeutic effect. The peptide should resist proteolysis in the circulatory system; be sufficiently hydrophobic to transverse the BBB by passive diffusion; be immune to degradation by cerebrovasculature peptidases; and preserve its active, native form, capable of binding to brain receptor sites upon arrival. Intensive attempts carried out along these lines included peptide lipidization, substitution of l- by d-amino acids to enhance stability against degradation, glycosylation, and the covalent linkage of such nontransportable neuropeptides to proteins that penetrate the BBB either by receptor or absorptive-mediated transendocytosis. All attempts made so far in this direction showed rather limited success. Derivatization of peptides to increase stability and lipophilicity often ended up with biologically inactive derivatives, and the same applies to neuropeptides covalently linked to BBB-transport vectors. The probability of releasing the neuropeptide from the inactive-conjugate by enzymatic cleavage in its active form in brain tissue is quite low (reviewed in refs (1−5)).
In previous studies, we have found that derivatization of peptides with 9-fluorenylmethoxycarbonyl-N-hydroxysuccinimide (Fmoc-OSu) as a reagent turns them into Fmoc-hydrophobic, inactive prodrug derivatives. Upon incubation at physiological conditions, the Fmoc moieties undergo spontaneous chemical hydrolysis, i.e., β-elimination, generating the nonmodified active parent peptides at a slow rate and in a homogeneous fashion with t1/2 values in the range of 30−70 h (6−10). On the basis of these findings, we have synthesized a heterobifunctional agent, 9-hydroxymethyl-2-(amino-3-maleimidopropionate)-fluorene-N-hydroxysuccinimide (MAL-Fmoc-OSu) which allows the covalent linkage of SH-containing compounds to the amino-side chains of peptides and proteins through a hydrolizable chemical bond. Peptides are released from such conjugates in their native active form with t1/2 values in the range of 20−70 h (11−14).
Taken together, the findings that lipophilicity as well as conjugation to transport vectors can promote BBB permeability and that conjugation through MAL-Fmoc-OSu produces a prodrug that releases the active peptide prompted us to investigate whether Fmoc-based peptide derivatization can turn a nontransportable neuropeptide into a lipophilic-prodrug derivative capable of reaching the brain, following peripheral administration. Similarly, we investigated whether the reversible linkage between such neuropeptides and a BBB transport vector through the Fmoc-type spacer can produce a prodrug that penetrates the BBB and confers a therapeutic effect upon the release of the active peptide in the brain.
Our in vivo experimental system was based on Leu-Enkephalin or Met-Enkaphalin. This BBB-nontransportable peptide facilitates an analgesic effect only if centrally administered to rodents (15). A variety of conjugates and derivatives, all linked to the single α-amino side chain of Enkephalin, were prepared and tested for their analgesic effect in mice following intraperitoneal administration.
As the covalent introduction of bulky groups to the α-amino side chain of Enkephalin inactivates this neuropeptide (16), we hypothesized that only those conjugates or derivatives capable of penetrating the BBB and releasing the peptide in brain tissue in its active form should be considered analgesic following peripheral administration.
Results
Reversible Lipophilization: Preparation of Fmoc Derivatives of Enkephalins
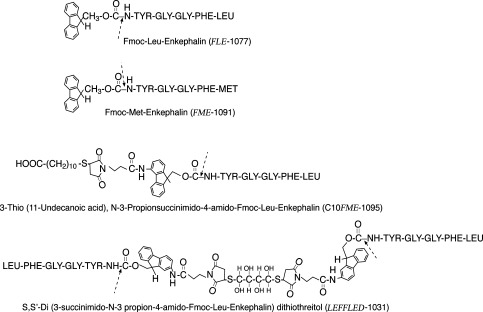
Figure 1 shows the structure of the Fmoc-Enkephalin analogues synthesized in the present study. The list includes Fmoc-Leu-Enkephalin (FLE-1077), Fmoc-Met-Enkephalin (FME-1091), 3-Thio (11-Undecanoic acid), N-3-propiosuccinimido-4-amido-Fmoc-Leu-Enkephalin (C10FME-1095), and S,S′-di-(3-succinimido-N-3-propion-4-amido-Fmoc-Leu-Enkephalin) dithiothreitol (LEFFLED-1031). All Fmoc-Enkephalin derivatives had decreased solubility in H2O, high solubility in organic solvents, such as dimethylsulfoxide (DMSO), and remained in solution upon 20 times dilution with 0.01 M NaHCO3. The concentration of each analogue could be determined either by acid hydrolizing an aliquot followed by amino acid analysis or simply by the absorbance at 280 nm. The molar extinction coefficient (ε280) for each analogue is the combined value for Enkephalin (ε280 = 1520), the Fmoc moiety (ε280 = 10,200), and/or the Fmoc-MAL spacer (ε280 = 21,200 (11)).
Figure 1.
Structures and nomenclatures of the Fmoc-Enkephalin analogues investigated in this study. Derivatives are Fmoc-Nα-leucine-Enkephalin (FLE-1077); Fmoc-Nα-methionine-Enkephalin (FME-1091); 3- Thio [11-undecanoic acid], N-3-propiosuccinimido-4-amido-Fmoc-Leu-Enkephalin (C10FME-1095); and S,S′-di-[3-succinimido-N-3-propion-4-amido-Fmoc-Leu-Enkephalin] dithiothreitol (LEFFLED-1031). Dashed arrows indicate the sites of cleavage that take place upon incubation at physiological conditions.
Table 1 summarizes the retention time (Rt) values, obtained by analytical HPLC analyses, for Met- and Leu-Enkephalins and for the Fmoc-Enkephalin analogues prepared by us, using a chromolite R.P18e column. This approach of hydrophobic chromatography yielded an estimate of the relative lipophilic degree for each analogue. Rt values, in an increasing order, were 5.31, 5.69, 8.56, 8.77, 9.14, and 9.58 min for Met-Enkephalin, Leu-Enkephalin, Fmoc-Met-Enkephalin, Fmoc-Leu-Enkephalin, LEFFLED-1031, and C10FME-1095, respectively (Table 1).
Table 1. Rt Values of the Low-Molecular-Weight Enkephalin Analogues on HPLC Chromatographya.
| Rt value (min) | |
|---|---|
| Met-Enkephalin | 5.31 |
| Leu-Enkephalin | 5.69 |
| Fmoc-Met-Enkephalin | 8.56 |
| Fmoc-Leu-Enkephalin | 8.77 |
| Leu-Enkephalin-Fmoc-Fmoc-Dimer(LEFFED-1021) | 9.14 |
| HOOC(CH2)10-Fmoc-Leu-Enkephalin(C10 FME-1095) | 9.58 |
| Rt values of pegylated enkephalin analogues | |
| PEG5-Leu-Enkephalin (irreversible) | 9.17 |
| PEG5-Fmoc-Leu-Enkephalin | 9.94 |
Analytic HPLC was performed using a prepacked chromolith R.P 18e column (4.0 mm × 100 mm Merck KGaA, Darmstadt Germany). The column was eluted with a binary gradient established between solution A (0.1% TFA in H2O) and solution B (acetonitrile/H2O; 75:25 in 0.1% TFA). A linear gradient from 0 to 100% of solution A to solution B was applied over a period of 10 min following 4 min in solution B at a rate of 3 mL/min.
Rate of Release of Met/Leu-Enkephalin under Physiological Conditions
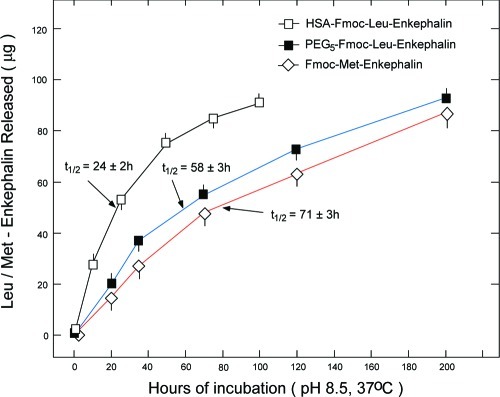
Incubation of all Fmoc-Enkephalin analogues at pH 10.3 for 30 h at 25 °C released the nonmodified Met/Leu-Enkephalin from the conjugates and/or from the Fmoc Enkephalin derivatives in a quantitative fashion (not shown). In Figure 2, three representative categories of the Enkephalin analogues studied, namely, HSA-Fmoc-Leu-Enkephalin, PEG5-Fmoc-Leu-Enkephalin, and Fmoc-Met-Enkephalin, were incubated in 0.1 M borate buffer (pH 8.5 and 37 °C), and the amount of released Enkephalin as a function of time was quantified by HPLC analysis. At pH 8.5, the rate of Fmoc hydrolysis was nearly identical to that obtained in normal human serum (NHS) at 37 °C (6,7). Previously, we found that the rate of peptide release from Fmoc upon incubation in enzyme-inactivated-heat-treated NHS is the same as that obtained in untreated NHS indicating that the hydrolysis is a spontaneous, nonenzymatic process (unpublished observations). As shown in Figure 2, Leu/Met-Enkephalin is released from the analogues in a slow and homogeneous fashion, with half-lives of 24 ± 2, 58 ± 3, and 71 ± 3 h for HSA-Fmoc-Enkephalin, PEG5-Fmoc-Enkephain, and Fmoc-Met-Enkephalin, respectively (Figure 2).
Figure 2.
Rate of Enkephalin released from HSA-Fmoc-Enkephalin; PEG5-Fmoc-Enkephalin; and Fmoc-Met-Enkephalin upon incubation at pH 8.5 and 37 °C. Solutions of HSA-Fmoc-Enkephalin, PEG5-Fmoc-Enkephalin, or Fmoc-Met-Enkephalin, each containing 100 μg/mL covalently linked Enkephalin, were incubated at 0.1 M sodium-borate buffer at pH 8.5 and 37 °C. At the indicated time points, aliquots (0.1 mL) were analyzed by HPLC for the amount of released Met/Leu-Enkephalin.
Antinociceptive Effect of Fmoc-Enkephalins
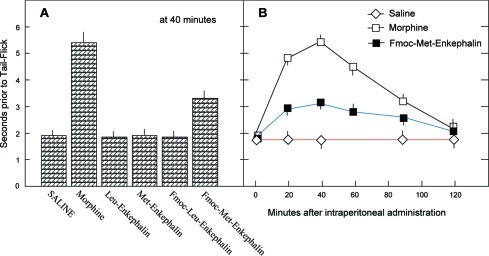
In Figure 3, Leu-Enkephalin, Met-Enkephalin, and the corresponding Fmoc-Enkephalin analogues were intraperitoneally administered to mice, at a dose of 20 μmol/kg body weight, and their analgesic effect was studied using the tail flick test (experimental part). Neither Met-Enkephalin nor Leu-Enkephalin nor Fmoc-Leu-Enkephalin was active at this dose (Figure 3A). Intraperitoneally administered Fmoc-Met-Enkephalin, although slightly less lipophilic than Fmoc-Leu-Enkephalin (Table 1), has facilitated an analgesic effect amounting to ∼35% of that induced by morphine (Figure 3A). The time course of this effect resembled that of intraperitoneally administered morphine (Figure 3B). The effect was first seen 20 min after administration, reaching its maximal response at 40 min, and returned to its basal level 2 h after administration (Figure 3B).
Figure 3.
Extent and time course of antinociceptive response following intraperitoneal administration of Enkephalins and derivatives. (A) Enkephalins and their Fmoc analogues were administered intraperitoneally (0.2−0.3 mL) to ICR male mice (n = 5 per group), at a dose of 20 μmol/kg body weight, and assayed by the tail flick test. Results are expressed as seconds prior to tail flick. (B) Time course of response after intraperitoneal administration. Each point is the arithmetic mean ± SEM from 5 male ICR mice.
Lipophilic Analogues of Leu-Enkephalin Are Analgesic
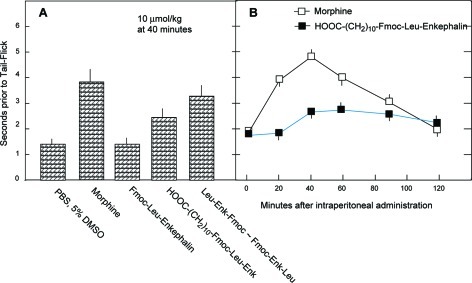
Since intraperitoneally administered Fmoc-Leu-Enkephalin was not analgesic (Figure 3), two Fmoc-Leu-Enkephalin derivatives were synthesized, both of which are more lipophilic than Fmoc-Leu-Enkephalin as judged by HPLC chromatography. The Rt value of Fmoc-Leu-Enkephalin has shifted from 8.77 to 9.14 min for LEFFLED-1021 and to 9.58 min for 10-FME-1095 (Table 1). Indeed, both of these analogues were markedly analgesic following intraperitoneal administration. At 40 min after administration, their antinociceptive effect amounted to 43% and 79% of that manifested by morphine, respectively (Figure 4A). Analysis of the time course of this effect revealed that it began following a lag period of about 20 min, reached its maximal effect at 40 min, and then slowly decreased over a period of 80 min, slightly exceeding at 2 h the antinociceptive effect of morphine (Figure 4B).
Figure 4.
Intraperitoneal administration of Fmoc-derived lipophilic Leu-Enkephalin analogues produces analgesia. (A) The indicated Enkephalins and analogues were administered intraperitoneally, each at a concentration of 10 μmol/kg body weight, and analyzed for their analgesic effect in mice, 40 min after administration. (B) Time course of the antinociceptive response following intraperitoneal administration of HOOC(CH2)10-Fmoc-Leu-Enkephalin (C10FME-1095). Each group consisted of 5 mice. Each point in the figure is the arithmetic mean ± SEM from 5 male ICR mice.
Cationized HSA-Fmoc-Enkephalin Is Analgesic Following Intraperitoneal Administration
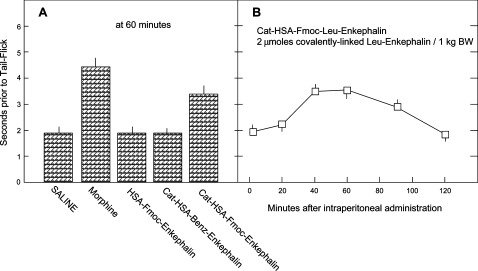
As shown in Figure 5, cationized HSA-Fmoc-Enkephalin has facilitated an antinociceptive effect following intraperitoneal administration. Analgesia was manifested at a low dose (2 μmol/kg body weight) and amounted to 55% of that obtained by intraperitoneally administered morphine. Maximal effect was obtained 40 min after administration (Figure 5B). Neither noncationized HSA-Fmoc-Enkephalin nor the irreversibly linked cationized HSA-Benz-Enkephalin was analgesic at this concentration (Figure 5A).
Figure 5.
Analgesic effect of peritoneally administered cationized HSA-Fmoc-Enkephalin. (A) The indicated Leu-Enkephalin analogues, were administered intraperitoneally, each at a concentration of 2 μmol per 1 kg body weight, and analyzed for their analgesic effect 60 min after administration. (B) Time course of the antinociceptive response following intraperitoneal administration of cationized HSA-Fmoc-Leu-Enkephalin. Each point in the Figure is the arithmetic mean ± SEM from 5 male ICR mice.
Peripherally Administered PEG5-Fmoc-Enkephalin Conjugates Produce an Analgesic Effect
Intraperitoneal administration of PEG5-Fmoc-Leu-Enkephalin at a concentration of 8 μmol/kg body weight facilitated an antinociceptive effect in mice, amounting to 30 ± 3% of maximal stimulation. A triple intraperitoneal dosage of irreversibly linked PEG5-Leu-Enkephalin was ineffective (Figure 6). Both PEG5-enkephalin analogues studied were considerably hydrophobic (Rt value > 9.15 min) on the basis of their migration pattern on a chromolite R.P18e column (Table 1).
Figure 6.
Reversibly pegylated derivatives of Leu-Enkephalin are analgesic following intraperitoneal administration. The indicated Leu-Enkephalin derivatives were administered at the indicated concentrations, and the antinociceptive response was assayed 60 min after ip administration and compared to that of intraperitoneally administered morphine (10 μmol/kg body weight) and PBS. Each determination is the arithmetic mean ± SEM from 5 male ICR mice.
Discussion
Lipid solubility is a major factor that determines the rate by which a drug passively crosses the BBB. Liphophilicity of peptides, however, is governed by multiple factors such as flexibility, conformation, as well as by the biochemical properties of the constituent amino acids and their arrangement. Elevating the liphophilicity of a given peptide agonist without losing its binding specificity and biological potency is in most cases a hit or miss process that must be assessed on a peptide by peptide basis (reviewed in ref (4)). This issue is further complicated with regard to peripherally administered peptides that should preserve some hydrophilicity in order to migrate from the intraperitoneal space into the bloodstream, before entering brain capillaries (20).
The same complexity is valid with regard to the issue of vector- mediated or conjugated peptides. If such conjugates are being inactivated upon conjugation, a reasonable solution might be a reversible linkage between the BBB-transport-vector and the nontransportable neuropeptide agonist. The peptide must be released unmodified in a slow, continuous fashion at physiological conditions.
Here, we describe strategies that enable a viable blood-to-brain peptide delivery. The first can be named induced reversible lipophilization. The introduction of an Fmoc to the α-amino side chain of Met-Enkephalin has elevated its lipophilicity, turned it into a prodrug releasing Met-Enkephalin at physiological conditions, and induced an analgesic response following intraperitoneal administration in mice (Figures 2 and 3). A similar derivatization of Leu-Enkephalin has produced an analogue that is slightly more lipophilic than Fmoc-Met-Enkephalin (Table 1), nevertheless ineffective following intraperitoneal administration. We therefore engineered more heavily lipophilic Leu- Enkephalin derivatives (C10FME-1095 and LEFFLED-1031), which proved to be markedly analgesic following peripheral administration (Figure 4). Thus, as peptide inactivation is of no obstacle with this approach, lipophilzation can be introduced to any peptide of interest to a level requiring enforcement of its passage through the BBB by passive diffusion.
With regard to vector-mediated brain delivery, the stable (nonhydrolizable) linkage between the vector and the peptide has been replaced by an Fmoc-based spacer. Cationized HSA linked to Leu-Enkephalin this way (cat HSA-Fmoc-Enkephalin) releases the covalently linked Leu-Enkephalin upon incubation at physiological conditions (t1/2 = 24 ± 2 h, Figure 2) and facilitates a marked analgesic effect following intraperitoneal administration in mice (Figure 5).
Of special interest is our finding of PEG5-Fmoc-Leu-Enkephalin being analgesic following intraperitoneal administration (Figure 6). PEG conjugation masks the peptide/protein surface and therefore reduces proteolysis, toxicity, immunogenicity, and clearance by renal ultrafiltration, and increases solubility (reviewed in refs (21−23)). In theory, pegylation, by virtue of the increase in molecular size and hydrophilicity is not expected to increase BBB permeability; however, this point has not been extensively studied (4).
As we have noted here, the PEG5-Enkephalin analogues prepared, elute from the RP-18e column with Rt values in the range of 9.17−9.94 min (Table 1), namely, within the range where the intraperitoneally administered low-molecular-weight Enkephalin analogues were markedly analgesic (Rt 9.14−9.6 min; Table 1 and Figure 4). We speculate that the addition of Fmoc to the PEG5-Leu-Enkephalin (Rt 9.169 min) adds lipophilicity (and reversibility) to PEG5-Fmoc-Enkephalin as judged by HPLC chromatography (Rt = 9.943 min) and therefore permits BBB penetration and a viable effect on brain tissue.
In summary, we have overcome the obstacles of BBB nontransportable peptides and of inactive conjugates that fail to release the active peptide in the brain tissue. As far as we know, our reversible-linking procedure is presently the only one capable of releasing the covalently linked peptide, in its native form, by spontaneous chemical hydrolysis, and the rate of release appears sufficient to maintain the nanomolar level of active Enkephalin that is required to promote an analgesic effect in brain tissue (24). This and other aspects brought forth in this study are currently under investigation.
Methods
Enkephalin Analogues
The enkephalin analogues applied in this study can be classified into low-molecular-weight derivatives (see structures and nomenclature in Figure 1) and high-molecular-weight Enkephalin analogues, namely, HSA-Fmoc-Enkephalin, cationized HSA-Fmoc-Enkephalin, cationized HSA-Benz-Enkephalin, PEG5-Enkephalin, and PEG5-Fmoc-Enkephalin. The synthesis, purification, and characterization of all studied Enkephalin analogues are described in detail in the Supporting Information. MAL-Fmoc-OSu was synthesized as described in detail in ref (11).
Antinociceptive Studies
Antinociceptive studies were carried out in male ICR-mice (20−30 g) using the tail-flick assay essentially as described in refs (17−19). All Enkephalin analogues used were administered intraperitoneally (0.2−0.3 mL). All experiments also included two control groups of mice, one receiving the medium (saline or PBS) and the other morphine-HCl at a dose of 10 or 20 μmol/kg body weight. Complete (100%) antinociceptive response was taken here as that obtained in the morphine-treated group minus that obtained in the PBS (or saline)-treated group of mice (n = 5 per group).
Acknowledgments
We thank Yigal Avivi for editing the manuscript.
Abbreviations
HSA, human serum albumin; HPLC, high-performance liquid chromatography; MIB-NHS, m-maleimido benzoic acid-N-hydroxysuccinimide ester; DTNB, 5,5′-dithiobis (2-nitrobenzoic acid); PEG, polyethylene glycol; PBS, phosphate buffer saline; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; Fmoc, 9-fluorenylmethoxycarbonyl; Fmoc-OSu, 9-fluorenylmethoxycarbonyl-N-hydroxysuccinimide; MAL-Fmoc-OSu, 9-hydroxymethyl-2-(amino-3-maleimidopropionate)-fluorene-N-hydroxysuccinimide; leu-enkephalin, leucine enkephalin; met-enkephalin, methionine enkephalin; PEG5-SH, a 5 kDa polyethylene glycol linked to reduce cystamine; PEG5-Fmoc-Enkephalin, a conjugate of PEG5-SH and Leu-Enkephalin covalently linked through the MAL-Fmoc spacer; HSA-Fmoc-Enkephalin, a conjugate of HSA linked to Leu-Enkephalin through a MAL-Fmoc spacer; Cat-HSA-Fmoc-Enkephalin, a conjugate of cationized HSA linked to Leu-Enkephalin via a MAL-Fmoc spacer; Cat-HSA-Benz-enkephalin, a conjugate of cationized HSA, linked to Leu-Enkephalin through a maleimido benzoate spacer.
Supporting Information Available
Preparation of PEG5-Enkephalin, PEG5-Fmoc-enkephalin, preparation of HSA-Fmoc-Enkephalin, cationized-HSA-Fmoc-enkephalin, cationization of HSA, cationized HSA-Fmoc-Enk, and preparation of cationized HSA-Benz-enkephalin, Fmoc-met-enkephalin and Fmoc-leu-Enk, 3-Thio (11-undecanoic acid) N-3-propionsuccinimido-4-amido-Fmoc-Leu-Enkephalin (10 FME-1095), and S,S′-Di-(3-succinimido-N-3-propion-4-amido-Fmoc-Leu-Enkephalin) dithiothreitol (LEFFLED-1031). This material is available free of charge via the Internet at http://pubs.acs.org.
Y.S. is the incumbent of the C.H. Hollenberg Chair in Metabolic and Diabetes Research established by the friends and associates of Dr. C. H. Hollenberg of Toronto, Canada, and M.F. is The Lester Pearson Professor of Protein Chemistry.
Supplementary Material
References
- Hruby V. J., Kazmierski W., Kawasaki A. M., and Matsunaga T. O. (1991) Synthetic Chemistry and the Design of Peptide-Based Drugs, in Peptide Pharmaceuticals (Ward D. J., Ed.) Chapter 5, pp 135−184, Open University Press, Buckingham, U.K. [Google Scholar]
- Knapp R. J., Davis T. P., Burks T. F., and Yamamura H. I. (1991) In Peptide Pharmaceuticals, ed. (Ward D. J., Ed.) Chapter 7, pp 210−242, Open University Press, Buckingham, U.K. [Google Scholar]
- Partridge W. M. (1991) Peptide Drug Delivery to the Brain, Raven Press, New York. [Google Scholar]
- Witt K. A.; Gillespie T. J.; Huber J. D.; Egleton R. D.; Davis T. P. (2001) peptide drug modifications to enhance bioavailablility and blood-brain-barrier permeability. Peptides 22, 2329–2343. [DOI] [PubMed] [Google Scholar]
- Partridge W. M. (2005) The blood-brain barrier: bottleneck in brain development. J. Am. Soc. Exp. Neuro Ther. 2, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershonov E.; Shechter Y.; Fridkin M. (1999) Spontaneous conversion of an inactive modified insulin to the active hormone in circulation: 9-fluorenylmethoxycarbonyl derivative of insulin. Diabetes 48, 1437–1442. [DOI] [PubMed] [Google Scholar]
- Gershonov E.; Goldwaser I.; Fridkin M.; Shechter Y. (2000) A novel approach for a water-soluble long-acting insulin prodrug: Design preparation and analysis of [2-sulfo-9-fluorenylmethoxycarbnyl]3-insulin. J. Med. Chem. 43, 2530–2537. [DOI] [PubMed] [Google Scholar]
- Shechter Y.; Goldwaser I.; lavon I.; Gershonov E.; Mester B.; Mironchik M.; Patt L.; Fridkin M. (2001) A new approach for prolonging the half-life of peptide, proteins and low-molecular-weight drugs in vivo. Drugs Future 26, 669–676. [Google Scholar]
- Shechter Y.; Patt P.; Schreiber G.; Fridkin M. (2001) Prolonging the half-life of human interferon-2 in circulation; design, preparation and analysis of (2-sulfo-9-fluorenylmethoxycarbonyl)7 interferon-2. Proc. Natl. Acad. Sci. U.S.A. 98, 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y.; Tsubery H.; Fridkin M. (2002) N-[2-sulfo-9-fluorenylmethoxycarbonyl]3-gentamicin C1 is a long acting prodrug derivative. J. Med. Chem. 45, 4264–4270. [DOI] [PubMed] [Google Scholar]
- Tsubery H.; Mironchik M.; Fridkin M.; Shechter Y. (2004) Prolonging the action of protein and peptide drugs by a novel approach of reversible polyethylene glycol modification. J. Biol. Chem. 279, 38118–38124. [DOI] [PubMed] [Google Scholar]
- Shechter Y.; Tsubery H.; Mironchik M.; Rubinstein M.; Fridkin M. (2005) Reversible pegylation of peptide PYY3−36 prolongs its inhibition of food intake in mice. FEBS Lett. 579, 2439–2444. [DOI] [PubMed] [Google Scholar]
- Shechter Y.; Mironchik M.; Saul A.; Gershonov E.; Sasson K.; Tsubery H.; Fridkin M. (2007) New technologies to prolong life-time of peptide and protein drugs in vivo (a review). Int. J. Peptide Res. Ther. 13, 105–117. [Google Scholar]
- Shechter Y.; Mironchik M.; Rubinraut S.; Saul A.; Tsubery H.; Fridkin M. (2005) Albumin-insulin conjugate releasing insulin slowly under physiological conditions; A new concept for long-acting insulin. Bioconjugate Chem. 16, 913–920. [DOI] [PubMed] [Google Scholar]
- Banks W. A.; Kastin A. J. (1990) Peptide transport systems for opiates across the blood-brain barrier. Am. J. Physiol. 159, E1–E10. [DOI] [PubMed] [Google Scholar]
- Egleton R. D.; Mitchell S. A.; Huber J. D.; Tanders J.; Strropova D.; Polt R.; Yamamura H. I.; Hurby v.J.; Davis T. P. (2000) Improved bioavailability to the brain of glycosylated met-enkephalin analogs. Brain Res. 881, 37–46. [DOI] [PubMed] [Google Scholar]
- D′Amour F. E.; Smith D. L. (1941) A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 72, 74–79. [Google Scholar]
- Gupta S.; Pasha S.; Gupta Y. K.; Bardway D. K. (1999) Chimeric peptide of met-enkephalin and FMRFa induces antinociception and attenuates development of tolerance to morphine antinociception. Peptides 20, 471–478. [DOI] [PubMed] [Google Scholar]
- Gupta S.; Pasha S.; Gupta Y. K.; Bardway D. K. (2001) Effects of intracerebro-ventricularly administered chimeric peptide of met-enkephalin and FMRFa-[D-Ala2]YFa-on antinociception and its modulation in mice. Brain Res. Bull. 55, 51–57. [DOI] [PubMed] [Google Scholar]
- Polt R.; Porreca F.; Szabo L. Z.; Bilsky E. J.; Davis P.; Abbruscato T. J.; Davis T. P.; Horvath R.; Yamamura H. I.; Hruby V. J. (1994) Glycopeptide enkephalin analogues produce analgesia in mice: Evidence for penetration of the blood-brain barrier. Proc. Natl. Acad. Sci. U.S.A. 91, 7114–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerteges F.; Abuchowski A. (1990) The clinical efficacy of poly(ethyleneglycol) modified proteins. J. Controlled Release 11, 139–148. [Google Scholar]
- Delegado C.; Francis G. E.; Derek F. F. (1992) The uses and properties of PEG-linked proteins. Crit. Rev. Ther. Drug Carrier Syst. 9, 249–304. [PubMed] [Google Scholar]
- Reddy K. R. (2000) Controlled-release, pegylation liposomal formulations: new mechanisms in the delivery of injectable drugs. Ann. Pharmacother. 34, 915–923. [DOI] [PubMed] [Google Scholar]
- Weber S. J.; Greene D. L.; Sharma D. D.; Yamamura H. I.; Kramer T. H.; Burks T. F.; Hruby V. J.; Hersh L. B.; Davis T. P. (1991) Distribution and analgesia of [3H][D-Pen2, D-Pen5]enkephalin and two halogenated analogs after intravenous administration. J. Pharmacol. Exp. Ther. 259, 1109–1117. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.